Abstract
A natural thermal habitat, Tulasi Shyam, was studied for the culture-dependent and culture-independent diversity of the thermophilic bacteria. Soil and water samples of winter, summer and monsoon seasons were analyzed to assess the impact of the seasonal variation on the microbial diversity. The soil was fertile and contained high humic substances. The temperature of the water was 50 °C, which did not significantly vary with seasons. Various methods were assessed for the extraction of the metagenomic DNA to obtain quality DNA in good quantity. The 16S rRNA genes were amplified using the metagenomic DNA as template and the products were analyzed further by the denaturing gradient gel electrophoresis (DGGE) for evaluating the bacterial diversity. The metagenomic diversity of the hot spring reservoir-1 as compared to reservoir-2 was less influenced by the seasonal variations. The DGGE patterns of the soil samples suggested significant effect of the seasonal changes. The metagenomic diversity was distinct when compared with the culture-dependent microbial diversity. The diversity was further analyzed by the principal component analysis (PCA) and diversity indices. The apparent shift in the diversity appears to be governed by the prevailing environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The studies on the microbial ecology have provided understanding of the microbial community structures and their evolution under various climatic, biotic and xenobiotic conditions. For a long period of time, however, the studies on the microbial diversity depended on the culture-dependent approaches. The cultivation based analyses are not sufficient to explore the microbial diversity as it does not reveal a clear picture of the community diversity. Moreover, approximately 99% of the microorganisms present in nature cannot be cultured under the laboratory conditions. Thereby, the genetic information and biotechnological potentials of majority of the organisms dwelling in a given habitat would remain unexplored, if only conventional approaches are employed (Streit and Schmitz 2004). As a result, the assessment of the culture-independent diversity is inevitable for the comprehensive understanding of the microbial diversity and phylogeny.
The concept of metagenomics includes the sequence and the function-based approaches. Albeit both the concepts are interconnected, the sequence-based metagenomics deals especially with the diversity and phylogeny of the microorganisms in a particular habitat, highlighting the population heterogeneity and phylogenetic status in totality (Galperin 2008; Purohit and Singh 2009). Furthermore, the advancement in the field of bioinformatics has significantly added to the success of the metagenomic approaches (Thomas et al. 2012).
In the present study, the properties of the soil and water were analyzed, followed by the sequence-based metagenomics to explore the culture-independent diversity of the natural hot spring reservoirs, Tulasi Shyam, which is located in the Gir Forest of the Gujarat State, India. Extracted of the intact and pure environmental DNA from a given habitat is the first and significant step in the metagenomics (Voget et al. 2003; Kennedy et al. 2007; Sharma et al. 2007; Purohit and Singh 2009; Siddhapura et al. 2010; Akbari et al. 2014). Various protocols have been developed and optimized to extract metagenomic DNA, based on the combinations of the enzymatic and mechanical methods to disrupt the rigid cell structure. The 16S rRNA gene was amplified with a pair of the universal eubacterial primers using the extracted megenomic DNA as the template. The polymorphism was assessed by the denaturing gradient gel electrophoresis (DGGE), using urea and formamide as the chemical denaturants. The impact of the seasonal variations and incubation temperatures on the microbial diversity was assessed using both, culture-dependent and metagenomic approaches. In addition, various statistical indices and the similarity matrix would significantly add to the understanding of the microbial diversity of the natural thermal habitat under seasonal alterations.
Materials and Methods
Materials
Agarose and lysozyme were purchased from Hi-Media Limited (Mumbai, India), whereas DNA markers were from Merck Limited, India and Thermo Scientific, India. Primers were procured from the Imperial Life Sciences, India. Microbiological filters (0.2 micron) were from Millipore, India. Other chemicals were of analytical grade and obtained from Rankem Laboratories, India.
Preparation of Reagents and Buffers
The extraction buffer contained 100 mM Tris-Cl, 100 mM EDTA and 1.5 M NaCl (pH 8). The TE buffer contained 1 mM EDTA in 10 mM tris-Cl buffer, pH 8. In addition, the chemical lysis buffer contained 20% (w/v) SDS, 20 mg/ml Lysozyme, 10 mg/ml Protinase-K, 10 mg/ml N-lauryl sarcosine and 1% (w/v) CTAB (cetyl trimethyl ammonium bromide) in the sterile MiliQ grade water.
Tulasi Shyam Hot Spring Reservoirs
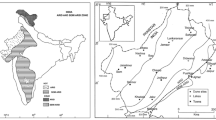
Tulasi Shyam is a natural thermal habitat, located in the middle of the Gir Forest in Gujarat, India (Longitude 71.02 and Latitude 21.05). It is the only thermal habitat in the Saurashtra region and not explored for the microbial diversity and phylogeny. The thermal habitat has religious significance as well due to the ancient temple of Lord Krishna in its vicinity.
To explore culture-dependent and culture-independent bacterial diversity, different soil and water samples were collected from the site in different seasons; winter, summer and monsoon. The physicochemical properties of the soil and water samples were measured immediately after sampling (Marx et al. 1996; McSweeney and Grunwald 1999).
Conventional Microbiological Approaches
Isolation of Thermophilic Bacteria
The soil and water samples were serially diluted in the sterile distilled water and 5% (v/v) of the diluted samples was inoculated into the modified thermophilic medium containing 0.7% (w/v) peptone, 0.5% (w/v) yeast extract, 0.3% (w/v) malt extract and 0.5% (w/v) NaCl, along with 1% (w/v) glucose, at different pH of 7–9. The flasks were incubated at 50 °C for 24–48 h. Thereafter, 0.1 ml of the enriched culture was inoculated on the modified thermophilic agar plates containing the same ingredients as mentioned above, supplemented with 5% (w/v) agar powder at pH 7–9. The inoculated plates were incubated at temperatures between 37 and 70 °C for 24–48 h. The isolated colonies were subsequently streaked repeatedly to obtain pure culture (Kikani et al. 2015).
Effect of Incubation Temperatures on the Bacterial Growth
The thermophilic bacterial isolates were individually inoculated in the medium and incubated at various temperatures for 24–48 h.
Sequence Based Metagenomic Approach
Extraction of the Metagenomic DNA: Establishment of the Protocols
For the standardization of the extraction protocols, 10% (w/v) of each soil sample was added into a 10 ml of the extraction buffer. On the other hand, 500 ml of the water sample was filtered aseptically, using the bacteriological filters (0.2 μ). The filter pads were added to 50 ml of the extraction buffer, pH 8. The sample was incubated with the extraction buffer at 50 °C with continuous shaking for 18 h. The samples were re-extracted in 1 ml of the same extraction buffer. The supernatants were collected by centrifugation at 8385 g for 10 min at 4 °C.
Soft Method
DNA Extraction using the Soft Lysis Method
To the supernatant, 4 ml of the Lysis buffer was added, followed by the incubation at 65 °C for 2 h with vigorous shaking at every 15 min. The samples were then centrifuged at 8385 g for 10 min at 4 °C. The upper aqueous phase was extracted with the equal volume of phenol, chloroform and iso-amyl alcohol (P:C:I) in the ratio of 25:24:1. The treated samples were centrifuged at 8385 g for 20 min at 4 °C. Consequently, the upper aqueous phase was re-extracted with the equal volume of choloroform and iso-amyl alcohol in the ratio of 24:1. As previously described, the tubes were centrifuged at 8385 g for 15 min at 4 °C. The DNA was treated with 7.5 M potassium acetate subsequently. The DNA was precipitated by chilled absolute ethanol. The tubes were kept at 4 °C for 1 h. Further, the precipitated DNA was recovered by centrifugation at 8385 g for 10 min at 4 °C. The DNA was air dried and suspended in the minimal volume of the sterile TE buffer, pH 8.
Harsh Methods
DNA Extraction Using Bead Beating Method
To the supernatant, 10% (w/v) glass beads with 5 mm diameter were added, followed by continuous blending for 15 min. The tubes were incubated at 65 °C for 2 h with vigorous shaking at every 15 min. The remaining protocol for the DNA extraction was as described for the soft lysis method.
DNA Extraction Using Sonication Method
The supernatant was further sonicated using a High Intensity Ultrasonic processor (Sartorious Make, India) with a standard 13 mm horn solid probe for 3 pulses of 30 s each in a chilled ice bath. The sample was cooled in the ice and repeatedly sonicated for 6 consecutive cycles. Later, the tubes were incubated at 65 °C for 2 h with vigorous shaking at every 15 min. The rest of the steps were as described above in the soft lysis method.
DNA Extraction by a Combination of Bead Beating and Sonication Treatment
The supernatant was treated with the beads followed by the sonication as described earlier. Later, the samples were incubated at 65 °C for 2 h with vigorous shaking at every 15 min. The rest of the extraction protocol was the same as described in the soft lysis method.
Soft and Harsh Methods in the Combination
DNA Extraction Using Bead Beating, Together with the Soft Lysis Method
The supernatants were further treated with the beads as described earlier. It was then subjected to the chemical lysis buffer. Later, the tubes were incubated at 65 °C for 2 h with vigorous shaking at every 15 min and the remaining protocol was followed as described for the soft lysis method.
DNA Extraction by Sonication in Combination with the Soft Lysis Method
The supernatants were subjected to sonication for 6 cycles as described earlier. Subsequently, it was treated with the chemical lysis buffer. The samples were then incubated at 65 °C for 2 h with vigorous shaking at every 15 min and the DNA was extracted as described for the soft lysis method.
Determination of Purity and Yield of the Extracted DNA
The humic acids and proteins are the major contaminants that generally co-extract with the environmental DNA from the soil and water samples. Evidently, the humic acids absorb at 230 nm, while DNA and protein at 260 and 280 nm, respectively. To evaluate the purity of the extracted DNA, the absorbance ratios at 260/230 nm (DNA/humic acid) and 260/280 nm (DNA/protein) were determined.Moreover, the DNA concentration can be calculated as:
Further, the metagenomic DNA extracted by different methods were analyzed on the agarose gel electrophoresis, with 0.8% (w/v) agarose.
PCR Amplification of 16S rRNA Gene
The 16S rRNA gene was amplified using the universal eubacterial primers and the metagenomic DNA as template. To 100 ng of DNA template, 25 pmol of each, forward (AGA GTT TGA TCC TGG CTC AG) and reverse (ACG GCT ACC TTG TTA CGA CTT) oligonucleotide primers (Imperial Life sciences, India) and 25 µl of 2X Red Mix (Master Mix) Plus (Merck, India) were added. The amplification protocol consisted of four steps: step-1, the initial denaturation at 94 °C for 1 min; step-2, the denaturation at 94 °C for 30 s; step-3, the annealing at 54 °C for 30 s and step-4, the extension at 72 °C for 2 min. Consequently, Steps 2 and 4 were repeated for 29 cycles; with the final elongation step at 72 °C for 2 min (Purohit and Singh 2009). Two negative controls, one without template DNA and another without any primer were also included. The amplified products and the 500 bp DNA marker (Merck, India) were loaded on the agarose gel electrophoresis, having 1.2% (w/v) agarose concentration. The amplified products were stored at −20 °C till further use.
Denaturing Gradient Gel Electrophoresis
The 16S rRNA amplicon, 50 µl were subjected to the increasingly higher concentrations of urea and formamide, which act as the chemical denaturants (20–50%). The amplicons migrated through the polyacrylamide gel containing denaturants (Muyzer and Smalla 1998; Purohit and Singh 2009). The gels were stained with ethidium bromide (5 µg/ml).
Statistical Analysis of the Diversity and Construction of the Dendrogram
The diversity index (Di) is used to explore the microbial complexity of each sample, which can be calculated using the formula, Di = n/nM, where i is the index number for each band present in a DGGE profile; n is the number of DGGE bands in a given DGGE profile; nM is the number of bands in the DGGE profile with the highest number of bands (Pongsilp et al. 2012). Various diversity indices were also calculated (Hammer et al. 2001). The patterns and DGGE bands were compared and scored. A matrix based on the presence and absence of the characters was constructed. The data was further analyzed using cluster analysis by the PAST program (Hammer et al. 2001). DGGE patterns were further analyzed for the relative similarity in the microbial diversity, using principal component analysis (PCA) and Pearson correlation analysis, using the Microsoft Xlstat software 2013.3 (Addinsoft, France). Later, the matrix was compared in BioEdit programme (v7.09). The similarity matrix was then transformed into a phenogram, using neighbour-joining methods (Hall 1999).
Results
Determination of the Properties of the Soil and Water Samples
The physicochemical properties of the soil and water samples were analyzed after each sampling and documented in Tables 1 and 2, respectively. The soil structure of the soil-C was considered as blocky, as the surface dried during the summer season and cracks appeared. In comparison, the soil-A was granular structured, as the soil particles remained aggregated and glued together by the organic matter. The granular structure of the soil indicated that the soil was rich in the organic matters, with good porosity and easy water–air exchange; whereas, the blocky soil indicated high clay content. It was further confirmed based on the analysis of the soil texture. The soil-A, the loamy textured soil is considered as the fertile soil with 40–45% sand, 30–40% silt and 20–25% clay. In comparison, the soil-C was clay type, indicated by the high clay content. With reference to the soil consistency, the soil-A remained firm during the winter and summer seasons, while due to heavy rainfall it apparently turned loose during the monsoon season. In contrast, due to the continuous contact of the effluent flow from the thermal habitat, the soil-C remained loose, irrespective of the seasonal variations. The organic carbon content indicated that the soil samples were quite fertile.
In addition, the water samples were semitransparent in nature. The pH values of the water samples were slightly acidic to neutral. Interestingly, the temperature of the water samples did not significantly fluctuate in response to the seasonal variations. This might be due to the natural volcanic and geothermal processes, continuously occurring at the particular thermal habitat. The temperature of the water samples was around 50 °C. Therefore, the inoculated extraction buffer was incubated at the same temperature, during the isolation of the environmental DNA. The water samples were slightly saline to non-saline in nature.
Microbial Diversity Using Conventional and Metagenomic Approaches
Extraction of the Metagenomic DNA
The protocols for the isolation of the metagenomic DNA were standardized. Cellular lysis of the microbial population was employed to extract metagenomic DNA from the soil and water samples. The extraction of the intact metagenomic DNA with minimum shearing and removal of the inhibitors are the major objectives behind standardization of the protocol. Various physicochemical methods were examined for the DNA extraction. Two approaches have been developed for extracting the environmental DNA with the desired purity and quantity. One is the direct extraction of the nucleic acids from the samples, after in situ cell lysis, followed by the DNA purification. While the second approach separate the cellular fractions from the sample, prior to the lysis of the cells, followed by the extraction of the nucleic acids. In the present study, we employed direct approaches to extract the metagenomic DNA from the soil and water samples. On the same note, the direct DNA extraction protocol involved soft and harsh lysis methods. The soft lysis method was based on the disruption of the microorganism solely by the enzymatic and the chemical means. The harsh lysis involved cell disruption by bead beating, sonication, freeze-thawing and grinding. The overall trends on the extraction of the metegenomic DNA revealed that the physical methods alone were not suitable for the extraction of the metagenomic DNA from the soil samples. This might be due to the fact that the soil samples were highly humified and the humic acids could not be removed, using the physical techniques alone. Additionally, sonication treatment together with the soft lysis was most suitable among the other explored methods for the water samples. This might be due to the fact that the microbial populations were comparatively lesser and more diluted in the water samples than the soil samples. Therefore, sonication achieved maximum cellular lysis, in combination with the soft lysis. Further, other contaminants were also removed, due to the chemical and enzymatic treatments. The findings also exemplified that the optimum method for a particular soil may vary as the season changes. Illustratively, the chemical lysis method was the best approach to isolate the metagenomic DNA from the soil type-A, collected during the winter and summer seasons. While, for the same soil, collected in the monsoon, the optimized method was chemical lysis in combination with the bead beating (Table 3).
Further, the retrieved DNA was assessed for the purity and yield, on the basis of the ratio of the absorbance at 260 and 230 nm (DNA versus Humic acids concentrations), and the absorbance ratio at 260 and 280 nm (DNA versus protein concentrations). The higher ratio (≥1.8) indicates extremely good purity of the extracted environmental DNA, whereas a lower ratio indicates the presence of either the proteins at 280 nm or the humic acids at 230 nm (Table 3). The isolated metagenomic DNA was further analyzed on the agarose gel electrophoresis, with 0.8% (w/v) agarose concentration (Fig. 1).
Analysis of the metagenomic DNA, isolated from the soil and water samples on 0.8% (w/v) agarose gel electrophoresis: Lane 1, 8 and 12 represent molecular marker (Broad-range DNA ruler; Thermo Scientific, India); Lane 2–4 represent metagenomic DNA of soil type A during monsoon, summer and winter, respectively; Lane 5–7 represent metagenomic DNA of water (hot spring reservoir-2) during monsoon, summer and winter, respectively; Lane 9–10 represent metagenomic DNA of soil type C monsoon, summer and winter, respectively; Lane 13–15 represent metagenomic DNA of water (hot spring reservoir-1) during monsoon, summer and winter, respectively
Sequence Based Metagenomic Approach
The sequence-based metagenomic approaches deal with the diversity, population dynamics and various inter- and intra-microbial relations in a particular habitat. Towards this end, the 16S rRNA gene was amplified, using a set of the universal eubacterial primers (Fig. 2). The bacterial heterogeneity and population dynamics was investigated using the fingerprinting technique, the denaturing gradient gel electrophoresis (DGGE). In DGGE, different sequences of the DNA denature at different denaturant concentrations, resulting in the generation of the varying band patterns. Theoretically, each band represents a distinct bacterial population present in the community. Evidently, varieties of the band patterns from the same sample, irrespective of the soil and water, collected during different seasons indicated the seasonal impact on the bacterial diversity and population dynamics as a whole (Fig. 3). The DGGE profile revealed a significant impact of temperature fluctuations over the culture-dependent diversity of the natural thermal habitat. The DGGE patterns revealed that the seasonal changes did not significantly affect the bacterial diversity of the hot spring reservoir-1 and the hot spring reservoir-2. As already described, the temperature of the water did not significantly change in different seasons. Therefore, a nearly constant temperature in various seasons may be responsible for no change in the metagenomic diversity of the water samples.
Amplification of the 16S rRNA gene as analyzed on the agarose gel electrophoresis, with 1.2% (w/v) agarose: Lane 1 and 9, DNA molecular marker (500 bp DNA ladder, Merck, India); Lane 2–4 and Lane 6–8 represent amplified products of soil type A and soil type C, respectively during monsoon, summer and winter seasons; Lane 10–13 and Lane 14–16 represent amplified products of water samples of hot spring reservoir-1 and water samples of hot spring reservoir-2, respectively during summer, monsoon and winter seasons, respectively
Analysis for the polymorphism of the amplified products on the denaturing gradient gel electrophoresis: Lane 1 represents DNA molecular marker (100 bp DNA ladder, Merck, India); Lane 2–4 represent hot spring reservoir-1; Lane 5–7 represent hot spring reservoir-2; Lane 8–10 represent soil type C and Lane 11–13 represent soil type A during summer, monsoon and winter seasons, respectively
As described in Table 1, the consistency of the soil-A varies significantly due to seasonal alterations. Due to heavy rainfall in the monsoon season, the soil-A got totally wet, which has contributed to change in its consistency. Besides, it is very much evident that the temperature of the soil would never remain constant upon seasonal variations. The temperature of the soil decreased in monsoon season as compared to that in the summer due to heavy rain fall. In addition, the temperature of the surrounding environment also decreased in the monsoon and winter seasons. This variation may contribute to the alterations in the microbial diversity. The fact may be correlated by the DGGE profiles of the soil samples, as revealed by the variations in the band patterns indicated distinct effect of the seasonal changes on the bacterial diversity.
Conventional Methods
We assessed the effect of incubation temperatures on the growth of thermophilic bacteria, as a part of the culture dependent approach (Fig. 4a, b). The absence of growth at 37 °C confirmed that all the bacterial isolates were true thermophilic in nature. The results also highlighted significant impact of the temperature and seasonal changes over the culture-dependent microbial diversity. However, the trends of the metagenomic diversity are not entirely reflected by the culture-dependent approaches (Kikani et al. 2015). This is likely due to the fact that some bacterial species might be competed out during enrichment under the laboratory conditions. Therefore, the sequence based metagenomics and culture based approaches, in combination would reveal the microbial diversity of the explored thermal habitat in a significant manner.
a Analysis of the growth temperature profile of bacteria isolated from the soil samples during summer (Dark bar), winter (Grey bar) and Monsoon (White bar) seasons. b Analysis of the growth temperature profile of bacteria isolated from the water samples during summer (Dark bar), winter (Grey bar) and Monsoon (White bar) seasons
Statistical Analysis of the Diversity
The species diversity can be mathematically measured by calculating the diversity index of a particular habitat or community. The diversity index furnishes some invaluable information about the community structure, its composition and species richness (Tables 4, 5). The diversity index (Di) corroborated the obtained trend (Table 4). The Di values generally lie between 0 and 1, reflecting lowest and maximum diversity, respectively. A Di value of 0.16 indicated the least metagenomic diversity of the soil sample-C during the monsoon season, as compared to the metagenomic diversity of the hot spring reservoir-1 during the winter season. In this context, the Simpson’s Index describes the probability of the similarity between the two randomly selected species. It ranges between 0, infinite diversity and 1, no diversity. In contrast, the Dominance Index signifies the inverse relationship with the Simpson’s Index. Higher Dominance Index indicates towards greater diversity. The values of the Simpson’s Index and the Dominance Index indicated high metagenomic diversity. However, the seasonal impact on the overall diversity of the site was evidently quite low (Table 5). On the similar note, the Shannon Index is commonly used to characterize the species diversity in a community with respect to the species richness and the evenness. A low value of the Shannon Index indicates uneven species distribution in a particular habitat. Similarly, the Menhinick Index describes the species richness, while the Equitability Index highlights the evenness in the distribution. Another parameter, the Berger-Parker Dominance Index indicates the proportional abundance of the most abundant species in comparison to other bacterial species in a particular community. The Ginni Coefficient is the area between the line of perfect equality and the absolute Lorenz curve.
The principal component analysis (PCA) was used to examine variation in the DGGE band patterns. The relationships between the ordination axes and measured environmental variables (both abiotic and biotic) were analyzed using correlation analysis (Table 6). The PCA analysis based on the DGGE band patterns easily distinguished the diversity of the water samples (winter season) of both hot spring reservoirs and the soil type C (summer and monsoon seasons) from other samples. However, it could not further distinguish the remaining two clusters, due to the identical band patterns (Fig. 5).
Computation of the Similarity Matrix and Construction of the Dendrogram
The DGGE band patterns were converted into a binary matrix. Using presence/absence of a particular band on the DGGE, a pair wise similarity of the band patterns of the different samples was calculated using the Jaccard coefficient. A value of zero indicated that the samples were completely different, whereas a value of 1 indicated complete similarity between the samples. Further, an UPGMA cluster analysis was conducted. One of the advantages of this presentation is the rapid assessment of the coherence of the fingerprinting patterns (Fig. 6). The dendrogram also supported the diversity profile, which was judged by the DGGE band profiles. As per the dendrogram, the diversity of the soil type-C during the winter season was quite distinct than other seasons. On the other hand, the effect of the seasonal changes was less visible on the diversity of the water samples.
Discussion
Culture-dependent approaches are not sufficient to judge the microbial diversity of any given habitat. This is primarily due to the fact that majority of the bacteria communities are unable to grow under laboratory conditions (Muyzer et al. 1993; Stewart 2012). Besides, the enrichment process selectively favors only a few strains of the bacteria of a specific phenotype. Some of the microorganisms are late growers, and, therefore, can be eliminated from the competition by the fast-growing species in a particular habitat (Dunbar et al. 1997). Moreover, the microorganisms are heterogeneously distributed inside microaggregates and in macroporosities outside microaggregates (Ranjard et al. 1998). The microorganisms are strongly bound with the soil particles through the various binding mechanisms, and thus denying access to the whole bacterial population (Bakken and Lindahl 1995; Blume and Reichert 2015; Bressan et al. 2015). Therefore, adaptations of the culture-independent approaches are essential for the comprehensive understanding of the microbial diversity and phylogeny of a given habitat.
The thermal habitats are scarcely attended with entirety as far as the microbial diversity is concerned. Nevertheless, there are some reports related to the metagenomic studies on some thermal habitats (Inskeep et al. 2010; Lewin et al. 2012). However, the reports correlating culture-independent and culture-dependent diversity are very rare. In addition, the present study has also included seasonal impact on the bacterial diversity. The proposed site, Tulasi Shyam (Gujarat, India) was never explored for the microbial diversity and biocatalytic profiling, except our recent reports on the characteristics of the thermostable α-amylases (Kikani and Singh 2011, 2012; Kikani et al. 2013; Kikani and Singh 2015). Our findings indicated that the studied habitat might serve as a marker niche for the amylase producing bacteria.
The analysis of the physicochemical properties of the water and soil samples of the study thermal habitat appeared significant in developing the methods for the extraction of the environmental DNA (McSweeney and Grunwald 1999). Inskeep et al. (2010) reported the geochemical controls on the microbial community structure and function from the high-temperature chemotrophic systems, using various metagenomic techniques. The pH of the soil samples was slightly acidic to neutral. The findings suggested that the water samples were non-saline in nature. Along the similar lines, Marx et al. (1996) described the water samples as slightly saline or non-saline, if the TDS values ranged within 640–1280 ppm, with the corresponding EC values in the range of 1000–2000 μS/cm. This was correlated by the lower concentrations of chloride and sulphates present in the sample.
As described earlier, the metagenomic DNA was extracted by either the direct (More et al. 1994) or indirect methods (Courtois et al. 2003). In comparison to the direct extraction methods, the indirect protocols involve blending, cation-exchange method (Desai and Madamwar 2007) or other new approaches, such as the use of super paramagnetic silica-magnetite nanoparticles for the extraction and purification of the DNA from soil samples (Sebastianelli and Bruce 2008). Both approaches have their own merits and demerits, with respect to the DNA yields, DNA purity, the amenability to the molecular applications and the unbiased representation of the entire microbial diversity (Courtois et al. 2003; Roose-Amsaleg et al. 2001). The main disadvantage of the indirect methods was little representation, only 20–25% of the total endogenous bacterial community in the recovered fraction (Bakken and Lindahl 1995; Purohit and Singh 2009). The direct extraction lysis method, supposedly recovered more than 60% of the total theoretical bacterial DNA (More et al. 1994). Moreover, the indirect extraction methods usually lead to the lower humic acid contamination, as compared to the direct extraction methods. Yet, both the approaches rely on the subsequent purification steps to obtain nucleic acids useful in molecular applications (Robe et al. 2003). However, the indirect methods are time consuming as compared to the direct methods.
In the direct extraction methods, various physical treatments tend to have greater access to the entire bacterial community, including bacteria associated with the soil microaggregates. The most commonly used physical disruption methods are freezing–thawing or freezing–boiling and bead-mill homogenization (Siddhapura et al. 2010; Delmont et al. 2011; Yuan et al. 2012). The physical methods alone could not remove the contaminants and sometimes, it may even cause DNA shearing. Therefore, it should be used in combination with the soft lysis method (Purohit and Singh 2009). The lysis buffer contains detergents and surfactants, such as sodium dodecyl sulfate (SDS), N-lauryl sarcosine, CTAB, lysozyme and Proteinase-K. Usually, the surfactants dissolve the hydrophobic components of the cell membranes. Besides, cetyl trimethyl-ammonium bromide (CTAB) or poly vinyl poly pyrrolidone (PVPP) partially removes humic compounds. Albeit, use of PVPP results in more DNA loss as compared to CTAB (Zhou et al. 1996). CTAB forms insoluble complexes with the denatured proteins, polysaccharides and cell debris, while the PVPP appears ineffective during the cell lysis (Saano et al. 1995). Among enzymatic lysis methods, various protocols have been developed yet lysozyme treatment is among the most commonly used methods (Niemi et al. 2001; Purohit and Singh 2009; Siddhapura et al. 2010). Moreover, proteinase K has also been used to digest the contaminating proteins (Niemi et al. 2001; Purohit and Singh 2009).
Importantly, a major soil component, humic acids inhibits restriction enzyme digestion of DNA and the polymerase chain reaction (Purohit and Singh 2009). Further, it may interfere with the quantitative membrane hybridizations by lowering the expected hybridization signal (Alm et al. 2000). The phenolic groups present in the humic acids denature the biological molecules by bonding to amides. Sometimes, it gets oxidized to form the quinine, which establishes the covalent bond with the DNA (Robe et al. 2003). In most studies, the first DNA purification step following cell lysis includes extraction using organic solvents, mainly phenol and/or chloroform. It is generally followed by ethanol, isopropanol or polyethylene glycol (PEG) assisted precipitation. An unbiased description of the microbial diversity depends on the optimization of the nucleic acid extraction protocol. Standardization of the metagenomic DNA extraction methods from the saline soil of the Coastal region of Gujarat (India) (Purohit and Singh 2009; Siddhapura et al. 2010) and from polluted habitats (Akbari et al. 2014), have been recently reported where the soft lysis method appeared as the most appropriate method for majority of the salt enriched soil samples.
Subsequent to isolation of pure and intact environmental DNA, the sequence based metagenomic approach generally employs various DNA fingerprinting techniques to explore microbial diversity and phylogeny. One of the fingerprinting techniques, denaturing gradient gel electrophoresis (DGGE) was used to explore the metagenomic diversity. DGGE separates PCR-generated DNA products, having different DNA sequences. As the PCR products from a given reaction are of similar size (bp), conventional separation by agarose gel electrophoresis results in a single DNA band, which is largely non-descriptive. In comparison, DGGE can overcome the limitation by separating the PCR products, based on the differences in their respective sequences, used to judge the microbial diversity and population dynamics (Singh et al. 2013). Thus, the present study demonstrated the advantages of the culture-independent techniques to explore the diversity. Similar studies on the exploration of the metagenomic diversity, using PCR-DGGE were earlier conducted (Purohit and Singh 2009), where the metagenomic diversity of the saline soil was judged on the basis of the polymorphism in the band patterns in DGGE. Similarly, Wang et al. (2007) analyzed the metagenomic diversity of the soil samples using DGGE. DGGE has also been used to explore the culture-dependent diversity (Pongsilp et al. 2012) and some other applications (Carmona et al. 2012). Besides DGGE, certain other molecular fingerprinting techniques can also be used such as TGGE, RFLP, ARDRA and ARISA for the assessment of the diversity (Kikani et al. 2015; Delmont et al. 2011; Singh et al. 2013). In addition to various DNA fingerprinting techniques, metagenomic libraries may also be constructed, using the suitable vector-host systems (Lewin et al. 2012). The nucleic acid hybridization may also yield valuable information on the microbial diversity in a given habitat (Alm et al. 2000; Rossello-Mora and Amann 2015; Jang et al. 2015; Zhou et al. 2015).
The metagenomic diversity of the studied natural thermal habitat was further analyzed statistically by calculating various diversity indices. The diversity indices indicated species richness and evenness in the microbial distribution. The Shannon Index was used to explain the microbial diversity of the Arabian Sea (Kapley et al. 2007). Wittebolle et al. (2009) has established a relationship between microbial distribution and Ginni Coefficient, as higher Ginni Coefficient indicate more unequal distribution. In another study, Islam and Sar (2011) analyzed the culture-dependent and culture-independent bacterial diversity of a uranium mine, using various statistical indices. The principal component analysis (PCA) allows ordering of the samples and taxa (i.e. bands) along axes (principal components) on the basis of the banding patterns (Van Der Gucht et al. 2001). In the present study, PCA could not distinguish the clusters which contained similar microbial diversity (Müller et al. 2001). The diversity patterns were also analyzed by calculating the similarity coefficient and UPGMA cluster analysis to determine whether the samples revealed a non-random pattern (Fromin et al. 2002).
Conclusion
Tulasi Shyam is a natural and only thermal habitat in the Saurashtra region of the Gujarat State, India. This study accounts as the first attempt on the microbial diversity and phylogeny of the proposed habitat. Different methods using the combinations of physicochemical parameters were assessed for the extraction of the quality environmental DNA. The sequence-based metagenomic approach was based on the 16S rRNA gene amplification, which followed the analysis of the bacterial diversity using DGGE and diversity indices. The variations in the DGGE band patterns indicated apparent polymorphism, which was also reflected in PCA. Various diversity indices suggested species richness and evenness in distribution. The study also highlighted the significant impact of temperature and seasonal variations on the cultivable bacterial diversity. The amalgamation of both, culture dependent and culture independent approaches in the light of the physicochemical changes of the habitat yielded a comprehensive view of the bacterial diversity of the hot spring.
Highlights of the study
-
Studied thermal habitat not explored earlier for microbial diversity and phylogeny
-
Extraction of the metagenomic DNA established & optimized from soil and water
-
Metagenomic diversity assessed by the sequence-based approach and PCR-DGGE
-
DGGE profile analyzed by PCA, Pearson’s correlation and dendrogram
-
Diversity indices computed for the species richness and evenness
-
Shifts in the metagenomic diversity of soil and water analyzed as a function of seasonal alterations
-
Comparative analysis of culture dependent and culture independent diversity
References
Akbari VG, Pandya RD, Singh SP (2014) Extraction of the metagenomic DNA and assessment of the bacterial diversity from the petroleum-polluted sites. Env Monit Assess 186(10):6351–6362
Alm EW, Zheng D, Raskin L (2000) The presence of humic substances and DNA in RNA extracts affects hybridization results. Appl Env Microbiol 66(10):4547–4554
Bakken LR, Lindahl V (1995) Recovery of bacterial cells from soil. In: Nucleic acids in the environment. Springer, Berlin, Heidelberg, pp 9–27
Blume E, Reichert JM (2015) Banana leaf and glucose mineralization and soil organic matter in microhabitats of banana plantations under long-term pesticide use. Environ Toxicol Chem 34(6):1232–1238
Bressan M, Gattin IT, Desaire S, Castel L, Gangneux C, Laval K (2015) A rapid flow cytometry method to assess bacterial abundance in agricultural soil. Appl Soil Ecol 30:60–68
Carmona M, Sepulveda D, Cardenas C, Nilo L, Marshall SH (2012) Denaturing gradient gel electrophoresis (DGGE) as a powerful novel alternative for differentiation of epizootic ISA virus variants. PLoS One 7(5):37353
Courtois S, Cappellano CM, Ball M, Francou FX, Normand P, Helynck G, Martinez A, Kolvek SJ, Hopke J, Osburne MS, August PR (2003) Recombinant environmental libraries provide access to microbial diversity for drug discovery from natural products. Appl Env Microbiol 69(1):49–55
Delmont TO, Robe P, Cecillon S, Clark IM, Constancias F, Simonet P, Hirsch PR, Vogel TM (2011) Accessing the soil metagenome for studies of microbial diversity. Appl Env Microbiol 77(4):1315–1324
Desai C, Madamwar D (2007) Extraction of inhibitor-free metagenomic DNA from polluted sediments, compatible with molecular diversity analysis using adsorption and ion-exchange treatments. Bioresour Technol 98(4):761–768
Dunbar J, White S, Forney L (1997) Genetic diversity through the looking glass: effect of enrichment bias. Appl Env Microbiol 63(4):1326–1331
Fromin N, Hamelin J, Tarnawski S, Roesti D, Jourdain-Miserez K, Forestier N, Teyssier-Cuvelle S, Gillet F, Aragno M, Rossi P (2002) Statistical analysis of denaturing gel electrophoresis (DGE) fingerprinting patterns. Environ Microbiol 4(11):634–643
Galperin MY (2008) Genomes of model organisms: know thy tools. Environ Microbiol. 10(6):1383–1391
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for education and data analysis, Palaeontologia. Electronica 4:1–9
Inskeep WP, Rusch DB, Jay ZJ, Herrgard MJ, Kozubal MA, Richardson TH, Macur RE, Hamamura N, Jennings RD, Fouke BW, Reysenbach AL (2010) Metagenomes from high-temperature chemotrophic systems reveal geochemical controls on microbial community structure and function. PLoS One 5(3):9773
Islam E, Sar P (2011) Culture-dependent and-independent molecular analysis of the bacterial community within uranium ore. J Basic Microbiol 51(4):372–384
Jang HM, Ha JH, Park JM, Kim MS, Sommer SG (2015) Comprehensive microbial analysis of combined mesophilic anaerobic–thermophilic aerobic process treating high-strength food wastewater. Water Res 73:291–303
Kapley A, Siddiqui S, Misra K, Ahmad SM, Purohit HJ (2007) Preliminary analysis of bacterial diversity associated with the Porites coral from the Arabian sea. World J Microbiol Biotechnol 23(7):923–930
Kennedy J, Marchesi JR, Dobson AD (2007) Metagenomic approaches to exploit the biotechnological potential of the microbial consortia of marine sponges. Appl Microbiol Biotechnol 75(1):11–20
Kikani BA, Singh SP (2011) Single step purification and characterization of a thermostable and calcium independent α-amylase from Bacillus amyloliquifaciens TSWK1-1 isolated from Tulsi Shyam hot spring reservoir, Gujarat (India). Intern J Biol Macromol 48(4):676–681
Kikani BA, Singh SP (2012) The stability and thermodynamic parameters of a very thermostable and calcium-independent α-amylase from a newly isolated bacterium, Anoxybacillus beppuensis TSSC-1. Process Biochem 47(12):1791–1798
Kikani BA, Singh SP (2015) Enzyme stability, thermodynamics and secondary structures of α-amylase as probed by the CD spectroscopy. Intern J Biol Macromol 81:450–460
Kikani BA, Pandey S, Singh SP (2013) Immobilization of the α-amylase of Bacillus amyloliquifaciens TSWK1-1 for the improved biocatalytic properties and solvent tolerance. Bioprocess Biosyst Eng 36(5):567–577
Kikani BA, Sharma AK, Singh SP (2015) Culture dependent diversity and phylogeny of thermophilic bacilli from a natural hot spring reservoir in the Gir Forest, Gujarat (India). Microbiol 84:687–700
Lewin A, Wentzel A, Valla S (2012) Metagenomics of microbial life in extreme temperature environments. Curr Opin Biotechnol 24:1–10
Marx ES, Hart JM, Stevens RG (1996) Soil test interpretation guide. Oregon State University Extension Service, USA, Oregon
McSweeney K, Grunwald S (1999) Soil morphology, classification and mapping. University of Wisconsin-Madison, USA
More MI, Herrick JB, Silva MC, Ghiorse WC, Madsen EL (1994) Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Env Microbiol 60(5):1572–1580
Muller AK, Westergaard K, Christensen S, Sorensen SJ (2001) The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiol Ecol 36(1):11–19
Muyzer G, Smalla K (1998) Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 73(1):127–141
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59(3):695–700
Niemi RM, Heiskanen I, Wallenius K, Lindström K (2001) Extraction and purification of DNA in rhizosphere soil samples for PCR-DGGE analysis of bacterial consortia. J Microbiol Methods 45(3):155–165
Pongsilp N, Nimnoi P, Lumyong S (2012) Genotypic diversity among rhizospheric bacteria of three legumes assessed by cultivation-dependent and cultivation-independent techniques. World J Microbiol Biotechnol 28(2):615–626
Purohit MK, Singh SP (2009) Assessment of various methods for extraction of metagenomic DNA from saline habitats of coastal Gujarat (India) to explore molecular diversity. Lett Appl Microbiol 49(3):338–344
Ranjard L, Poly F, Combrisson J, Richaume A, Nazaret S (1998) A single procedure to recover DNA from the surface or inside aggregates and in various size fractions of soil suitable for PCR-based assays of bacterial communities. Eur J Soil Biol 34(2):89–97
Robe P, Nalin R, Capellano C, Vogel TM, Simonet P (2003) Extraction of DNA from soil. Eur J Soil Biol 39(4):183–190
Roose-Amsaleg CL, Garnier-Sillam E, Harry M (2001) Extraction and purification of microbial DNA from soil and sediment samples. Appl Soil Ecol 18(1):47–60
Rossello-Mora R, Amann R (2015) Past and future species definitions for bacteria and archaea. Syst Appl Microbiol 38(4):209–216
Saano A, Tas E, Pippola S, Lindström K, Van Elsas JD (1995) Extraction and analysis of microbial DNA from soil. In: Nucleic acids in the environment. Springer, Berlin, Heidelberg, pp 49–67
Sebastianelli A, Bruce IJ (2008) Extraction of DNA from soil using nanoparticles by magnetic bioseparation. Lett Appl Microbiol 46(4):488–491
Sharma P, Capalash N, Kaur J (2007) An improved method for single step purification of metagenomic DNA. Mol Biotechnol 36:61–63
Siddhapura PK, Vanparia S, Purohit MK, Singh SP (2010) Comparative studies on the extraction of metagenomic DNA from the saline habitats of Coastal Gujarat and Sambhar Lake, Rajasthan (India) in prospect of molecular diversity and search for novel biocatalysts. Intern J Biol Macromol 47(3):375–379
Singh SP, Shukla RJ, Kikani BA (2013) Molecular diversity and biotechnological relevance of thermophilic actinobacteria. In: Thermophilic Microbes in Environmental and Industrial Biotechnology. Springer, Netherlands, pp 459–479
Stewart EJ (2012) Growing Unculturable Bacteria. J Bacteriol 16:4151–4160
Streit WR, Schmitz RA (2004) Metagenomics–the key to the uncultured microbes. Curr Opin Microbiol 7(5):492–498
Thomas T, Gilbert J, Meyer F (2012) Metagenomics-a guide from sampling to data analysis. Microb Inform Exp 2(3):1–2
Van Der Gucht K, Sabbe K, De Meester L, Vloemans N, Zwart G, Gillis M, Vyverman W (2001) Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ Microbiol 3(11):680–690
Voget S, Leggewie C, Uesbeck A, Raasch C, Jaeger KE, Streit WR (2003) Prospecting for novel biocatalysts in a soil metagenome. Appl Env Microbiol 69(10):6235–6242
Wang CY, Ng CC, Chen TW, Wu SJ, Shyu YT (2007) Microbial diversity analysis of former salterns in southern Taiwan by 16S rRNA-based methods. J Basic Microbiol 47(6):525–533
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N (2009) Initial community evenness favours functionality under selective stress. Nature 458(7238):623–626
Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ (2012) Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS One 7(3):33865
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62(2):316–322
Zhou J, He Z, Yang Y, Deng Y, Tringe SG, Alvarez-Cohen L (2015) High-throughput metagenomic technologies for complex microbial community analysis: open and closed formats. MBio 6(1):1–17. doi:10.1128/mBio.02288-14
Acknowledgements
The authors are thankful to the University Grant Commission (UGC), New Delhi, India and Saurashtra University, Rajkot (Gujarat, India) for the financial and infrastructural support. BAK acknowledges Senior Research Fellowship by the Council of Scientific and Industrial Research (CSIR-SRF), the Government of India, New Delhi. AKS gratefully acknowledges Research Fellowship in Sciences for Meritorious Students by UGC, New Delhi, India. BAK also acknowledges the Department of Science and Technology (DST), the Government of India, New Delhi, for the International Travel Grant to present his work in 9th International Congress on Extremophiles 2012, held in Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no potential sources of conflict.
Rights and permissions
About this article
Cite this article
Kikani, B.A., Sharma, A.K. & Singh, S.P. Metagenomic and Culture-Dependent Analysis of the Bacterial Diversity of a Hot Spring Reservoir as a Function of the Seasonal Variation. Int J Environ Res 11, 25–38 (2017). https://doi.org/10.1007/s41742-017-0003-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0003-9