Abstract
The α-amylase of Bacillus amyloliquifaciens TSWK1-1 (GenBank Number, GQ121033) was immobilized by various methods, including ionic binding with DEAE cellulose, covalent coupling with gelatin and entrapment in polyacrylamide and agar. The immobilization of the purified enzyme was most effective with the DEAE cellulose followed by gelatin, agar and polyacrylamide. The K m increased, while V max decreased upon immobilization on various supports. The temperature and pH profiles broadened, while thermostability and pH stability enhanced after immobilization. The immobilized enzyme exhibited greater activity in various non-ionic surfactants, such as Tween-20, Tween-80 and Triton X-100 and ionic surfactant, SDS. Similarly, the enhanced stability of the immobilized α-amylase in various organic solvents was among the attractive features of the study. The reusability of the immobilized enzyme in terms of operational stability was assessed. The DEAE cellulose immobilized α-amylase retained its initial activity even after 20 consequent cycles. The DEAE cellulose immobilized enzyme hydrolyzed starch with 27 % of efficiency. In summary, the immobilization of B. amyloliquifaciens TSWK1-1 α-amylase with DEAE cellulose appeared most suitable for the improved biocatalytic properties and stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thermophilic bacteria can grow and produce industrially valuable compounds optimally at high temperatures [1]. The ability to grow at high temperatures is associated with the stability of the macromolecules. The thermophilic enzymes could provide potential models for understanding the thermostability. The thermophilic processes are stable, rapid and less expensive, which ultimately leads to the improved activity and product recovery [2, 3].
The amylases hydrolyze starch to form diverse products, which include dextrin and successively smaller polymers of glucose [4]. The α-amylase (E.C.3.2.1.1) family comprises of a group of enzymes with a variety of different specificities that act on a substrate, which contains glucose residues linked through α-1-1, α-1-4, α-1-6, glycosidic bonds [5, 6]. It shares approximately 30 % of the enzyme market demand [5], with potential applications in the starch liquefaction, manufacturing of maltose, high fructose-containing syrups (HFCS), oligosaccharides mixtures, maltotetrose syrups, high molecular weight-branched dextrins, in the removal of starch sizer from textiles, direct fermentation of starch to ethanol and in the treatment of starch processing waste water [7]. The thermostable α-amylases of other Bacillus sp. with fairly good activity and stability at high temperatures are also reported [8–12]. In general, the thermostable α-amylases are considered to be calcium dependent in nature. However, the demand for calcium-independent α-amylase in the starch industries is ever increasing.
The immobilization of microbial cells [13] or enzyme [14–19] is a value maximization approach for the efficient utility, improved quality and better applications in the industries. The advantages of enzyme immobilization include enzyme re-utilization, enhanced stability, easy separation from the reaction mixture and ready applications in the automated continuous processes [20]. The factors affecting the yield of the enzyme immobilization are mainly, type of enzyme and carrier used, type of immobilization method used, enzyme concentration, concentration of the carrier, utilization of any cross-linking agent and its concentration and bead/block size. Besides, the amount of beads/blocks utilized in the reaction mixture also significantly influence the substrate hydrolysis. The process variables affecting the immobilization of α-amylase on gelatin were also optimized by the response surface methodology (RSM) [15]. Another study was focused on the optimization of the immobilization of α-amylase using calcium alginate [18]. The immobilized system should also be stable for a longer time without any toxic and hazardous effects. The stability parameters relate to the resistance against various physicochemical factors including mainly temperature, pH, denaturants, surfactants and organic solvents. In addition, to the advantage of easy recovery of the enzyme, the immobilization may also protect enzyme from deactivation, destabilization and denaturation in organic solvents [21].
The present study aimed at the immobilization of purified α-amylase of B. amyloliquifaciens TSWK1-1 (GenBank Number, GQ121033) on various carriers using ionic binding, covalent binding and entrapment methods. The properties of free and immobilized enzymes were compared with respect to the effect of pH, temperature, surfactants and organic solvents on enzyme activity and stability.
Materials and methods
Materials
Starch, acrylamide, bis-acrylamide, TEMED and DEAE cellulose were purchased from Merk, Mumbai, India. The medium components and agar were purchased from Hi Media Laboratories, Mumbai, India. The gelatin and other chemicals were purchased from Rankem Laboratories, Mumbai, India, which were of the analytical grade.
Purification of α-amylase of B. amyloliquifaciens TSWK1-1
The single-step purification of the α-amylase of a thermophilic bacterium, B. amyloliquifaciens TSWK1-1 (GenBank Number, GQ121033) was achieved on the phenyl Sepharose 6FF column (Sigma, India; 1 cm × 6.5 cm) by the hydrophobic interaction chromatography. The bacterium was isolated from a hot spring reservoir, located in Tulsi Shyam, Gujarat, India [22].
The immobilization strategies
The ionic binding of the α-amylase with DEAE cellulose
The DEAE cellulose in various amounts (1–3 g) was added to 20 ml of 20 mM phosphate buffer, pH 7 and incubated overnight with the continuous stirring for swelling and mixing of the slurry. The swelled DEAE cellulose was filtered on a Buchner funnel and was incubated with 20 ml 0.5 N HCl for 1 h. It was then washed with distilled water thoroughly till pH 7. The HCl-treated DEAE cellulose was further treated with 20 ml 0.5 N NaOH, followed by the continuous slow stirring at the room temperature for 1 h. It was then washed with distilled water until pH 7. The activated DEAE cellulose was preserved in 20 ml of 20 mM phosphate buffer, pH 7 at 4 °C [23]. The purified α-amylase (2,400 U/g of carrier) in 1 ml was mixed with the activated DEAE cellulose, prewashed with the assay buffer and stirred for overnight at 4 °C. The unbound enzyme was washed out extensively with the assay buffer.
The covalent coupling of the α-amylase with gelatin
The gelatin powder in various concentrations from 5 to 15 % was swelled in 10 ml of 20 mM phosphate buffer, pH 7. To ensure the complete solubilization, the preparation was heated at 50 °C for 10 min. After sufficient cooling, 1 ml of the purified α-amylase (2,400 U/g of carrier) was mixed. This followed by the addition of 0.6 % (w/v) gluteraldehyde, an organic cross-linker. After continuous stirring, the preparation was poured on a glass plate to prepare thin film of immobilized enzyme and preserved at 4 °C. The unbound enzyme was washed out extensively with the assay buffer and cut into small uniform blocks for further applications [15, 16, 24].
The entrapment of the α-amylase in polyacrylamide and agar
The enzyme immobilization in polyacrylamide gel was achieved by mixing 3 ml of the acrylamide: bis-acrylamide mixture, 4 ml of water, 2 ml of 100 mM Tris–Cl buffer, pH 8 and 1 ml of the purified α-amylase (2,400 U/g of carrier). The polymerization was achieved by the addition of 100 μl of 1 % (w/v) ammonium persulfate and 6 μl of TEMED. The gel film was polymerized at 4 °C on a glass plate. The unbound enzyme was washed out with the assay buffer. The immobilized gel was cut into small uniform blocks before further utilization [24].
The various concentrations of agar from 1 to 5 % were prepared in 20 mM phosphate buffer, pH 7. It was heated at 100 °C for 10 min followed by cooling till 50 °C. The purified α-amylase (2,400 U/g of carrier) in 1 ml was mixed with 5 ml of the cooled mixture and allowed to solidify. The unbound enzyme was washed out extensively with the assay buffer. The immobilized enzyme was preserved at 4 °C before cutting into small uniform blocks for further applications [20].
Amylase activity
The film of immobilized enzyme was cut into 24 small uniform blocks before use. The amylase activity was assayed using half of the blocks of the immobilized enzyme and 2 % (w/v) starch as substrate, dissolved in 20 mM phosphate buffer, pH 7. The reaction mixture was further incubated at 70 °C for 20 min. It was followed by the dinitrosalicylic acid method [22]. One unit was defined as the amount of enzyme liberating 1 μmol of maltose per minute under the assay conditions. Maltose (100–1,000 μg) was used as standard. The protein content of the purified enzyme was measured according to the Bradford method using bovine serum albumin (10 μg/ml) as a standard.
The preparations after each immobilization process were assessed for the immobilization and activity yield.
where, A is the activity (unit/g of carrier) of the free enzyme before immobilization; B is the activity (unit/g of carrier) of the unbound wash out and C is the activity (unit/g of carrier) of the immobilized enzyme.
The enzyme characterization
The determination of kinetic parameters
The immobilized enzyme preparations were assayed at substrate concentrations between 0.1 and 3 % (w/v) of starch. The K m and V max were estimated from the reciprocal plot of the substrate concentration versus the velocity.
The temperature and pH profiles of the immobilized amylase
The amylase activity was measured by incubating reaction mixtures, containing immobilized enzyme and 2 % starch dissolved in 20 mM phosphate buffer, pH 7 at temperatures ranging from 10 to 100 °C for 20 min to generate the temperature profile. Similarly, for pH range and pH optima, the immobilized enzyme was incubated with 2 % soluble starch dissolved in various buffers, including 20 mM citrate buffer, pH 3–4; 20 mM acetate buffer, pH 5; 20 mM phosphate buffer, pH 6–7; 20 mM Tris–HCl buffer, pH 8; 20 mM glycine–NaOH buffer, pH 9 and 20 mM NaOH–Borax buffer, pH 10–12, followed by the incubation at the optimum temperature (70 °C) for the enzyme activity.
The thermostability and pH stability of immobilized amylase
To assess the thermostability and pH stability, the immobilized enzyme was incubated in various buffers, including 20 mM phosphate buffer, pH 6–7; 20 mM Tris–HCl buffer, pH 8 and 20 mM glycine–NaOH buffer, pH 9 at temperatures, 37 and 60 °C. The aliquots were withdrawn at regular interval of 3 h for 12 h for the enzyme activity, followed by the estimation of residual activities. The residual activities were computed by comparing the enzyme activity at 0 min (100 %) with the activities at various time intervals after incubation.
The determination of decay constant (κ) and half-life (t 1/2)
The values of decay constant (κ) and half-life (t 1/2) of the enzyme were calculated as follows:
At t = 0; E = E 0. Integrating…
where, E 0 is the initial activity, while t denotes to the time elapsed during the reaction. The residual activity A r is directly proportional to the concentration of the active enzyme (E):
From the Eqs. (2) and (3) [18],
This is known as the exponential decay model.
Therefore,
The half-life of the enzyme (t 1/2) is the time required for the enzyme activity to decrease 50 % of the initial value.
Therefore [25],
The effect of surfactants on enzyme activity
The effect of surfactants on enzyme activity was assessed by incubating the free and immobilized enzymes for 1 h in the ionic surfactant, 20 mM sodium dodecyl sulfate (SDS) and non-ionic surfactants, including Tween-20, Tween-80 and Triton X-100 in 5 % (v/v) concentration. The relative activities were calculated by comparing the activities with control, without any surfactant as 100 %.
The effect of solvents on enzyme activity
The range of solvents was selected on basis of their interaction with water and logP ow values. The selected solvents were as follows: acetone (−0.24), diethyl ether (0.8), benzene (2.13), toluene (2.73) and cyclohexane (3.3). The effect of these solvents on amylase activity was followed by monitoring activities of free and immobilized enzymes in various solvents at various concentrations ranging between 10 and 50 % (v/v). The substrate concentration was 2 % starch dissolved in 20 mM phosphate buffer, pH 7. The reaction mixtures were incubated at 70 °C for 20 min under the slow shaking condition (80 rpm) to ensure proper mixing. The relative activities were calculated by comparing the activity with control, without any solvent as 100 %.
The effect of solvents on enzyme stability
The stability profiles of the free and immobilized α-amylase against the above listed solvents were investigated. The free and immobilized enzymes were incubated separately with the above-stated solvents in 10 % (v/v) concentration at 60 °C under the slow shaking condition at 80 rpm for proper mixing. The activities were monitored at regular intervals of 3 h for 12 h. The residual activities were calculated thereafter.
The operational stability
The immobilized α-amylase was incubated with 500 μl of 2 % (w/v) starch, dissolved in 20 mM phosphate buffer, pH 7 at the optimum temperature, 70 °C for 20 min. The immobilized enzyme was separated and washed thoroughly with the assay buffer before the next reaction. The protein contents were measured by the Bradford method, which was related to the activity. The procedure was repeated for 20 subsequent cycles.
Application of the DEAE immobilized α-amylase in the starch hydrolysis
The DEAE cellulose immobilized α-amylase was incubated with 10 ml of 2 % starch dissolved in 20 mM phosphate buffer, pH 7 at 100 °C for 10 min. The gelatinized starch was further incubated at 80 °C for 1 h for the starch liquefaction. At last, the liquefied starch was subjected to starch saccharification by incubating with the DEAE cellulose immobilized α-amylase at 50 °C for 4 h. The immobilized enzyme was removed and thoroughly washed. The hydrolyzed products were analyzed for the hydrolysis efficiency by measuring the reducing sugars produced after each step. The reducing sugars were estimated by DNS method using maltose as a standard.
The hydrolysis efficiency can be expressed as
In other words [26],
Results and discussion
The present study focused on the immobilization of purified α-amylase of B. amyloliquifaciens TSWK1-1 to achieve improved functioning for the industrial applications.
Immobilization strategies
The B. amyloliquifaciens TSWK1-1 α-amylase was immobilized with different matrices by various methods, including ionic binding by DEAE cellulose, entrapment by agar and polyacrylamide and covalent coupling by gelatin. Table 1 indicates the immobilization and activity yields using various concentrations of the support matrices. While immobilization was achieved by all the methods, the immobilization with DEAE cellulose emerged as the most suitable support. Quite low yield was evident for immobilization with polyacrylamide. The trend of the efficacy of the immobilization was also reflected in protein contents and specific activities highlighted in Table 2. The peroxidase of bitter gourd was successfully immobilized with DEAE cellulose [23]. The studies with the β-xylosidase of Talaromyces thermophilus, for the production of xylose and xylooligosaccharide reflected that chitosan was the best carrier for the immobilization, while DEAE cellulose was also fairly good support for immobilization [24].
In the present study, the immobilization yield increased with increasing concentrations of carrier, with the exception of gelatin. Besides, the trend of the present study contradicts the immobilization of soybean α-amylase on gelatin, where the increase in gelatin and gluteraldehyde concentration leads to the enhanced immobilization yield [16]. However, both the trends contradict a report on the immobilized amylase of Bacillus sp. [20], where increasing concentrations of carrier led to decreased immobilization yield by the entrapment method.
The enzyme characterization
The determination of kinetic parameters
The kinetic parameters, including K m and V max are presented in Table 2. The K m and V max values of the purified B. amyloliquifaciens TSWK1-1 α-amylase were 0.6 mg/ml and 2,632 mol/ml/min, respectively [22]. In general, the K m values exhibited by the immobilized amylase were higher than that of the free form, which was most likely due to either conformational changes or lower accessibility of the substrate to the active site of the enzyme in its immobilized form. This is likely to be supported by decreased V max of the immobilized enzyme. The present study revealed that the lowest K m, 0.8 mg/ml, was achieved with DEAE cellulose and gelatin, while the highest K m, 1.2 mg/ml, was apparent with polyacrylamide. Similarly, the V max value was highest at 2,186 mol/ml/min with DEAE cellulose, and lowest, 1,563 mol/ml/min, with polyacrylamide. Similar trends were also reflected on the immobilization of amylases of thermophilic Bacillus sp. [20], Bacillus subtilis [27] and Aspergillus oryzae [28].
The temperature and pH profiles of the immobilized enzyme
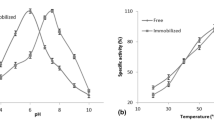
The α-amylase of B. amyloliquifaciens TSWK1-1 was active over a wide range of temperatures, from 37 to 100 °C, with an optimum at 70 °C. Similarly, the enzyme was also active over a broad range of pH from 5 to 10. The highest activity was achieved in 20 mM phosphate buffer, pH 7 [22]. The optimum temperature and pH remained unaltered after immobilization. However, the temperature and pH profiles of the immobilized α-amylase turned broader, i.e., the temperature between 10 and 100 °C, while the pH from 3 to 12 (Fig. 1a, b). However, at 10 °C and also in 20 mM NaOH–Borax buffer, pH 12, the activity of the enzyme immobilized on polyacrylamide was completely lost. Similar findings on α-amylase immobilized on polyacrylamide were reported at high temperatures [19]. According to Raviyan et al. [19], the possible reason was due to the changes in the microenvironment of the gel matrix at various temperatures, which induced conformational changes in the enzyme. The changes might have further enhanced its susceptibility to the inactivation. Moreover, the stabilized hydrogen-bonded structure formed between amide groups in acrylamide and α-amylase might have been affected. The trends of the present study was also similarly observed for an immobilized peroxidase with DEAE cellulose, where temperature and pH optima of the enzyme in immobilized and free states did not alter. Nevertheless, the immobilized enzyme had significantly greater catalytic activity at higher temperatures and pHs [23]. The starch converting enzymes were immobilized on DEAE cellulose and the co-immobilized system of DEAE cellulose and silica gel. Interestingly, the optimum temperature of the native and the immobilized enzymes were 50 and 60 °C, respectively. The pH optimum of the native enzyme was 5, which was shifted to 5.5 for immobilized enzymes [29].
a Temperature optimization profile of the immobilized and free B. amyloliquifaciens TSWK1-1 α-amylase; immobilized on DEAE cellulose (open diamonds), agar (open triangles), polyacrylamide (times) and gelatin (filled circles), respectively, b pH optimization profile of the immobilized and free B. amyloliquifaciens TSWK1-1 α-amylase; immobilized on DEAE cellulose (open diamonds), agar (open triangles), polyacrylamide (times) and gelatin (filled circles), respectively
The thermostability and pH stability of the immobilized enzyme
The purified α-amylase of B. amyloliquifaciens TSWK1-1 was thermally stable and calcium independent [22]. However, the thermostability was significantly increased after immobilization on various supports. The increase in the thermostability is reflected by the decreased decay rate and increased half-life of the enzyme. The half-life of the purified α-amylase of B. amyloliquifaciens TSWK1-1 was 31.5 h in 20 mM phosphate buffer, pH 7 at 60 °C, while, it increased to 38.5, 45, 62 and 86.5 h upon immobilization with polyacrylamide, agar, gelatin and DEAE cellulose, respectively [Table 3]. The thermostability of α-amylase of B. subtilis ITBCCB148 increased upon immobilization with CM cellulose [14]. In the same way, the thermostability of the α-amylase was enhanced upon immobilization at 60 °C after 1 h of incubation. The residual activity of α-amylase was 26.93 %, which was further enhanced upon immobilization with AS-alumina, DEAE cellulose, chitin and polyacrylamide to 70.53, 76.42, 98.0 and 88.1 %, respectively [26].
Similarly, the pH stability profiles, illustrated in Table 3 pointed out that the immobilized enzyme had greater stability than the free form. However, the immobilization with DEAE cellulose was the most effective among the different methods with respect to the thermal and pH stability. As indicated in Table 3, the enzyme was more stable at 60 °C as compared to 37 °C, which highlighted the thermophilic nature of the enzyme. While the stability of the enzyme was the highest in 20 mM phosphate buffer, pH 7 as compared to other buffer systems, the enzyme had a broad pH range for the stability. Similar report on immobilization of another α-amylase with gum arabica and agar gum highlighted that gum arabica was the best support leading to lower decay constant and thereby higher half-life [30]. On a similar note, the immobilized peroxidase [23] and β-xylosidase [24] displayed enhanced temperature and pH stability. The stability generally results from the molecular rigidity introduced by attachment to a rigid support and creation of a protected microenvironment. In case of multimeric enzymes, it was found that the immobilization of the enzyme may prevent subunit dissociation by inter-subunit cross-linking while simultaneously reducing conformational inactivation by intra-subunit crosslinking [17].
The effect of surfactants on the immobilized enzyme
After 1 h of incubation with various surfactants, the resistance of the immobilized B. amyloliquifaciens TSWK1-1 α-amylase against various ionic and non-ionic surfactants was enhanced as compared to its free form. However, 20 mM SDS inhibited the enzyme greatly as compared to other non-ionic surfactants. The results are illustrated in Fig. 2. The immobilization with agar and DEAE cellulose was most suitable as compared to gelatin and polyacrylamide. Similar results were obtained with the immobilized β-xylosidase [24, 31, 32]. The amylases are useful in the detergent industry. However, to have potential applications in detergent, the amylase must be alkali tolerant and should be stable in various detergent ingredients, such as various surfactants [8]. The inherent properties of the α-amylase and enhanced stability after immobilization make it a promising candidate for detergent supplement.
The effect of various solvents on the enzyme activity and stability
The solvent tolerance is among the key features of the enzymes required for the field applications. The solvent-tolerant enzymes must be active and stable in the presence of toxic solvents, providing a promising tool for biocatalysis in non-aqueous systems [33–37]. Most of the studies on solvent-tolerant microorganisms have focused on the Gram-negative bacteria, which display effective adaptive mechanisms to acclimatize in organic solvents. However, very limited information is available on organic solvent tolerance in Gram-positive bacteria [38]. The vast majority of the synthetic enzyme reactions are performed in the organic media. The biotransformation processes in the organic solvents offer unique advantages as compared to the traditional aqueous-biocatalysis. The advantageous features include increased solubility of the non-polar substrates and the products; enhancing the overall reaction rates; reversal of the thermodynamic equilibrium in favor of the synthesis over hydrolysis; suppression of undesirable water-dependent side reactions, which can degrade common organic reagents and reduced risk of the microbial contamination in the reaction mixture [39]. However, low specific activity in the organic solvents is a major drawback of non-aqueous catalysis. As water is responsible to maintain the structural flexibility and mobility of the protein molecule, the organic solvent may cause deamidation of Asn and Gln residues and hydrolysis of the peptide bond, which ultimately leads to unfolding of enzyme molecules and loss of enzymatic activity [40]. Although hydrolases, such as proteases and lipases are extensively studied [41], only limited attention has been paid to the immobilized α-amylase with respect to enhanced solvent tolerance.
In addition to other stabilization techniques, the immobilization enhances the thermostability and solvent stability of the enzyme [17]. As indicated in Fig. 3 and Table 4, the B. amyloliquifaciens TSWK1-1 α-amylase was active against both, hydrophilic and hydrophobic solvents. The results contradict the general perception that the hydrophobic solvents with lower log P ow are usually not suitable for enzymatic catalysis [42]. In the present study, the α-amylase was active against various solvents. Nevertheless, the activity was further enhanced through immobilizing. In the presence of 50 % (v/v) benzene; 84, 74, 68 and 82 % of the original activities were retained upon immobilization of the enzyme with DEAE cellulose, gelatin, polyacrylamide and agar, respectively, as compared to the free form of the purified enzyme with the residual activity to be 51 %. Similar trends were also reflected with other solvents; toluene, cyclohexane, diethyl ether and acetone, where the enzyme catalysis of the α-amylase was quite effective after immobilization on various supports as compared to the free form of the enzyme (Fig. 3).
The immobilization of enzymes by multipoint attachment protects them from denaturation induced by the organic solvents. Enzyme immobilized by adsorption on Eudragit S-100, chitin and chitosan exhibited enhanced activity in organic co-solvent mixtures at around 10–20 % solvents (v/v) [43, 44]. In comparison, the immobilized amylase in the present study was active even at 50 % (v/v) solvents.
The loss of stability of α-amylase of Haloarchaeon, Haloarcula sp. S-1 was reported in presence of various solvents [45]. We have not come across with any other study on the solvent stability of the immobilized α-amylase. However, the effect of various solvents on the amylase activity has recently been reported [46]. Table 4 illustrates that the B. amyloliquifaciens TSWK1-1 α-amylase was fairly stable in 10 % (v/v) benzene, toluene, cyclohexane, diethyl ether and acetone for 12 h, when immobilized with DEAE cellulose and agar.
As illustrated in Fig. 3 and Table 4, the DEAE cellulose immobilized α-amylase was quite active and stable against various solvents at 10 and 50 % (v/v) concentrations, as compared to other immobilized forms and that of the free native form. Similarly, other reports have revealed the enhanced enzyme stability against various denaturants after immobilization with DEAE cellulose [47–49]. However, DEAE cellulose immobilized enzymes have only non-covalent forces between the support and enzyme molecules, hence it may lead to desorption of the enzyme from the support. However, the adsorbed enzyme can be cross-linked with bifunctional or multifunctional reagents to prevent desorption of the enzyme [48].
The operational stability of the immobilized enzyme
The operational stability of an immobilized preparation is one of the most important factors in the enzyme-based bioconversion processes. Therefore, the aspect of operational stability of the immobilized enzyme on various supports was extensively studied in the present work. The DEAE cellulose immobilized enzyme was reused repeatedly for 20 successive cycles at its optimum temperature and pH with a marginal loss of 4 % in its original activity. This was followed by the immobilization with gelatin, where 83 % of the original activity was retained after 20 cycles. However, immobilization with agar and polyacrylamide were comparatively less stable with 71 and 65 % of original activity, respectively, after 20 cycles (Fig. 4). The decrease in the activity and stability after successive cycles could have been due to enzyme denaturation and/or physical loss of enzyme from the carriers.
In another study, the α-amylase immobilized with gum arabica and agar gum, retained 98 and 13 % of its original activity after four subsequent cycles, respectively [30]. The α-amylase of Bacillus amyloliquifaciens, immobilized on calcium alginate beads with 5 % (w/v) CaCl2 were suitable for up to seven repeated uses and 38 % of the initial activity was retained [50]. However, the capsules prepared from 2 % (w/v) sodium alginate and 5 % (w/v) CaCl2 to immobilize the α-amylase of B. subtilis were suitable for up to 20 repeated uses, losing only 30 % of their initial efficiency [51]. Similarly, the B. circulans α-amylase immobilized by entrapment in calcium alginate beads retained 83 % of the initial activity after seven cycles [52]. The findings would play key role in developing a bioreactor for the continuous applications.
Application of the DEAE immobilized α-amylase in starch hydrolysis
The DEAE cellulose immobilized α-amylase fairly hydrolyzed starch to maltooligosachharides. In general, the α-amylase acts randomly on the starch molecule, as compared to β-amylase which acts on the non-reducing ends. Thereby, the α-amylase generates maltose as major, while glucose as minor end product. Besides, the hydrolysis efficiency also indicates the rate of starch hydrolysis. The efficiency values of pure starch and maltose are 0 and 50 %, respectively, while the value, 100 % indicates complete theoretical conversion of starch to glucose, indicating that the total number of glycosidic bonds present before the enzyme treatment equals the total number of glycosidic bonds broken during the treatment. In the present study, the hydrolysis efficiency values after gelatinization and liquefaction were 1.5 and 4 %, respectively. While after the starch saccharification, the value was 27 %, which indicates its potential application in the high maltose syrup preparation.
Conclusion
The present study focused on the immobilization of a purified α-amylase from B. amyloliquifaciens TSWK1-1 by ionic binding, covalent coupling and entrapment methods to achieve enhanced catalytic properties, in terms of activity and stability. Among these, DEAE cellulose proved as the best method in terms of activity yields and stability at various temperatures and pH and in various surfactants and solvents. The immobilized enzyme with enhanced properties can be successfully explored in industries and also in the waste treatment contaminated with the solvents. To the best of our knowledge, this report is the first on the immobilized α-amylase with the enhanced solvent stability. Besides, the operational stability of the immobilized amylase was excellent even after 20 successive cycles. The DEAE cellulose immobilized enzyme satisfactorily hydrolyzed starch, which directs its potential application in the maltooligosaccharide production. The findings are of great interest toward the development of a bioreactor for the continuous applications.
References
Brock T (1986) In: Thermophiles: general, molecular and applied microbiology. Wiley, New York, pp. 2–15
Haki GD, Rakshit SK (2003) Bioresour Technol 89:17–34
Singh SP (2006) In: Environmental microbiology, National Science Digital Library (CSIR): E-Book. Council of Scientific and Industrial Research, India, pp 1–35
Reddy NS, Nimmagadda A, Rao KRSS (2003) African J Biotechnol 2:645–648
Van der Maarel MJEC, Van der Veen B, Uitdehaag H, Leemhuis H, Dijkhuizen L (2002) J of Biotechnol 94:137–155
Shivramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) Food Technol Biotechnol 44:173–184
Aiyer PV (2005) Afr J Biotechnol 4:1525–1529
Kikani BA, Singh SP (2012) Process Biochem (in press)
Bozic N, Ruiz J, Lopez-Santin J, Vujcic Z (2011) Biochem Eng J 53:203–209
Mollania N, Khajeh K, Hosseinkhani S, Dabirmanesh B (2010) Int J Biol Macromol 46:27–36
Kikani BA, Shukla RJ, Singh SP (2010) In: Current research, technology and education topics in applied microbiology and microbial biotechnology, vol 2. Formatex Research Center, Badajoz, pp 1000–1007
Asgher M, Asad MJ, Rahman SU, Legge RL (2007) J Food Eng 79:950–955
Mamo G, Gessesse A (1997) Biotechnol Tech 11:447–450
Yandri SD, Suhartati T, Hadi S (2012) Mod Appl Sci 6:81–86
Jaiswal N, Prakash O, Talat M, Hasan SH, Pandey RK (2012) J Genet Eng Biotechnol (in press)
Jaiswal N, Prakash O (2011) Asian J Biochem 6:337–346
Iyer PV, Ananthnarayan L (2008) Process Biochem 43:1019–1032
Ertan F, Yagar H, Balkan B (2007) Prep Biochem Biotechnol 37:195–204
Raviyan P, Tang J, Rasco BA (2003) J Agric Food Chem 51:5462–5466
Sumen S, Ramesh K (2009) Int J Pharm Sci 1:315–319
D’Souza SF (1999) Curr Sci 77:69–79
Kikani BA, Singh SP (2011) Int J Biol Macromol 48:676–681
Kulshreshtha Y, Husain Q (2006) Enzyme Microb Technol 38:470–477
Guerfalia M, Maaleja I, Gargouria A, Belghith H (2009) J Mol Catal B Enzym 57:242–249
Fabrisco MG, Ernanades BP, Heizo F (2004) Biomacromolecules 5:17–23
Jana M, Maity C, Samanta S, Pati BR, Islam SS, Das PK, Mohapatra MKC (2012) Ind Crops Prod 41:386–391
Abdel-Naby MA, Hashem AM, Esawy MA, Abdel-Fattah AF (1998) Microbiol Res 153:1–7
Siso MIG, Graber M, Condoret IS, Combes D (1990) J Chem Technol Biotechnol 48:185–200
Park D, Haam S, Jang K, Ahn IS, Kim WS (2005) Process Biochem 40:53–61
Egwim EC, Oloyede OB (2011) J Biochem Tech 3:222–224
Abdel-Naby MA (1999) Process Biochem 34:399–405
Gouda KM, Abdel-Naby MA (2002) Microbiol Res 157:275–281
Castro GR (1999) Enzyme Microb Technol 25:689–694
Takeda Y, Aono R, Doukyu N (2006) Extremophiles 10:269–277
Gupta A, Khare SK (2009) Crit Rev Biotechnol 29:44–54
Pandey S, Singh SP (2010) Appl Biochem Biotechnol 166:1747–1757
Pandey S, Rakholiya KD, Raval VH, Singh SP (2012) J Biosci Bioeng 114:251–256
Torres S, Pandey A, Castro G (2011) Biotechnol Adv 29:442–452
Torres S, Castro GR (2004) Food Technol Biotechnol 42:271–277
Affleck R, Zu-feng X (1992) Biochemistry 89:1100–1104
Gupta MN, Roy I (2004) Eur J Biochem 271:2575–2583
Laane C, Boeren S, Vos K, Veeger C (2005) Biotechnol Bioeng 30:81–87
Batra R, Gupta MN (1994) Biotechnol Appl Biochem 19:209–215
Batra R, Tyagi R, Gupta MN (1997) Biocatal Biotransf 15:101–119
Tadamasa F, Toru M, Akinobu E, Akira I, Ron U (2005) Extremophiles 9:85–89
Akkaya B, Yenidunya AF, Akkaya R (2012) Int J Biol Macromol 50:991–995
Sakharov IY, Castillo JL, Areza JC, Galaev IY (2000) Bioseperation 9:125–132
Musthapa MS, Akhtar S, Khan AA, Husain Q (2004) J Sci Ind Res 63:540–547
Reddy RCK, Srivastava PK, Dey PM, Kayastha AM (2004) Biotechnol Appl Biochem 39:323–327
Demirkan E, Dincbas S, Sevinc N, Ertan F (2011) Romanian Biotechnol Lett 16:6690–6701
Konsoula Z, Liakopoulu-Kyriakides M (2006) Process Biochem 41:343–349
Dey G, Singh B, Banerjee R (2003) Braz Arch Biol Technol 46:167–176
Acknowledgments
The authors are highly thankful to University Grant Commission, New Delhi, India and Saurashtra University, Rajkot, India for the financial and infrastructural support. Mr. Bhavtosh Kikani acknowledges the award of Senior Research Fellowship from the Council of Scientific and Industrial Research, New Delhi, India. Mr. S. Pandey is thankful to UGC, New Delhi for the award of Meritorious Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kikani, B.A., Pandey, S. & Singh, S.P. Immobilization of the α-amylase of Bacillus amyloliquifaciens TSWK1-1 for the improved biocatalytic properties and solvent tolerance. Bioprocess Biosyst Eng 36, 567–577 (2013). https://doi.org/10.1007/s00449-012-0812-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0812-3