Abstract
Highly efficient low-cost alum sludge-based sorbents, (termed as AS-Sorbents), derived from alum sludge by-product waste have shown a significant potential for treating wastewaters contaminated by textile dyes. Their application for wastewater treatment can be termed as industrial ecology approach. X-ray diffraction and scanning electron microscope were used to investigate the structural and morphological properties of these sorbents (AS, AS400, AS600, AS800). The results revealed the presence of the highly efficient adsorbent, zeolite in AS400. The experimental data revealed that AS-Sorbents exhibited a reasonable textile dye removal from real jeans dying effluent. The highest removal rate was corresponding to AS400 due to the presence of graphite and zeolite. Compared to the commercially available silica gel (Sgel) and activated carbon (Norit), AS-Sorbents are exhibited a superior removal rate of Procion Blue dye from textile effluent. The adsorption efficiency is in the order of AS400 ≫ AS > AS600 > AS800 > Norit > Sgel. The adsorption matrix was well fitted by Langmuir isotherm and followed second-order kinetic model; the maximum monolayer adsorption capacity was 6.5 mg/g. Thermodynamic parameters show that the process follows the physical adsorption matrix with a spontaneous and exothermic nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Presently, the goal of every industry should be adopting sustainable technologies at the highest possible rate. To satisfy the required standards, industrial discharges must undergo through a costly treatment prior to the final disposal into the water bodies (revivers, lakes and seas). In view of this, wastewater recovery techniques and solid waste management are considered substantial sustainable evolutions for reusing such water (Hessel et al. 2007; Tony et al. 2016; Manera et al. 2018).
Textile industry is a vast consumer of water and chemicals that is required for process preparation and cloth dying. Therefore, a significant amount of wastewater is discharged including unconsumed dyes and some other chemicals. Nowadays, among the various types of dyes used in such industry, reactive dyes are potentially widely applied for its chemical stability (Hessel et al. 2007). However, there are impacts associated with those dyes released into the environment such as posing significant carcinogenic effects and toxicity (Alencar et al. 2012). Physical, chemical or biological management techniques are available for treating such dyes. Physical adsorption is considerably an efficient approach for removing different pollutants from wastewater. For instance, Alencar et al. (2012) and Inyinbor et al. (2016) applied the adsorption technique for removing dyes from textile wastewater effluents; however, Tony et al. (2018) applied such technique for removing organics from launderette wastewater. In addition, Parker et al. (2012) used adsorption method for treating phenolic compounds in wastewater. However, this technique is still considered a limited solution due to the cost, availability and practical applications because of pore sizes that cause an increase in the amount of the required adsorbent to improve the adsorption rate (Parker et al. 2012).
Alum sludge is an inevitable by-product in waterworks plants resulting from potable water treatments (Babatunde et al. 2009). Aluminium-based waterworks sludge is generated through the flocculation/sedimentation processes where aluminium sulphate salt is applied as a primary coagulant (Tony et al. 2011; Zhao et al. 2009). Generally, for decades, alum sludge is treated as a waste. Although this sludge is exposed for landfilling, the handling cost and the insufficient land space are still a concern (Hou et al. 2018). In Egypt, alum sludge is produced everyday as a by-product from waterworks plants. This sludge is released to the environment as it is subjected to a direct disposal to the environment without treatment (Tony and Tayeb 2016).
In recent years, researchers have attracted much attention for using alum sludge in the adsorption matrix (Babatunde et al. 2009; Hou et al. 2018). Up to now, the most studies are focused on the heavy metals removal specially phosphorus from wastewater, nevertheless less literature is published regarding the effect of alum sludge with other pollutants (Babatunde et al. 2009). For instance, Jangkorn et al. (2011) used alum sludge with fresh alum for coagulating anionic surfactant from industrial wastewater. Tong et al. (2012) transformed alum sludge by precipitation and calcination onto a double layer of magnesium–aluminium hydroxides for removing acid red G dye. Doherty et al. (2015) achieved organic removal using constructed wetlands from alum sludge incorporating microbial fuel cell technology. Cheng et al. (2016) treated ammonium-rich wastewater by alum sludge in the presence of sulphuric acid. Devi and Saroha (2017) found alum sludge could eliminate various heavy metals from wastewater. Additionally, Chu (2001) used the recycled alum sludge combined with polymer, which are leftover from different experiments for dye removal from wastewater. But, using alum sludge in its treated form to produce zeolite and applying it as an adsorbent material have a few applications on real textile dying wastewater.
To the best of the author’s knowledge, there is a limited data in the existing literature dealing with the preparation and application of calcined real alum sludge from drinking water treatment plant for industrial textile dying wastewater management. Calcination temperature affects the microstructure of the solid waste, which may improve the engineering properties of the material (Kasina et al. 2019). Hence, it is an interesting research topic to use waste alum sludge as a pre-treatment stage for textile effluents containing reactive dyes as a win–win technology for both waste management and water treatment.
Herein, the goal of this study is to characterize the calcined alum sludge residue formed as a by-product from potable water treatment plant located in Menoufia Governorate in the north of Egypt to estimate its potential as an adsorbent material for dye uptake from textile effluent. The calcination process produces valuable products, i.e. zeolite and graphite for wastewater treatment. The characteristics and the maximum Procion reactive textile dye capacity of four alum sludge adsorbent materials were investigated. Additionally, isotherm models, i.e. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich were applied to define the adsorption mechanism.
Materials and methods
Materials
Real jeans-textile dyeing wastewater
Wastewater samples were collected from a dying facility in northern Egypt that is dealing with Jeans-textile dyeing and finishing processes. Wastewater was collected from the last rinse cycle of jeans washing basin that used to remove the excess dye. This dying system was operated using a powder Procion Blue MX-7RX (reactive blue 161) dye. Samples that were collected contain fibres, salts, other chemicals from dyeing and finishing processes and high organic matter content. The main characteristics of wastewater are suspended solids 1210 mg/L, chemical oxygen demand (COD) 596 mg-COD/L, turbidity 36 NTU, pH 7.5 and a dye load in the range of 7.3–27.8 mg/L.
Adsorbents
Preparation of alum-derived materials, AS-Sorbents
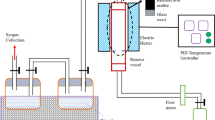
Aluminium-based waterworks sludge residue was collected from the largest water treatment plant in Southern Shebin El-Kom City, Menoufia governorate, Egypt. The raw water flows from the River Nile reservoir to the plant through pumping. The plant produces about 40,000 m3 potable water daily for western side of the city where aluminium sulphate is used as the primary coagulant for flocculating reservoir water. The fresh sludge was directly collected from the underflow channel of the sedimentation tank and transported to laboratory for analysis. Subsequently, the sludge is subjected to gravity settling for excess water removal followed by air-drying to reduce the moisture content to 10.8%. Thereafter, the produced sludge cake is washed to remove impurities. Afterwards, it is exposed to overnight oven drying at 105 °C to remove any water content at the time of being used. The resulting dried sludge is subjected to ball mill grinding for 1 h to obtain a fine powder, and the sample is called raw alum sludge (AS). AS has a specific surface area Brunauer–Emmett–Teller (BET), SBET of 61.58 cm2/g. Raw sludge is then calcined in an electrical furnace for 2 h at 10 °C/min to 400, 600 or 800 °C, and the materials are refereed as AS400, AS600 or AS800. Schematic overview of the process description is illustrated in Fig. 1.
Further adsorbents
For a comparison basis, activated charcoal (Norit) and silica gel (Sgel) that are commercially available porous materials were applied to examine the effect of AS-Sorbents adsorbents type, calcination temperature and adsorption time. Norit purchased from Fluka with a BJH (Barrett, Joyner, Halenda) pore volume 0.3 (cm3/g)BJH and 1.3 nm pore diameter with a specific surface area (BET), SBET 744 cm2/g. Sgel purchased from Sigma-Aldrich in high purity grade with a 0.7(cm3/g)BJH pore volume and 10 nm pore diameter with a specific surface area, SBET 299 cm2/g. All reagents were used as received without further purification.
Dye adsorption studies
To determine the adsorption isotherms and the sorption kinetics, vessels were filled with dye-containing solution in a volume of 10 mL prior, the adsorbents (AS, AS400, AS600, AS800, Norit or Sgel) were added and the vessels were sealed. Isotherm time was investigated by subjecting the wastewater samples contacted with adsorbents for different stirring time ranged from 5 min to 24 h at 298 K. AS-Sorbents (AS, AS400, AS600, AS800) concentrations ranged from 0.5 to 5 g/L were also studied. For the pH variation test, prepared solutions of sodium hydroxide (1 N) and diluted sulphuric acid that were purchased from Alpha Scientific were used. UV–Vis analysis was performed using Unico UV-2100 spectrophotometer, USA to determine the extent of Procion Blue dye removal, which was identified at a maximum wavelength of λ = 570 nm. The adsorption capacity of the materials (qe) was calculated by:
where Co and Ct are the initial and final concentrations of the aqueous dye stream, V is the solution volume (L) and m is adsorbent mass used (g).
Mathematical models
Isotherm models
In order to determine the adsorption capacity of AS-Sorbent materials, four isotherm models were applied, i.e. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) to investigate the Procion Blue uptake.
Langmuir isotherm
The Langmuir adsorption isotherm is based on the assumption of a maximum adsorption capacity, which is related to the monolayer adsorption on a homogeneous surface. The linearized form of Langmuir model was applied in this investigation (Kim et al. 2012) as shown in the following equation:
where Ce is the Procion Blue concentration at equilibrium (mg L−1), qe is the equilibrium adsorption capacity, and aL and KL are Langmuir adsorption constants. Qo is the monolayer adsorption capacity of the solid (the mass of dye adsorbed per unit mass of adsorbent) (mg g−1).
Freundlich Isotherm
The Freundlich model is applicable for the highly heterogeneous surface systems in which it is categorized by the heterogeneity constant (1/n). The linear form of the model can be written as the following:
where KF is Freundlich constant that relates to the adsorption capacity of the solid (L g−1). 1/n is the heterogeneity constant that measures the adsorption intensity and gives a significance of how the adsorption is favourable. n greater than unity represents favourable adsorption conditions (Parker et al. 2013).
Dubinin–Radushkevich (D–R) Isotherm
D–R isotherm is applied to investigate the nature of the adsorption process. The D–R isotherm assumes the adsorption process is limited to a monolayer adsorption capacity; however, it does not assume a homogeneous surface or constant sorption potential. This isotherm model can be used to evaluate the adsorption energy (Kim et al. 2012; Parker et al. 2013). The linear form of D–R model can be expressed as the following:
where qm is the monolayer saturation capacity (L g−1) and KDR is the D–R isotherm constant of adsorption energy, which gives the mean free energy (E) of sorption per molecule of the sorbate and can be estimated from the relationship in Eq. (7). The calculated E value signifies the type of adsorption, chemical or physical adsorption process.
Temkin isotherm
This model describes the adsorption in the layer decreased by the coverage due to the interactions of adsorbent and adsorbate. The linear form applied in this study is expressed according to the following equation:
where B is related to the heat of adsorption \(\left( {B = \frac{RT}{b}} \right)\), T is the absolute temperature (K), R is a gas constant (8.314 J mol−1 K−1), A is the equilibrium binding constant (Kim et al. 2012).
Kinetic models
To further understand the mechanism and rate controlling steps of the dye adsorption via AS-Sorbents, kinetic models were applied to the adsorption system. Lagergren’s pseudo-first order, pseudo-second order and Bangham were applied to study the adsorption mechanism.
Lagergren’s pseudo-first-order kinetic model (Eq. 9) signifies that one sorptive site in the adsorbent material adsorbing one dye molecule (Parker et al. 2013).
where qe is the amount of the dye adsorbed at equilibrium (mg g−1); qt is the amount of the dye adsorbed at time t (mg g−1) and K1 is the adsorption pseudo-first-order rate constant.
The pseudo-second-order kinetic model (Eq. 10) assumes that the occupation rate of sorption sites is proportional to the square number of vacant sites (Dawodu and Akpomie 2016).
where K2 expressed the adsorption pseudo-second-order rate constant.
Banghams’s model (Eq. 11) is further used to investigate if the pore diffusion is solely limiting the rate of the adsorption process.
where KB and α are the Banghams’s model constants and (α < 1), m is the mass of adsorbent (g L−1) and V is the volume of solution (L). If the data fit Eq. (11), this means the pore diffusion is the only step that controlling the adsorption reaction. Thereby, if the data are not fitted with Eq. (11), this is demonstrating the adsorption is not limited to the diffusion of the adsorbate into the pores of the adsorbent material (Srivastava et al. 2006).
Characterization study
The morphologies of the prepared sludge samples as reported previously in Sect. 2.1.2 were investigated and imaged by field-emission scanning electron microscope (SEM) (FE-SEM, Quanta FEG 250). The used typical magnifications were x8000 and x60000. This was accompanied by energy-dispersive X-ray spectroscopy (EDX). The principal oxides content in the alum sludge was examined by energy-dispersive spectrum. The crystal structure of the prepared alum sludge adsorbent samples was characterized by single-crystal X-ray diffraction (XRD) measurements that performed on a Bruker–Nonius Kappa CCD diffractometer with CuKα radiation source (λ = 1.5406 Å). This XRD measurement was done under step-scan mode, and the registered intensities of the diffracted X-rays were detected every 0.026° over 2θ range of 15–75°. The diffractometer works at 45 kV with a step-scan time of 18.87 s.
Results and discussion
Characterization of AS-Sorbents
XRD patterns of the prepared powder, AS-Sorbents, from raw alum sludge (AS) and that calcined at 400–800 °C are given in Fig. 2. XRD data reveal the presence of sharp peaks in the AS-sorbent or those thermally treated at different temperatures indicate the presence of the crystalline structure. The main crystalline inorganic matter in such sludge is quartz (silicon oxide, SiO2) with a hexagonal crystal structure. The highest intense peaks of SiO2 are assigned as seen in Fig. 2a–d according to the hkl planes. Since only aluminium sulphate is applied in this water treatment plant as the primary coagulant, the suspended materials such as sand and clay in the raw water may be the source of those quartz particles (Fungaro and Silva 2014).
Also, anorthic crystal structure of calcium aluminosilicate (CaAl2Si2O8) appears in the four AS-Sorbents types with a remarkable increase in its amount with the increase in the calcination temperature according to the identified peaks as shown in Fig. 2. This is due to the calcination of CaCO3 and Al2Si2O5(OH)4 occurs at 800 and 600 °C, respectively, that may help in establishing more CaAl2Si2O8 (Traore et al. 2003). Graphite (C) in a hexagonal crystal structure appears in all AS-Sorbents and allocated with its planes for all sorbents.
The crystalline rich-silica zeolite, ZSM-12 (sodium aluminium silicate, Na1.16Al2Si77.4O158.38) with a monoclinic crystal structure appears in the sludge calcined at 400 °C (AS400). SiO2 and Al2O3 are considered the main zeolite precursors. Those compounds are rich in alum sludge because of the presence of sand and using Al2(SO4)3 through the water treatment with the presence of NaOH. Calcination of such precursors at 400 °C resulting in the formation of zeolite (ZSM-12) which is characterized by its peaks in Fig. 2b and that does not appear in the raw sludge (AS). However, increasing the calcination temperature more than 400 °C resulting in a deleterious effect on the zeolite formation and its peaks disappeared in the AS600 and AS800 (Fig. 2c, d). This is likely because of the zeolite formation that is affected by both, the ratio of SiO2 and Al2O3 besides the loss of ignition (Barrer 1982).
A notable increase in the amount of quartz is obtained with the increase in the calcination temperature. This could be attributed by the crystallization temperature of amorphous silica into quartz, which occurs from 245 °C (ASTM 1998). Thus, the calcination of sludge at 400 °C results in the formation of ZSM-12. However, this zeolite-based material is disappeared when the calcination temperature is increased.
The physical morphology of the prepared AS-Sorbent material particles is represented by the SEM micrographs (Fig. 3). The figure shows the SEM images of AS400 sorbent material before and after adsorption technique. Figure 3a reveals the porous structure of the prepared AS-Sorbent (AS-400) that is heterogeneous in nature with non-uniform porous shape mixture. Thus, the sludge has uneven structure nature. Observation of SEM images after adsorption (Fig. 3b) gave a visual representation of the sludge that is loaded with the dye molecules. A noticeable smoothing of the sharp surface of the material to be more outstretched can be seen after adsorption, and the morphology became such a petal shaped. This could be illustrated by the dye molecules adsorbed on the AS-Sorbent solid material penetrating the surface to the pores of the material and therefore is leading to the expansion of AS-Sorbent surface that is reconstructing its structure.
The crystallite size of the synthesized AS-Sorbent micro-particles was calculated via mean linear intercept method, in such method random lines were drawn in the SEM photograph then counting the number of crystallite boundaries. Therefore, the average crystallite size was obtained after dividing the length of the straight line over the number of crystal boundaries (van der Zaag et al. 1993). The produced AS400 micro-particles contain irregular shape and non-uniform particle size ranged from 0.181 to 1.8 μm as shown in Fig. 4. The typical mean particle size is 0.99 μm; however, the predominant size is 0.545 μm.
Elemental analysis of alum sludge, AS-Sorbent, calcined at 400 °C (AS400) as determined by EDX is illustrated in Table 1. Calcined sludge powder composes of the main C component, along with Si and Al, with the presence of small amounts of S, Ca, Na and Fe. The application of aluminium sulphate as a primary coagulant in the potable water treatment plant was reflected in the composition of the sludge cake produced. However, calcination of the sludge resulted in a reduction in the aluminium hydroxide.
Dye removal by AS-Sorbent adsorption
Dye adsorption related to contact time
Firstly, before designing the adsorption matrix, it is needed to examine the adsorption equilibrium time. The time-profile of Procion Blue dye adsorption using the six adsorbents (AS, AS400, AS600, AS800, Norit and Sgel) was studied at room temperature and presented in Fig. 5. AS400 showed the highest dye adsorption capacity overall, with the majority of the dye adsorbed within the first 2 h. This could be attributed by the presence of zeolite (ZSM-12) in AS400, according to the X-ray diffraction result (Fig. 2b), through calcination and crystallization of alum sludge. Such zeolite is effective in dye adsorption due to the ion exchange characteristics (Wang and Peng 2010). Therefore, the formation of the ZSM-12 amount in the calcined sludge at 400 °C is directly proved as expected its importance in increasing the adsorption capacity. ZSM-12 appears as a cation exchanger due to the negative charge of its framework. Thus, water molecules could be bonded onto the framework of the ZSM-12 and then the dye molecules are exchangeable (Pinar et al. 2013). The significance of zeolite in improving the adsorption capacity is in accordance with previous investigations of Tiwari et al. (2017) and Gomez et al. (2017). Tiwari et al. (2017) reported that the aluminosilicate zeolite is a significant low-cost adsorbent for removing dyes from textile dyeing effluents. Also, Gomez et al. (2017) investigated that the zeolite-based adsorbent is an efficient option for removing insecticide from wastewater. Furthermore, the presence of graphite in all AS-Sorbents (AS, AS400, AS600 and AS800) supports the adsorption process since it shows a high effective reactive dye uptake. Graphite surface is effective in adsorbing the organic molecules by the hydrophobic interactions (Carvallho et al. 2016). This result gives an evidence of the importance of both the porous nature of adsorbents besides the effective presence of zeolite (ZSM-12) as it allows higher dye uptake. Sgel is the slowest adsorbent of Procion Blue dye because of its small surface area that inhibited the dye diffusion. A similar investigation is observed by Tony et al. (2018) in treating launderette wastewater effluent using a mesoporous material derived from polysaccharides. They found that more than 80% of the organics were removed from the wastewater by applying the porous material within 1 h of contact time.
Noticeably, for all adsorbents, there is no further dye uptake with the prolonged reaction time more than 2 h, since the available active sites of the adsorbent materials are saturated with the dye molecules.
Effect of initial dye concentration on adsorption capacity
The effect of the initial dye concentration is given in Fig. 6. The results show the effect of the initial Procion Blue dye load which is ranged from (7.3–27.8 mg/L) at 298 K on the adsorption capacity using different adsorbents. Overall, for all the used adsorbents, a noticeable increase in the adsorption capacity is observed with increasing the dye load. This could be attributed by, at higher dye concentration, the enhancement in the interaction between the dye molecules and AS-Sorbents or other adsorbents. AS400 presented higher adsorption capacities overall; this could be illustrated by the presence of ZSM-12 (zeolite). Also, porous nature and higher surface area may allow further available adsorption sites to be utilized and thus increasing the adsorption capacity. The unit adsorption capacity of AS400 is increased from 2.9 to 6.5 mg g−1 with increasing the Procion Blue concentration from 7.3 to 27.8 mg L−1, respectively. This performance may be illustrated by the increase in the initial Procion Blue dye concentration resulted in an excess in dye molecules that are surrounding the AS-Sorbent sites and thus enhancing the mass transfer rate between the adsorbent material and the adsorbed solute, the result is an improvement in the adsorption capacity.
These results of increasing the adsorption uptake with increasing the adsorbate are in accordance with that previously investigated by Mittal and Gupta (2010) in using bottom ash and de-oiled soya in adsorption of Eriochrome Black T and Geng et al. (2018) who used a composite TiO2 and alum sludge for adsorption of methylene blue from wastewater. They found that the increase in the initial organics concentration results in more organic molecules surrounding the adsorbent surface. Consequently, this is improving the mass transfer rate between the adsorbate and the surface of adsorbent material, thus enhancing the adsorption rate (Geng et al. 2018).
Adsorption performance at different adsorption conditions
Procion Blue dye uptake for the range of AS-Sorbents dosages is studied, and the results are given in Fig. 7a where the other parameters are kept constant. It can be seen from Fig. 7a that the adsorption capacity of Procion Blue increased with an increase in AS-Sorbents amount up to 2 g L−1 due to the increase in the available adsorption sites. However, increasing the adsorbent dose more than 2 g L−1, the result is a decrease in the dye uptake. This may be due to the increase in adsorbent dose at a constant adsorbate concentration is leading to unsaturation of sorption sites. Additionally, the excess of the adsorbent dose may cause aggregation (i.e. particulate interaction); thereby, the overall surface area of the adsorbent material is decreased and the diffusional path length is increased (Tony et al. 2018). This concluded the adsorbent dose is basically proportional to the dye uptake. Furthermore, the dye removal drop was mainly due to the restabilization of the dye particles (Chu, 2001). Thus, 2 g L−1 of AS-Sorbent materials was used in the subsequent experiments. This investigation is in accordance with that previously stated by Chu (2001) and Tony (2019) for dye uptake with different adsorbents.
In order to maximize the adsorption capacity, the effect of the pH on the adsorption capacity was also tested. Experiments were carried out with various initial pH values (4, 6, 7.5 and 9) with fixing other parameters. It is apparent from Fig. 7b that for all AS-Sorbents applied, the adsorption capacity increases with increasing the pH value and the neutral pH is preferred. However, further increase in the pH value to the alkaline range, no apparent adsorption uptake has occurred. This loss in the adsorption capacity at the alkaline pH attributed to the loss of the active sites of the adsorbent material. Additionally, the pH value affects the surface charge of the aluminium-based sludge. The surface charge of alum sludge varies with the change in the pH value. According to the previous studies (Kim et al. 2012), the measure of surface charge of alum sludge (zeta potential) has observed to become positive as the point of zero charge (PZC) of the alum sludge reached at a pH higher than the solution’s pH. However, it became negative once the solution pH is higher than PZC. Hence, Procion Blue dye adsorption is related to the surface charge of AS-Sorbents in comparison with the solution pH. Since PZC of Al(OH)3 is in the pH range of 8.5–9.5 (Kim et al. 2012), the surface charge of the AS-Sorbent materials below pH 8.5 might be a positive charge. Consequently, increasing the solution’s pH weakens the attraction between the adsorbent and the adsorbate and thus the overall adsorption uptake is decreased. Chu (2001) reported 9.13 to be the pH of the maximum hydrophobic dye removal using recycled alum sludge that is augmented with polymer. Chu (2001) results mean that the point of zero charge of alum sludge may be affected by the polymer addition; therefore, the electrostatic attraction phenomenon for adsorption was occurred at pH 9.1.
Temperature effect on Procion Blue adsorption capacity was investigated. The reaction temperature was adjusted over the range of 26–60 °C. The results presented in Fig. 7c demonstrating that the dye sorption capacity is decreased with the increase in temperature. This could be illustrated by the enhancement of the dye desorption stage in the adsorption process confirming that the process is exothermic (Parker et al. 2013). Besides, the temperature increase weakens the sorptive forces between the adsorption active sites on AS-Sorbent material and the dye molecules, and also between adjacent Procion Blue dye species on the sorbed phase. Similar results were recorded by Parker et al. (2012) who found that phenol removal is deteriorated by the increase in wastewater temperature.
Adsorption isotherms
To investigate the adsorption mechanism of Procion Blue dye on the AS-Sorbent materials, the experimental data were applied to the function isotherm models (Langmuir, Freundlich, Temkin and D–R) (Fig. 8). The constant parameters of the linearized form equations of these isotherm models were calculated and listed in Table 2. Assessment of AS-Sorbent adsorption process was based on comparing the correlation coefficients, R2 values, of those models (Table 2).
The experimental results were tested over the selected models, and the equilibrium data of AS-Sorbents showed a good correlation to Langmuir isotherm (Table 2). It is signified that the adsorption surface is a homogenous one. Additionally, the dye adsorption on AS-Sorbents is going to be monolayer coverage until the saturation of the active sites is occured.
From the correlation coefficient values, R2, Freundlich isotherm is not adequate as Langmuir or D–R isotherms and the lowest R2 values were to Temkin model. The adsorption is favourable when the n value is ranged from 1 < n < 10, as shown from Table 2, n values are more than unity which signifies the adsorption is favourable. Interestingly, the small values of the mean free energy of adsorption (E) not more than 0.91 kJ mol−1, assuming the dye adsorption on AS-Sorbents is a physisorption type; since the forces involved in physisorption are weak Van der Waals forces (Ashour et al. 2014). The low values of B, the heat of adsorption, confirm the physical adsorption process.
Thermodynamic study of adsorption
Analysis of the adsorption thermodynamic parameters was investigated in order to further clarify the adsorption of the dye molecules onto the AS-Sorbents. The thermodynamic parameters such as enthalpy, Gibbs free energy and entropy changes were calculated and listed in Table 3.
The van’t Hoff equation was applied to calculate the values of enthalpy (ΔH) and entropy (ΔS) as given in Eq. (11). Since the equilibrium constant (KL) can be used to investigate the thermodynamic parameters, the change in Gibbs free energy of adsorption is given as Eq. (12) (Parker et al. 2013).
where R is universal gas constant (8.314 J/mol K) and T is the absolute temperature (K). Thus, the change in enthalpy (∆H) and entropy change (∆S) is calculated from the slope and intercept of the plot of lnKL against 1/T, respectively (Fig. 9).
For all AS-Sorbents applied for treatment, the calculated negative Gibbs free energy and negative enthalpy values across all temperatures specify that the adsorption is spontaneous, favourable and exothermic in nature. Although the experimental data indicate that AS400 has high adsorption capacity overall especially at low temperature, low ∆G value is corresponding to AS600 at 60 °C which indicates that the adsorption is more spontaneous. Although the reason of the disagreement between the experimental data and the model predictions remains unclear in the current study without further investigation, that may be attributed to the fact that carbonate concentrations were not constrained experimentally in the case of AS600 as adsorbent material (Hyun et al. 2001; Tournassat et al. 2018; Giraldo et al. 2019). Also, positive entropy change values reflect an irregular increase in the degree of randomness at the solid-adsorbate interface through the adsorption process.
Kinetics of dye adsorption characteristics
The adsorption kinetic models evaluate the magnitude and the mechanism of the proposed dye adsorption process via AS-Sorbents. The comparative estimation of the applicability of the Lagergren’s pseudo-first order, pseudo-second order and Bangham’s equation was analysed, and the parameters of each model are computed in Table 4.
Comparing the correlation coefficient values, R2, of the pseudo-first-order model by Lagergren’s and the pseudo-second-order model in Table 4 represents Lagergren’s model is not a well fit of the adsorption process as the pseudo-second-order model. For all adsorbents, excellent R2 of the pseudo-second-order model ranged from 0.98 and 1.00 is displayed. Also, the calculated equilibrium uptake of Procion Blue onto AS-Sorbents (qe) of the pseudo-second-order model is closer to the data attained from the experimental results with slightly lower values that signify the process is more complicated than the model described. Further, comparing the kinetic constants of the suggested model, K2, with those in the literature for similar adsorption systems indicates a reasonable comparable (El-Mekkawi et al. 2016; Li et al. 2016; Poormand et al. 2017).
Although Bangham’s model represents a reasonable fitting to the experimental data (Table 4), it is not sufficient to recommend the adsorbate pore diffusion step is only the controlling step of adsorption. This investigation indicates the adsorption is a multistep process involving not only the adsorption onto the required adsorbent material but also, desorption of the water molecules that adsorbed onto the adsorbent surface, hence, it depends on several aspects (Liu et al. 2010).
Conclusion
Although sludge waste from waterworks plants has been applied for different wastewater treatments, no attention has been paid on using it on its thermally activated form to produce ZSM-12 for applying on real textile dying wastewater uptake. In this study, the preparation and characterization of the drinking water plant waste as a low-cost adsorbent was studied and ZSM-12 zeolite was obtained. The experiments showed the highest adsorption capacity reached to 6.5 mg/g for AS400. Neutral pH of the solution was favourable for jeans dying wastewater removal from solution by all adsorbents. The results showed that high temperature was not vital for high adsorption capacities. Equilibrium sorption isotherm studies showed that Langmuir isotherm model satisfactorily described the adsorption data. This study was also dealt with comparing alum sludge adsorbents with commercially available adsorbents, and the results demonstrated that AS-sorbents have a superior role in textile dye removal. A pseudo-second-order adsorption model was well fitted for the adsorption data compared to other kinetic models. Thermodynamic analysis of the data with the negative values of Gibbs free energy and enthalpy indicates the spontaneous and endothermic nature of adsorption. Due to the worldwide availability of waste alum sludge, this could be a cheap and accessible solution as a pre-treatment step for dyed wastewater uptake alternating the high cost adsorbents.
References
Alencar WS, Lima EC, Royer B, Santos BD, Calvete T, Silva EA, Alves CN (2012) Application of aqai stalks as biosorbents for the removal of the dye Procion Blue MX-R from aqueous solution. Sep Sci Technol 47:513–526
Ashour EA, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: Kinetics and isotherm studies. Am J Chem Eng 2(6):92–98
ASTM designation: C 618–89. (1998). Standard specification for coal fly ash and raw or calcined natural pozzolana for use as a mineral admixture in concrete. Annual Book of ASTM standards
Babatunde AO, Zhao YQ, Burke AM, Morris MA, Hanrahan JP (2009) Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ Poll 157(10):2830–2836
Barrer RM (1982) Hydrothermal chemistry of zeolites. Academic Press, USA, p 360
Carvallho MN, Silva KS, Sales DS, Mauricio EF, Ghislandi AG (2016) Dye removal from textile industrial effluents by adsorption on exfoliated graphite nanoplatelets: kinetic and equilibrium studies. Water Sci Technol 73(9):2189–2198
Cheng WP, Chen PH, Yu RF, Ho WN (2016) Treating ammonium-rich wastewater with sludge from water treatment plant to produce ammonium alum. Sust Environ Res 26(2):63–69
Chu W (2001) Dye removal from textile dye wastewater using recycled alum sludge. Water Res 35(13):3147–3152
Dawodu MO, Akpomie KG (2016) Evaluating the potential of a Nigerian soil as an adsorbent for tartrazine dye: isotherm, kinetic and thermodynamic studies. Alex Eng J 55:3211–3218
Devi P, Saroha AK (2017) Utilization of sludge based adsorbents for the removal of various pollutants: a review. Sci Total Environ 578:16–33
Doherty L, Zhao YQ, Zhao XH, Wang W (2015) Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem Eng J 266(15):74–81
El-Mekkawi DM, Ibrahim FA, Selim MM (2016) Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J Environ Chem Eng 4(2):1417–1422
Fungaro DA, Silva M (2014) Utilization of water treatment plant sludge and coal fly ash in brick manufacturing. Am J Environ Prot 2:83–88
Geng Y, Zhang J, Zhou J, Le J (2018) Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv 8:32799–32807
Giraldo L, Rodriguez-Estupina P, Moreno-Piraja JC (2019) Isosteric heat: comparative study between clausius–clapeyron, CSK and adsorption calorimetry methods. Processes 7:203–228. https://doi.org/10.3390/pr7040203
Gomez S, Lerici L, Saux C, Perez AL, Brondino CD, Peirella L, Pizzio L (2017) Fe/ZSM-11 as a novel and efficient photocatalyst to degrade Dichlorvos on water solutions. Appl Catal 202:580–586
Hessel C, Allegre C, Maisseu M, Charbit F, Moulin P (2007) Guidelines and legislation for dye house effluents. J Environ Manag 83:171–180
Hou O, Meng P, Pei H, Hu W, Chen Y (2018) Phosphorus adsorption characteristics of alum sludge: adsorption, capacity and the forms of phosphorus retained in alum sludge. Mater Lett 229:31–35
Hyun SP, Cho YH, Hahn PS, Kim SJ (2001) Sorption mechanism of U(VI) on a reference montmorillonite: binding to the internal and external surfaces. J Radioanal Nucl Chem 250:55–62
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modelling of liquid phase adsorption of Rhodamine B dye on to Raphia hookerie fruit epicarp. Water Res Ind 15:14–27
Jangkorn S, Kuhakaew S, Theantanoo S, Klinla-or H, Sriwiriyarat T (2011) Evaluation of reusing alum sludge for the coagulation of industrial wastewater containing mixed anionic surfactants. J Environ Sci 23(4):587–594
Kasina M, Wendorff-Belon M, Kowalski PR, Michalik M (2019) Characterization of incineration residues from wastewater treatment plant in Polish city: a future waste based source of valuable elements? J Mater Cycles Waste Manag 21:885–896
Kim D-H, Yang J-S, Baek K (2012) Adsorption characteristics of As(III) and As(V) on alum sludge from water purification facilities. Sep Sci Technol 47:2211–2217
Li L, Liu F, Duan H, Wang X, Li J, Wang Y, Luo C (2016) The preparation of novel adsorbent materials with efficient adsorption performance for both chromium and methylene blue. Colloid Surf B 141:253–259
Liu Q-S, Zheng T, Wang P, Jiang J-P, Li N (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157:348–356
Manera CA, Tonello PA, Perondi D, Godinho M (2018) Adsorption of leather dyes on activated carbon from leather shaving wastes: kinetics, equilibrium and thermodynamics studies. Environ Technol. https://doi.org/10.1080/09593330.2018.1452984
Mittal A, Gupta V (2010) Adsorptive removal and recovery of the azo dye Eriochrome Black T. Toxil Environ Chem 92:1813–1823
Parker HL, Hunt AJ, Budarin VL, Shuttleworth PS, Miller KL, Clark JH (2012) The importance of being porous: polysaccharide-derived mesoporous materials for use in dye adsorption. RSC Adv 2:8992–8997
Parker HL, Budarin VL, Clark JH, Hunt AJ (2013) Use of starbon for the adsorption and desorption of phenols. ACS Sustain Chem Eng 1:1311–1318
Pinar AB, Gómez-Hortigüela L, Lynne B, McCusker LB, Pérez-Pariente J (2013) Controlling the aluminum distribution in the zeolite ferrierite via the organic structure directing agent. Chem Mater 25(18):3654–3661
Poormand H, Leili M, Khazaei M (2017) Adsorption of methylene blue from aqueous solutions using water treatment sludge modified with sodium alginate as a low cost adsorbent. Water Sci Technol 75(2):281–295
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf A 272:89–104
Tiwari EM, Shukla SP, Dhiman N, Mohan D, Kisku GC, Roy S (2017) An efficient removal of disperse dye from wastewater using zeolite. J Hazard Toxic Raddioact 21(4):5. https://doi.org/10.1061/(asce)hz.2153-5515.0000369
Tong DS, Liu M, Lin CX, Yu WH, Zhi PX, Zhou CH (2012) Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red G dye. Appl Clay Sci 70:1–7
Tony MA (2019) An industrial ecology approach: green cellulosebased bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2019.1661397
Tony MA, Tayeb A (2016) Response surface regression model in optimization of alum sludge drying facility: solar-Fenton’s reagent dewatering. Int J Chem Eng Appl 7(5):331–335
Tony MA, Zhao YQ, El-Sherbiny MF (2011) Fenton and Fenton-like AOPs for alum sludge conditioning: effectiveness comparison with different Fe2+ and Fe3+ salts. Chem Eng Commun 98(3):442–452
Tony MA, Parker HL, Clark JH (2016) Treatment of Laundrette wastewater using Starbon and Fenton’s Reagent. J Environ Sci Health A 23(11):974–979
Tony MA, Parker HL, Clark JH (2018) Evaluating Algibon adsorbent and adsorption kinetics for launderette water treatment: towards sustainable water management. Water Environ J. https://doi.org/10.1111/wej.12412
Tournassat C, Tinnacher RM, Grangeon S, Davis JA (2018) Modeling uranium(VI) adsorption onto montmorillonite under varying carbonate concentrations: a surface complexation model accounting for the spillover effect on surface potential. GCA 220:291–308
Traore K, Kabre TS, Blanchart P (2003) Gehlenite and anorthite crystallisation from kaolinite and calcite Mix. Ceramic Inter 29:377–383
Van der Zaag PJ, Ruigrok JJ, Noordermeer A, Van Delden A (1993) The effect of intragranular domain walls in MgMnZn-ferrite. J Appl Phys 74(6):4085
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Zhao YQ, Keogh C, Tony MA (2009) On the necessity of sludge conditioning with non-organic polymer: AOP approach. J Res Sci Technol 6(3):151–155
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Tony, M.A. Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Int. J. Environ. Sci. Technol. 17, 2485–2498 (2020). https://doi.org/10.1007/s13762-020-02646-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02646-8