Abstract
The accessibility of clean water and green environment are the major requirements for survival and sustainable development. In this study, iron-coated sand, derived from acid mine drainage effluent, has been applied as a heterogeneous catalyst. Treatment with H2O2, iron-coated sand, and iron-coated sand-Fenton processes is compared for the COD removals from municipal wastewater effluent. The results showed that the iron-coated sand catalyzed Fenton process could generate hydroxyl radicals (•OH) and oxidize the organic pollutants. Fenton process based on the iron-coated sand catalyst proved to be the most efficient process. The effect of operating conditions such as initial pH and Fenton’s reagent doses, i.e. initial H2O2 and iron on the organics oxidation from such wastewater was investigated. The results showed that 70% removal efficiency of COD was obtained within 30 min under optimized conditions (pH 3.0, H2O2 400 mg/L and iron 40 mg/L). The rate equation of iron-coated sand-Fenton system was simply expressed by the second-order equation and the model was found to fit well the data. Thermodynamic analysis of the results indicated that the iron-coated sand-Fenton oxidation is non-spontaneous and endothermic in nature. Regeneration of iron-coated sand was attempted and the catalyst had a good stability and reusability for successive treatments and reducing the quantity of sludge produced in Fenton reactions. Thus, expanding the sustainability scope of iron-coated sand based Fenton catalyst and offer new sustainable and inexpensive alternatives for the classical Fenton process.
Article Highlights
-
Iron recovered from acid mine drainage to prepare iron coated-sand as a Fenton source.
-
Novel Fenton reaction is proposed for treating polymer industry wastewater.
-
The system avoids iron sludge by-product by providing catalyst reusability.
-
The approach points the competitive novel iron waste source to applied as a Fenton technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is an increase in metals released from industrial waste into watercourse causing considerable concern for both aquatic organisms and human health (Ashour et al. 2014; Tony et al. 2015; Sylwan et al. 2016). One of those metals is iron that is a major source of water deterioration in coal and rock mining regions. Acid mine drainage (AMD) containing dissolved ferrous iron solution and sulphuric acid is formed through the interaction of iron pyrite with water (Bwapwa et al. 2017; Deng et al. 2019; Tony 2020a). Hence, the polluted AMD stream consists of various heavy metals and damages the surrounding environment. For a sustainable environment, the recovery of precipitated metals from AMD is a research objective for gaining both valuable products and meeting the discharging limitations. Therefore, effective different techniques, i.e. physical, chemical, and biological approaches have been applied for metal recovery from AMD (Wei et al. 2005). Chemical precipitation of AMD to recover iron and aluminum metals is considered a typical way due to the suitability for large or small flow, inexpensive and easy to operate (Wei et al. 2005). But, the additional concern is still associated with the disposal of the concentrated metal laden sludge (Lu et al. 2012; Tony 2020b).
Improvement of domestic wastewater management has received increasing attention throughout the world since houses generate a considerable amount of contaminated waste (Huling et al. 2000; Tony et al. 2019). Municipal wastewater effluents release constantly complex organic mixture containing suspended and dissolved materials (Vlyssides et al. 2003; Zhao et al. 2009). Those contaminants should undergo a significant treatment prior to the release into the environment. According to the literature, many of the treatment technologies available for wastewater treatment include physical and chemical treatments such as adsorption (Ashour and Tony 2017), membrane separation (Tatsi et al. 2003), filtration (Peng et al. 2011; Tony et al. 2012; Nitoi et al. 2013), ion exchange (Cantinh et al. 2016), chemical precipitation (Rule et al. 2006), and oxidation (Najjar et al. 2001). However, those treatment approaches produce a secondary sludge besides the lack of complete removal. Recently, advanced treatment techniques have emerged to be a better alternative to avoid the problem associated with the solid waste pollution and its disposal (Mohan et al. 2011; Tony et al. 2011; Liu et al. 2018).
Among the advanced techniques able to oxidize wastewater, advanced oxidation processes (AOPs), which are able to oxidize almost any organic molecule through the generation of highly reactive intermediates “hydroxyl radicals”, is a particularly attractive option. The most versatile and efficient among AOPs is Fenton’s reagent (Fe2+/3+/H2O2). Fenton’s reagent has evolved as the front line of AOPs (Tony et al. 2016). However, based on the previous reports, there are disadvantages of AOPs that are associated with their high operating costs due to the relatively large amounts of oxidants and/or catalysts consumed. Although application of the Fenton reaction in wastewater treatment is well developed, it is necessary to improve the system. Controlling the process and introducing a sustainable catalyst may move the process for commercial applications (Tony 2019). Thus, searching for an efficient and sustainable modified Fenton system is gaining a concern.
Previous work has already shown that iron could be recovered from AMD (Tony and Lin 2020a, b) and this is introduced to produce iron-coated sand that is an efficient absorbers of large organic molecules; however, their affinity to oxidize pollutants as the source of modified Fenton’s reagent has not before been investigated. Additionally, for practical applications and due to the particles agglomeration, iron from AMD should be coated onto some supports such as sand, bentonite, perlite etc. Furthermore, the support material overcomes the difficulty of catalyst separation, low hydraulic conductivity and excessive pressure drops when applied in flow through systems (Rasheed and Meera 2016).
Iron-coated sand from AMD is expected to achieve an efficient organic removal from wastewater due to using the waste iron source, AMD. Additionally, it is expected that this system is more applicable for sustainable treatment processes due to its granular nature. Herein, the goal of this investigation is to examine the feasibility of the modified Fenton’s system based on AMD to oxidize organics in municipal wastewater effluents. Optimization of operational parameters of the modified Fenton system is examined and reaction kinetics and thermodynamic parameters are reported. Also, the reusability of the iron-coated sand catalyst is investigated to confirm the catalyst sustainability.
Materials
Iron material
Iron source is precipitated from acid mine drainage (AMD) from Morgantown, West Virginia, USA (Marion Meadows site), using a selective precipitation method. To overcome the problems associated with using iron oxide powders in wastewater oxidation treatments and for the sustainable catalyst use through successive treatments, coating iron oxide on sand surface is designed. The iron-coated sand was previously prepared in laboratory in West Virginia University, USA. The pH of the AMD was adjusted for iron precipitation and the metal is allowed to settle overnight. The supernatant was siphoned off and the precipitate is applied to the sieved sand in the standard coating procedure by mixing the iron material selectively extracted from the raw AMD (Wei et al. 2005) with grey sand particles, followed by drying the mixture in an oven at 103 °C. Thereafter, such procedure was further repeated for three times followed by heating the coated sand particles in a muffle furnace at 500 °C. The amount of iron concentration on the sand surface was examined and found to be 9.5 mg-Fe/gm-sand. Also, the material analysis showed the presence of silica, iron and aluminum oxides. Additionally, the crystal structure of hematite, oxygen ions are hexagonally close packed, and iron is present in octahedral sites.
Wastewater sampling
On-site municipal wastewater samples were collected from Star City wastewater treatment plant in Morgantown, West Virginia, USA after the primary clarifier. Samples were taken to laboratory and kept at 4 °C for storing in closed high-density polyethylene tanks according to the standard methods (APHA 2005) without a pre-treatment. Samples were analyzed in the WVU laboratories in accordance with Standard Methods for the Examination of Water and Wastewater (APHA 2005). The parameters measured included chemical oxygen demand (COD), total oxygen demand (TOC), total phosphate, ammonia, sulphate and pH. The main characteristics of the wastewater are presented in Table 1.
Methodology and analytical determination
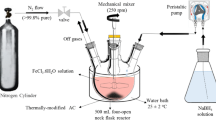
The Fenton process was performed in a magnetically stirred Fenton reactor (0.5 L). The wastewater was subjected to the reactor after analysis. The pH of the wastewater was adjusted, if needed, using sulfuric acid (H2SO4). Then, the required iron-coated sand is added thereafter the Fenton’s reaction is initiated with the addition of H2O2 for oxidizing the wastewater. All chemicals used in this study are of analytical-grade. The graphical representation of the treatment mechanism is illustrated in Fig. 1.
According to the standard protocol determinations (APHA 2005), wastewater samples were examined. Chemical Oxygen Demand (COD) concentration was investigated by COD analyser (HACH, DR1900 spectrophotometer). Total Organic Carbon (TOC) was analyzed using TOC analyser (TOC-L CHS/CSN Shimadzu, Japan). Phosphate, ammonia, and sulphate were performed spectrophotometrically (Genesys 10uv, Thermo Electron Corporation, USA). Also, iron is quantified by atomic absorption spectrophotometer with an acetylene flame (Perkin-Elmer, 3100). Samples for SEM/EDAX were performed using ((FE-SEM, Quanta FEG 250) field-emission scanning electronic microscope with an energy dispersive X-ray spectroscopy (EDAX) analysis for investigating the Fe content in the iron-coated sand catalyst after treatment were examined for catalyst sustainability. After attaining the organics oxidation, samples were filtered through a micro filter and the residual organics concentration was determined using COD analyzer.
Results and discussion
Effect of reaction time on different oxidation systems
Comparison of efficiencies of H2O2, Fenton based iron-coated sand and iron-coated sand processes on the organics removal from municipal wastewater is presented in Fig. 2, simultaneously with the investigation of the reaction time. For the object of comparison of these processes, the optimal concentrations of iron (40 mg/L) and H2O2 (400 mg/L) in each case are used at a fixed pH of 7.1.
For all the system examined, after 30 min of reaction time, the iron and/or H2O2 are mainly consumed. Therefore, the production of •OH radicals for H2O2 and Fenton systems was high and the COD removal rate grew faster during initial oxidation period. But, the •OH concentration is dramatically decreased as the reaction proceeds and hence COD removal rate is declined with the time increase. Furthermore, other radicals are formed which inhibit the reaction rate rather than enhancing the COD removal (He and Lei 2004). However, for the solo iron-coated sand material, the treatment could be done by the adsorption of organics onto the surface of the iron-coated sand which is stopped by the surface saturation (Huling et al. 2000).
Among the processes tested here for organics oxidation, the ranking of efficiency of COD removal is as follows: Fenton process > H2O2 > iron-coated sand for organics oxidation in municipal wastewater. The corresponding COD removal rates are 54%, 37% and 27%, respectively. From these results, it can be concluded that the Fenton process is efficient for oxidizing organics in municipal wastewater.
Effect of Fenton catalyst
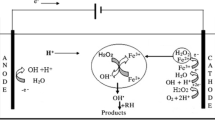
As known for the Fenton reaction, iron and H2O2 content has a significant effect on the system efficiency, since H2O2 is catalyzed by iron to produce the highly reactive intermediates (•OH), which oxidizes the organic pollutants. Mixture of H2O2 and iron in acidic solution generates the •OH radicals that consequently attack the organic pollutants present in the aqueous media and mineralizing them (Eqs. 1–3) (Nitoi et al. 2013). Thus, the initial H2O2 and iron content was investigated by one catalyst changing and keeping all the other parameters constant.
Firstly, to investigate the effect of iron concentration on the organics oxidation, a series of experiments were conducted by varying its concentration from 10 to 80 mg/L, at fixed pH and initial concentration of H2O2. It can be seen (Fig. 3a) that there is an increase in the Fenton’s oxidation rate of COD with the different concentrations of iron from 10 to 40 mg/L. The organics removal rate in wastewater is high for various concentrations of iron especially at the first 10 min of oxidation as presented in Fig. 3a. The results showed that the COD removal increased from 37 to 70% with the iron-coated sand removal from 10 to 40 mg/L. Since Fe2+ acts as a catalyst it is generated through the reaction according to Eqs. (4) and (5) (Liu et al. 2018).
Although iron increase results in an increase in the oxidation rate, further reagent increase more than 40 mg/L results in a decrease in the COD oxidation in municipal wastewater for only 54% organics oxidation. This could be illustrated by the increase in the •OH radicals generated at the optimal iron dose in the Fenton system. Furthermore, unfavorable effect is generated in the reaction system with the excess iron concentration on the media since the excess iron species in the solution reacts with the hydroxyl radicals and renders their performance (Eq. 6) (Rodrıguez-Chueca et al. 2014). Hence, the overall reaction rate is decreased. Therefore for further experiments as an optimal concentration of iron was used 40 mg/L to decrease risk of the excess ions and eventual forming of iron sludge.
To investigate H2O2 reagent effect on the oxidation efficiency, its concentration was changed from 100 to 800 mg/L at pH 3.0 and iron dose of 40 mg/L. As seen in Fig. 3b, the addition of H2O2 from 100 to 400 mg/L increases the COD removal rate from 53 to 70%, respectively. This could be attributed to the increase in produced •OH concentration. But at higher H2O2 concentration (800 mg/L) a decrease in the COD removal is attained due to the •OH radical scavenging effect. This could be attributed to the fact that the recombination of the •OH radicals and the reaction of H2O2 with the •OH radicals that lessening the •OH radicals produced in the media (Eqs. 7–9) (Goto et al. 1998; Janik et al. 2007). This verified the decreasing reaction rate according to the reaction optimum conditions, which may make the best use of •OH. Hence, 400 mg/L of hydrogen peroxide appear as the optimal dosage for the Fenton oxidation system.
Thus, •OH radicals were vastly reduced in this system as a result of the quenching effects of the non-optimal doses of iron ions and H2O2 on such radicals and even those between free radicals were effectively weakened.
Effect of pH
The Fenton oxidation process is extremely dependent on the solution pH. Unfavorable pH of the aqueous media causes diminishing •OH radicals’ concentration and therefore reducing the removal efficiency (Liu et al. 2018; Ashour and Tony 2020). Influence of the municipal wastewater pH in the oxidation rate was examined in this study by varying the initial pH of the aqueous media at fixed Fenton’s catalyst dose (iron 40 mg/L and H2O2 400 mg/L). The results in Fig. 4 reveal that the change of pH from the nature pH of the attained wastewater (7.1) to the acidic pH (3.0) increases the oxidation rate. The COD removal is increased from 54 to 70% with the decrease in the pH from 7.1 to 3.0, respectively. In addition, also the alkaline pH results in a decrease in the COD removal rate as seen from Fig. 4. This could be due to the optimum pH helping in the presence of •OH radicals that are the main responsible of the COD oxidation. Moreover, the •OH radicals’ concentration is sensitive to the pH value. Thereby, Fenton oxidation is insensitive at the alkaline pH medium as the •OH radicals’ concentration is increased to its maximum value at the acidic pH and thus the organics removal rate increased (Najjar et al. 2001; Tony et al. 2015). Also, at the acidic pH media the aqueous solution containing the organometallic complex where either hydrogen peroxide was regenerated which increases the oxidation rate. Additionally, increasing the pH into the alkaline range is unfavorable since it results in the formation of undesired radicals rather than the •OH radicals that reduces the overall reaction rate. This Fenton’s oxidation reaction sensitivity to the acidic pH has been previously reported in the literature by various investigators (Stock et al. 2000; Villegas-Guzmana et al. 2017; Klamerth et al. 2012; Tony and Lin, 2020a; Divyapriya and Nidheesh, 2020).
Additionally, in a similar study (Devi et al. 2014), it was stated that the dissolutions of iron from sand is related to the medium pH. In the low pH range between 2.0 and 5.0, the iron coated on sand is more stable and therefore a larger quantity of iron is coated onto sand is observed. However, a greater amount of iron is dissolute from sand at the alkaline pH. Thus, this confirms the suggested acidic pH is favorable for the iron-coated sand based Fenton’s reaction through the current study.
Temperature effects on kinetics and thermodynamic parameters
The effect of temperature at 289, 313, 323 and 333 K on the municipal wastewater oxidation via Fenton system was investigated. It can be seen from Fig. 5 that increasing temperature from 298 to 313 K has a positive effect on the organics oxidation. The COD removal efficiency is increased from 70 to 80% with the temperature increase from 298 to 313 K, respectively. This could be attributed by the fact that temperature affects the reaction between H2O2 and iron ions that increasing the •OH radicals generation rate and the therefore that Fenton’s reaction is accelerated and thus the organic oxidation in the form of COD removal is increased. Although, further increase in temperature more than 313 K results in a decrease in the COD removal rate that is reached to only 64 and 61% at 323 and 333 K, respectively. This is due to the fact that H2O2 undergoes self-accelerating decomposition at temperature higher than 313 °C. These results are in accordance with that previously reported in the literature by Dutta et al. (2010) for treating olefins contaminating wastewater with such reagent.
For the object of implementation of Fenton oxidation at an industrial level, other reactor configurations such as the continuous or semi-continuous application should be tested. For predicting such reactors’ performance, the knowledge of the reaction kinetics is crucial. So, zero-, first- and second-order reaction kinetic models are used to investigate the organics oxidation in municipal wastewater by iron-coated sand-Fenton oxidation process. The regression analysis based on the kinetic reaction orders for the COD oxidation was conducted and the results are tabulated in Table 2. Generally, regression coefficients (r2) of the linear reaction kinetic formula of the models are compared and the higher correlation coefficient are corresponding to the best fit model. As illustrated in Table 2, the values of correlation coefficient for second-order model are mostly higher than those of the zero-order and the first-order models. Therefore, second-order kinetic model is the best model to describe the oxidation of organics by the modified Fenton’s system. The obtained parameters seen in Table 2 show that as temperature increases from 298 to 313 K, the rate constants of the second-order model increase from 0.0009 to 0.0016 L mg−1 min−1, respectively. But, it decreases again to 0.0008 and reached to 0.0006 L mg−1 min−1 for the temperate raise to 323 and 333 K, respectively. This similar to House (House 1962; Huang et al. 2002) rule, the rate of that reaction doubles for a temperature increase from 295 to 305 K. Moreover, the half-life time (t1/2) of the COD oxidation that corresponding to the second order model increased with the temperature increase up to 313 K followed by a decrease in the half-life time with further temperature increase. Hence, this verified that a temperature controlled the reaction up to 313 K and the COD removal performance declined above this optimal temperature. Similar observation is previously stated in the literature (Anabela et al. 2003). It is also noted that the higher rate constant is related to the increase in temperature till a certain limit which means that the reaction favors a definite high temperature value.
Thermodynamic parameters including Gibbs free energy of activation (ΔG#), activation enthalpy (ΔH#) and activation entropy (ΔS#) are generally the parameters to well understand the temperature effect on the organics oxidation over the oxidation system. The variation of thermodynamic parameters with temperature for the iron-coated sand oxidation process studied and the results are summarized in Table 3. The temperature dependence on the observed second-order kinetic constant can be represented by Arrhenius equation (\({k}_{2}=A exp(\frac{{-E}_{A}}{RT}))\), where A is the frequency factor, EA is the activation energy, R is the gas constant and T is the temperature. The slope (EA/R) of the Arrhenius plot (plot is not shown) of the reaction rate constant for iron-coated sand-Fenton oxidation gives the corresponding activation energy for COD oxidation of 11.104 kJ/mol. This low activation energy observed indicates that the oxidation process requires low energy barrier and can be easily achieved which recommends the high effectiveness of organics oxidation due the •OH attack towards the modified Fenton reaction (Sun et al. 2007). Such observed low activation energy is in well matched with the previous results obtained in other studies using Fenton’s reagent. Previous findings of Lin et al. (1999) experienced activation energies of 10.62 and 8.08 kJ/mol for different types of benzene sulphonate oxidation with Fenton’s reaction. Also, Lin and Lo (1997) described low activation energy for COD oxidation in treating wastewater.
Gibbs free energy of activation (∆G#) is calculated by using equilibrium constant k2 equation: \(\Delta {G}^{\#}=RT\times \left[\mathrm{ln}\left(\frac{{k}_{B}T}{h}h\right)-lnk\right]\) where kB is Boltzmann’s constant, h is Planck’s constant and R is the gas constant. Gibbs free energy of activation (∆G#), the fundamental principal for the oxidation non-spontaneity must be positive for a feasible oxidation to occur. Positive ∆G# values showed that the organics oxidation process is non-spontaneous in nature and that the degree of non-spontaneity of the reaction increased with the temperature increase. This result also supported the suggestion that the resulting reaction mechanism was energetically stable and that the rate of COD oxidation reaction primarily increased with increasing temperature.
The variations of activation enthalpy (∆H#) and of entropy ((∆S#) were calculated as the slope and intercept of van’t Hoff equation (\(\mathrm{ln}{k}_{2}= \frac{-\Delta {H}^{\#}}{RT}+\frac{\Delta {S}^{\#}}{R}\)) plot of lnk2 versus 1/T. The variations of activation enthalpy (∆H#) shows positive values indicate that reaction is endothermic. Moreover, the estimated negative values of entropy of activation (∆S#) confirmed the non-spontaneously nature of the modified Fenton oxidation reaction. This exhibited a decline in the degree of freedom of the organic molecules and maintained a high oxidation yield between the organic molecules and the·OH radicals species. Similar results were also reported by the other researchers Argun and Karatas (2011) and Xu and Lu (2013).
Sustainability test of iron-coated sand
Iron sludge usually forms after the homogenous Fenton’s process, which needs process neutralization before the final disposal is carried out. However, in the heterogeneous modified Fenton’s process, iron catalyst could be separated to reuse for appropriate disposal. The final products of organics oxidation via Fenton’s reaction are carbon dioxide, water and inert salts.
To explore the stability of the catalyst, the organics removal efficiency over the modified Fenton process are assessed and the results are presented in Fig. 6. After each cycle, the catalyst was just recycled by filtration after the former cycle. Then, washing regenerates iron-coated sand catalyst with deionized water followed by drying in oven at 105 °C.
It is observed that the modified Fenton process displayed a slight decay in the iron-based sand catalyst activity between the former six cycles. When the catalyst was reused three times, the COD removal efficiency still remained more than 65%. Although a significant decline in the organics removal efficiency was observed at the fifth reuse, high removal efficiency was restored by regeneration. The COD removal efficiency remained more than 60% after six times of reuse with regeneration. Such decrease in the activity may be due to occupying the active sites of the iron-coated sand by the organics intermediates from municipal wastewater. Iron concentration in the fresh iron coated sand is 9.5 mg-Fe/gm-sand, thereby, after use the exhausted iron oxide-coated sand is regenerated for successive use. After the first use, the average iron dissolution in the wastewater is found to be about 0.3 mg/L after the Fenton’s oxidation reaction. The quantitative analysis of iron showed the iron concentration is reduced to 9.2 mg/L after the first cycle f oxidation. This may be illustrated by the complex formation in the aqueous solution could results in weakening the attractive force between the iron surface and neighboring O ions and, thereby, facilitate the breakdown of Fe–O bonds and hence, helping in the iron dissolution (Sidhu et al. 1981).
The spectrum of iron-coated sand by using elemental microprobe analysis of SEM/EDAX is illustrated in Fig. 7. The SEM micrographs in Fig. 7a, c illustrate that there were a difference after the first use and the sixth cycle. SEM images after the first and sixth cycle of oxidation provide a visual representation of the iron-coated sand particles. Observation of these micrographs identify the slightly reduction of iron coating available for oxidation as a result of Fenton reaction. Noticeably the surface of the catalyst becomes slightly smooth after the Fenton oxidation as the iron catalyst is reduced after the successive cycles.
EDAX analysis signified that the retained catalyst contained four main elements, namely O, Al, Si and Fe signals. Figure 7b, d show the energy dispersion X-ray spectrometer (EDAX) spectra for the iron-coated samples after the first and sixth oxidation cycles. As seen in the results of EDAX analysis after the first cycle of use and the sixth oxidation cycle, the percentage of Fe by weight deposited on sand surface is decreased from 21.49 to 17.61%, respectively. This illustrates the coated iron usage on the Fenton’s reaction and this reduction on the iron content reduces the reaction efficiency after each cycle. The slight difference intensities of the peaks for iron indicate that the small differences in the iron content resulted in a slight higher amount of iron coating on the sand and hence attributed in the reduction of the process efficiency from 70% into 61%.
The concentrations of regenerated coated sand after each cycle were determined and its total amount after sixth cycles of reaction was less than 0.6%. Thus, Fenton process based on using AMD wastewater as an iron source tends to be less expensive to build and operate than conventional treatment systems. Hence, this process is a promising sustainable environmental engineering technology. Although on the other hand, the operations of Fenton-type systems usually require strict pH control as they are working efficiently at pH around 3.0.
Conclusion
Overall, the study has proved that iron-coated sand can effectively be used as a source of modified Fenton’s reagent to oxidize organics from municipal wastewater and the organics removal efficiency depends on a range of experimental conditions. In the applied iron-coated sand-Fenton process, organics in wastewater are oxidized through Fenton process and the optimum pH was at 3.0 which corresponding to the utmost COD removal (70%) using 40 and 400 mg/L of iron and H2O2 doses, respectively. This investigation is also supported by the second-order kinetic. The optimum operating temperature was 313 K which corresponding to the highest COD removal and this is proven by its kinetic constant that was outstandingly higher than at other temperatures. Thermodynamic parameters were also studied and the activation energy for COD oxidation by the modified Fenton process was estimated to be in a low energy barrier (11.104 kJ/mol). Additionally, the catalyst reusability investigated a good stability over the iron-coated sand regeneration. Notably, this study proved that this modified iron-coated sand-Fenton system based on the acid mine drainage waste which overcome the drawback of the homogeneous catalyst, could clarify the increase in applicability of the Fenton oxidation with a great oxidation efficiency.
References
APHA (2005), Standard Methods for the Examination of Water & Wastewater (Port City Press, Baltimore, MD, 2005). American Water Works Association; Water Environment Federation
Ashour EA, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: kinetics and isotherm studies. Am J Chem Eng 2(6):92–98
Ashour A, Tony MA (2017) Equilibrium and kinetic studies on biosorption of iron (II) and iron (III) ions onto eggshell powder from aqueous solution. Appl Eng 1(3):65–73
Ashour A, Tony MA (2020) Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: activation and modification effects. SN Appl Sci 2:2042. https://doi.org/10.1007/s42452-020-03873-x
Anabela MFM, Guedes AM, Madeira LM, Boaventura RA, Costa CA (2003) Fenton oxidation of cork cooking wastewater—overall kinetic analysis. Water Res 37:3061–3069
Argun ME, Karatas E (2011) Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: kinetics and thermodynamics. Environ Prog Sus Ener 30(4):540–548
Bradu C, Frunza L, Mihalche N, Avramescu S, Neata M, Udrea I (2010) Removal of reactive black 5 azo dye from aqueous solutions by catalytic oxidation using CuO/Al2O3 and NiO/Al2O3. Appl Catal B 96(3–4):548–556
Bwapwa JK, Jaiyeola AT, Chetty R (2017) Bioremediation of acid mine drainage using algae strains: a review. S Afr J Chem Eng 24:62–70
Cantinh P, Matos M, Trancoso MA, Correia dos Santo MM (2016) Behaviour and fate of metals in urban wastewater treatment plants: a review. Int J Environ Sci Technol 13:359–386
Deng D, Lin O, Rubenstein A, Weidhaas JL, Lin L-S (2019) Elucidating biochemical transformations of Fe and S in an innovative Fe(II)-dosed anaerobic wastewater treatment process using spectroscopic and phylogenetic analyses. Chem Eng J 358:1208–1217
Devi RR, Umlong IM, Das B, Borah K, Thakur AJ, Raul PK, Banerjee S, Singh L (2014) Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Appl Water Sci 4:175–182
Divyapriya G, Nidheesh PV (2020) Importance of graphene in the electro-Fenton process. ACS Omega 5(10):4725–4732
Dutta B, Jana S, Bhattacharjee A, Gutlich P, Iijima S, Koner S (2010) γ-Fe2O3 nanoparticle in NaY-zeolite matrix: preparation, characterization and heterogeneous catalytic epoxidation of olefins. Inorg Chim Acta 363:696–704
Goto JJ, Gralla E, Valentine JS (1998) Reactions of hydrogen peroxide with familial amyotrophic lateral sclerosis mutant human copper-zinc superoxide dismutases studied by pulse radiolysis. J Bio Chem 273(46):30104–30109
He F, Lei L (2004) Degradation kinetics and mechanisms of phenol in photo-Fenton process. J Zhejiang Univ 5(2):198–205
House DA (1962) Kinetics and mechanism of oxidations by peroxydisulfate. Chem Rev 62:185–203
Huang K-C, Couttenye RA, Hoag GE (2002) Kinetics of heat assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 49:413–420
Huling S, Arnold R, Sierka R, Jones P, Fine D (2000) Contaminant adsorption and oxidation via Fenton reaction. J Environ Eng 126(7):595–600
Janik I, Bartels DM, Jonah CD (2007) Hydroxyl radical self-recombination reaction and absorption spectrum in water up to 350 °C. J Phys Chem A 111(10):1835–1843
Klamerth N, Malato AS, Aguuera A, Fernandez-Alba A, Mailhot G (2012) Treatment of municipal wastewater treatment plant effluents with modified photo-fenton as a tertiary treatment for the degradation of micro pollutants and disinfection. Environ Sci Technol 46(5):2885–2892
Lin SH, Lin CM, Leu HG (1999) Operating characteristics and kinetic studies of surfactant wastewater treatment by Fenton oxidation. Water Res 33(7):1735–1741
Lin SH, Lo CC (1997) Fenton process for treatment of desizing wastewater. Water Res 31(8):2050–2056
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46:854–862
Liu Q, Qian K, Qi J, Li C, Yao C, Song W, Wang Y (2018) Improving the efficiency of Fenton reactions and their application in the degradation of benzimidazole in wastewater. RSC Adv 8:9741–9748
Mohan D, Rajput S, Singh VK, Steele PH, Pittman CU Jr ((2011) Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J Hazard Mater 188: 319–333.
Najjar W, Chirchi L, Santosb E, Ghorhel A (2001) Kinetic study of 2-nitrophenol photodegradation on Al-pillared montmorillonite doped with copper. J Environ Monit 3:697–701
Nitoi I, Oncescu T, Oanc P (2013) Mechanism and kinetic study for the degradation of lindane by photo-Fenton process. J Ind Eng Chem 19:305–309
Peng G, Tian G, Liu J, Bao Q, Zang L (2011) Removal of heavy metals from sewage sludge with a combination of bioleaching and electrokinetic remediation technology. Desal 271:100–104
Rasheed R, Meera V (2016) Synthesis of iron oxide nanoparticles coated sand by biological method and chemical method. Procedia Technol 24:210–216
Rodrıguez-Chueca J, Polo-Lopez MI, Mosteo R, Ormad MP, Fernandez-Ibanez P (2014) Disinfection of real and simulated urban wastewater effluents using a mild solar photo- Fenton. Appl Catal B 150–151:619–629
Rule KL, Comber SDW, Ross D, Thornton A, Makropoulos CK, Rautiu R (2006) Diffuse sources of heavy metals entering an urban wastewater catchment. Chemosphere 63:64–72
Sidhu PS, Gilkes RJ, Cornell RM, Posner AM, Quirk JP (1981) Dissolution of iron oxides and oxyhydroxides in hydrochloric and perchloric acids. Clay Clay Miner 29(4):269–276
Sylwan I, Thorin E, Zambrano J (2016) Biochar adsorption for separation of heavy metals during municipal wastewater treatment. Inter J Environ Sci Technol 13(1):359–386
Sun J, Su SP, Fan MH, Guo HQ, Qiao IP, Sun RX (2007) A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J Hazard Mater 148:172
Tatsi AA, Zouboulis AI, Matis KA, Samaras P (2003) Coagulation flocculation pretreatment of sanitary landfill leachates. Chemosphere 53:737–744
Tony MA, Lin LS (2020a) Iron recovery form acid mine drain sludge as a Fenton source for municipal wastewater treatment. Inter J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1734196
Tony MA, Lin LS (2020b) Attenuation of organics contamination in polymers processing effluent using iron-based sludge: process optimization and oxidation mechanism. Environ Technol. https://doi.org/10.1080/09593330.2020.1803417
Tony MA, Parker HL, Clark JH (2019) Evaluating Algibon adsorbent and adsorption kinetics for launderette water treatment: towards sustainable water management. Water Environ J 33(3):401–408
Tony MA, Parker HL, Clark JH (2016) Treatment of Laundrette wastewater using Starbon and Fenton’s reagent. J Environ Sci Health A 23(11):974–979
Tony MA, Purcell PJ, Zhao Y (2012) Oil refinery wastewater treatment using physicochemical Fenton and photo-Fenton oxidation processes. J Environ Sci Health A 47(3):435–440
Tony MA, Zhao YQ, El-Sherbiny MF (2011) Fenton and Fenton-like AOPs for alum sludge conditioning: effectiveness comparison with different Fe2+ and Fe3+ salts. Chem Eng Commun 98(3):442–452
Tony MA, Purcell PJ, Zhao YQ, Tayeb AM, El-Sherbiny MF (2015) Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process. Environ Eng Manag J 14(1):11–16
Tony MA (2019) An industrial ecology approach: green cellulose based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Inter J Environ Anal Chem. https://doi.org/10.1080/03067319.2019.1661397
Tony MA (2020a) Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Inter J Environ Sci Technol 17:2485–2498. https://doi.org/10.1007/s13762-020-02646-8
Tony MA (2020b) Central composite design optimization of Bismarck Dye oxidation from textile effluent with Fenton’s reagent. Appl Wat Sci 10(5):108
Xu H, Lu G (2013) On-line spectrophotometric method for decolourizing reaction kinetics of reactive black 5 by Fenton oxidation. Asian J Chem 25(14):7989–7992
Villegas-Guzmana P, Giannakis S, Rtimi S, Grandjeanc D, Bensimonc M, Alencastro I, Torres-Palma R, Pulgarin C (2017) A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl Cat 219:538–549
Vlyssides AG, Loukakis H, Karlis PK (2003) Small sewage treatment works using a Fenton oxidation method. Environ Technol 24:931. https://doi.org/10.1080/09593330309385631
Wei X, Jr CV, Buzby K (2005) Recovery of Iron and Aluminium from Acid Mine Drainage by Selective Precipitation. Environ Eng Sci, 22(6):745-755
Zhao YQ, Keogh C, Tony MA (2009) On the necessity of sludge conditioning with non-organic polymer: AOP approach. Res Sci Technol 6(3):151–155
Acknowledgements
The author Maha Tony acknowledges the financial support by Ministry of Higher Education, Mission Department, Egypt grant through the postdoctoral research fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tony, M.A., Lin, LS. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int J Environ Res 15, 191–201 (2021). https://doi.org/10.1007/s41742-020-00309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00309-7