Abstract
Purpose
Neutrons detection is of paramount importance in nuclear physics experiments, reactor physics, monitoring and control of nuclear reactors and in radiation protection. Among the neutron detectors used are Proportional Counters (PC to BF3, boron deposition, 3He), Our Detection and Measurements laboratory has embarked on a project to develop, design and build proportional counters with boron deposition mainly for gas filling, several prototypes of which have been produced.
Method
This work presents the Boron-10 Lined Proportional Counters (PC), developed and produced in the Detection and Measurements Department (DDM19). The PC was designed by adopting, for the electrodes, a stainless steel cathode 0.5 mm thick and a gold-plated tungsten anode with diameter of 25 µm. The detectors are filled with an Ar-CH4 mixture (10%) at a pressure of 20 cmHg. The boron deposition is performed by the technique of electrophoresis.
Result
The results obtained using the DDM19 prototype PC (Detector produced in our Detection and Measurement laboratory, 2019), show the advantage of electrophoresis as a better boron deposition technique, and the limitation of the surface mass of 10B to optimal values is around 0.5 at 0.6 mg/cm2.
Conclusions
The characteristics of the gas amplification in the mixture (Ar-CH4 (CH4 10%)), were measured by different methods and compared with the values of the proportional reference counter LND232. The counting plates for the two reference meters LND232 and our DDM19 prototype from 200 to 300 V have slopes ranging from 6.5 to 10%/100 V, and gas amplification factors from 1436 to 1380, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
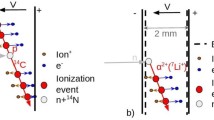
Neutrons are relatively massive particles that make up the nucleus with essentially the same mass as the proton. As they are not electrically charged, they do not react with the electrons of atoms and can only be detected through their interaction with a converter material during which charged particles are produced and detected.
These ionizing secondary particles will degrade their energy in the counter by interaction the electrons located near their orbits, based on the medium crossed, it produces pairs of ions in a gas; then they collected as an electrical signal. The famous reactions used in neutron detectors are: the 10B (n, α) 7Li, which interact in proportional counters of BF3, proportional counters with boron deposition and ionization chambers. In pulse mode neutron detectors, the count rate is proportional to the neutron flux, where the discrimination of gamma radiation takes place in pulse amplitude, generally, they operate from 10 to 106 c/s [1,2,3,4,5,6,7,8,9].
The sensitivity of a neutron detector depends on the longitudinal section with the converter material, on the quantity actually used and on the way in which the reaction is operated in the detector. The detectors operating in pulse mode are generally more sensitive to neutrons than ionization chambers with direct collection of average electric current. In this work, several processes have been developed (boron deposition, degassing and filling, etc.), all the electrical and nuclear tests were carried out in the DDM and sub-critical reactor laboratory AURES laboratory, their characteristics were noted and compared with those of the LND232 reference detector. In the nuclear detectors designing process, many criteria and operating requirements should be taken into account, among them: the type of thermal neutron flux in the range of 0.1 to 106 n/cm2 s of radiation to be measured, the range of flux or power, maximum temperature and relative temperature variations, the response time required for control and safety, the size of the detector (to avoid disturbance of flow in the reactor), resistance to shocks, vibrations and humidity; radiation resistance, the precision required for the measurement and the reproducibility of the measurements, Cost, availability and other economic factors [10, 11].
In this work, the thermal neutron capture reaction by the 10B isotope; was taken into consideration the most used conversion reaction to thermal neutron, as well known, the cross section of this capture is around 4010 barns, the reaction proceeds according as the following [1]:
The rules of energy conservation and momentum cause an energy distribution \( E_{\alpha } = 1.47 \) MeV and ELi = 0.84 MeV in the most probable mode (93.7%) [11]. Note that boron 10 only constitutes barely 18.8% of natural boron [12]; however, it has the advantage of not being toxic or radioactive. It is separated from natural boron by very complicated methods and techniques, among these techniques the fractional distillation of an etherate of boron trifluoride to obtain enrichments of about 95% in 10B. It is used either in solid form in a crystalline state or in amorphous powder with a particle size of the order of a micron.
Materials and methods
Technology adopted
The DDM19/1 prototype counter, produced by our Detection and Measurement Laboratory was designed by using gold-plated tungsten anode, (electrodes, a 0.5 mm thick stainless steel cathode and a 25 µm diameter) [13]. The detectors are filled with an Ar-CH4 mixture (10%) at a pressure of 20 cm Hg, after a long degassing under secondary vacuum at a temperature of 80 °C [11, 14]. The deposit of natural boron with 10B at 18.8% was carried out by the electrophoresis technique. The thickness of the deposit is 0.6 to 0.5 mg/cm2 [10, 11, 14]. Figure 1 shows a sectional view of PC with boron deposition designed, Figs. 2, 3 and 4 show the different stages of the realization of our prototype PC.
Boron deposition by electrophoresis
The choice of electrophoresis was done due its advantages compared to the other methods, the most important advantages of deposition by electrophoresis are the quality of the deposit (uniformity and adhesion), ease of implementation, reproducibility, high deposit rate and low product consumption [4, 5].
Experimental protocol of electrophoresis
Figure 5 shows a general diagram of the experimental device for depositing boron by electrophoresis; this device includes (high voltage–power supply, a stainless steel or aluminum cathode Φ = 2.44 cm and L = 15 cm, a stainless steel or copper anode (rod). The chemicals used in this experiment are natural amorphous boron (from LABOSI), distilled isopropyl alcohol, pure crystallized magnesium chloride (Mgcl2) and pure methanol (CH3OH), nitric acid (HNO3), acetone (C3H6O) were used for the cleaning, degreasing and stripping. The quantity of boron was calculated taking into account the thickness of the deposit (surface density), the surface of the cathode (internal surface of the tube) and the loss of a certain quantity of boron (approximately 20%). Otherwise, the coated boron weight can also be estimated by weighing the coated samples before and after coating (Fig. 6). 0.5 mg/cm2 of boron surface mass was taking because the best sensitivity, good quality of spectrum with boron deposition are obtained with this thickness [7, 9], the mass of boron is calculated as follows:
where mB: mass of boron, S: the internal surface of the tube, ms: mass per unit area.
The boron 10 should be diluted to 0.3 mg/ml; the volume of isopropyl alcohol should be mB/0.3, because MgCl2 acts as the source of electrical charge for the boron particles, for this, 23 ppm MgCl2 is needed in the solution. The methanol (CH3OH) must have 0.1% from the boron mass. In this work, we have followed the next procedure, firstly, we weighing the mass of boron mB and the mass of Mgcl2, then measure the volume of methanol and the volume of isopropyl alcohol, after that mix Mgcl2 with methanol. Add this mixture to the boron and mix in the mortar. Isopropyl alcohol is added in a test tube; it should be able to contain the electrophoresis solution and the electrodes with the possibility of the stirring. Put the contents of the mortar in the test tube and then rinse the mortar with isopropyl alcohol. After that mount the two perforated caps on the electrodes and connect the electric wires, finally, place the electrodes in the test tube. At the end of this experiment, we have obtained the tube with a thin layer of boron deposit (Fig. 6).
Experimental
The electrical and nuclear tests of the PC prototype and reference were carried out under ideal conditions, corresponding to low-intensity neutron flux, room temperature, without vibrations, shocks and electromagnetic disturbances, these conditions are obtained in our sub-critical reactor laboratory (AURES), using a source of 241Am-Be (2Ci) (Fig. 7) [4]. The high-performance electronic pulse measurement chain consists of a 2006E Canberra type charge preamplifier (PA), selected for its variable gain (1 or 5), its low noise and its speed (Rise Time < 20 ns). The search amplifier used (A450 EGG) helped to optimize the formatting constant suitable for this type of experience. The discrimination and plateau curves are deduced using a single-channel analyzer type SCA 550 EGG. The Canberra Dual Counter Timer 2070 type counting scale and a multi-channel analyzer (MCA 35 + Canberra) are used to collect energy spectra. These are systematically transferred to a computer for processing and analysis. Two high voltage precision modules (TENNELEC) were used for the polarization of the counters. The PC-PA connecting cable has a length of around 70 cm. The signal, high voltage and test cables, connecting the PA to the rest of the chain, installed in the AURES control room (Table 1).
The data obtained for the DDM/19 prototype proportional counter show very interesting results compared to the reference LND232. Figures 8 and 9 show the evolution of plateau curves and discrimination plateau of CP with boron deposition prototype DDM19. The counting plateau at S = 200 mv is 300 V and a slope of 6.53%/100 V (400–“700 V) therefore the plateau is located between 400 and 700 V of length 300 V, for the reference detector the slope is 5%/100 V < 10% 100 V. For the discrimination curves, two curves were recorded, one at 700 V for the prototype counter and the other at 750 V for the reference counter, on the first and second, a discrimination level does not appear, the discrimination threshold could be set at 200 mv for the two prototype and the reference LND232 detectors.
Distribution of energy pulses in PCBD
In this work, the average of recoil particles in solid boron 10B (density = 2.45 g/cm3), were 0.88 and 0.47 mg/cm2 for α (Eα = 1.49 MeV) and 7Li (ELi = 0.85 MeV), respectively, noted that, the optimal thickness for better conversion of α and 7Li particles were found in the range of 0.4 0–8 mg/cm2 [5, 7].
From Fig. 10 we noticed that proportional counter PC DDM19 presents a better quality of energy spectrum compared to the standard counter (LND232), Fig. 11 represents the Amplitude displayed by the oscilloscope of PC DDM19.
Gas amplification
The gas amplification is measured first by the technique in current mode I (V), the measurement of the gas amplification by I (V)/Is was carried out by polarizing the detector negatively in 10 V steps on the cathode of the PC and by measuring on the anode, the current was generated using a programmable digital electrometer (Keithley617). The precision measurement of the gas amplification obtained during the digital acquisition of the currents at different voltages was excellent; the reproducibility reached during the realization of the PCDB DDM19, was evaluated by the PCDB LND232. Figure 12 shows the results of measuring the characteristics of the gas amplification of two detectors (DDM19 and LND232 according to the formulations of DIETHORN [6] and SHALEV & HOPSTONE [2] (Figs. 13, 14).
Table 2 gives a comparison of the characteristics of the two proportional counters (PCs), measured for amplification factors ranging from 1 to 1436 (LND232 detector), and from 2 to 1380 for (DDM19 detector). The characteristic \( (\Delta V) \) of the formulation of DIETHORN represents the potential difference allowing an electron to cover an average path [6], it must be close to the necessary energy required to produce a pair of ions. K represents the critical value of the field strength per unit of pressure (E/p). The comparison of the characteristics (\( \Delta V \), K) of DIETHORN [6] and (A6, B6) of SHALEV et al., shows the excellent reproducibility between the two counters LND232 and our prototype DDM19 (Figs. 12, 14) (Table 3).
The gradient of the gas multiplication curve (dM/dV) is inversely proportional to A6 and high values of this term would mean a slow increase in gas multiplication, as a function of the anode voltage [3], compared to PC (90% Ar + 10% CH4) for example (A6 and B6 is 110, 2.8, respectively) [3]. Figure 12 represents the evolution of the gas amplification factor as a function of high voltage.
Discussion
We can conclude from the general results obtained for PCDB-DDM19 (terms of plateau curves, neutron-noise signal, discrimination curves and spectral energy quality), the feasibility of producing this type of detector in our laboratories. The discrimination characteristic allows the appropriate choice of the threshold compared with the reference detector S = 300 mv, in order to obtain a good signal-to-noise ratio without sensitivity losing (Figs. 6 and 7). The energy spectra measured show a low thermal neutron flux of natural boron, despite the low counting statistics compared to the reference detector, for this reason, it is necessary to increase the useful surface of the detector. The rise times of the pulses delivered by the PCDB-DDM19 were estimated at 200 ns, the discrimination curves obtained using the PCDB-DDM19 and for the standard detector are similar (Fig. 7), the energy spectrum presents a good quality compared to the spectra of the PC LND232. The optimal product (pressure-inter-electrode distance) was determined to be approximately 33.86 K Pascal cm. The Ar-CH4 (10%) or P10 mixture, showed some superiority in terms of charge collection speed, compared to the mixtures (Ar-N2 (4%), Ar-CO2 (10%) [7, 10], the optimal conditions determined correspond to a filling pressure of Ar-CH4 (10%) or Ar-N2 (4%), of 26.66 K Pascal (200 torr), a bias voltage of 700 V, collection of charges is then less than 200 ns.
Conclusions
The quality of the results obtained in this work allowed us to confirm that our designed detectors meeting the quality criteria required by the control and monitoring requirements of nuclear reactors, and on the other hand, the possibility of its development. The characterization work on prototype detectors was done under high neutron flux, in the laboratory of the sub-critical assembly, we have a source of 241Am-Be neutron with an activity of 2Ci, it is considered as a mixed neutron-gamma field, this is for the confirmation of the absence of influence of gamma radiation effect on the performance of these prototype counters. All this for the aim to obtain better accuracy in the evaluation of the characteristics. In the future, we will be working on producing other prototypes (deposit of 90% enriched boron) and improving the sensitivity of PCDB prototypes, ensuring reproducibility in the technological realization of this type of detector and the reliability of its response in actual operating conditions, parallel to that, launching of program continuity intended for the development of meters operating under working conditions (temperature, humidity, vibration, etc.) under intense gamma flux and provided with integrated cables with mineral isolation and high noise immunity.
References
G.F. Knoll, Radiation Detection and Measurement (Wiley, New York, 2010)
A. Williams, R.I. Sara, Parameters affecting the resolution of a proportional counter. Int. J. Appl. Radiat. Isot. 13, 229–238 (1962)
S. Shalev, P. Hopstone, Empirical expressions for gas multiplication in 3He proportional counters. Nucl. Instrum. Methods 155, 237–247 (1978)
M. Baaliouamer, C. Belaragueb, D. Mazed, Gas gain measurement in helium-isobutane mixtures filled-proportional counters. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 382, 490–494 (1996)
D. Mazed, Experimental gas amplification study in boron-lined proportional counters for neutron detection. Radiat. Meas. 42, 245–250 (2007)
W. Diethorn, A Methane Proportional Counter System for Natural Radiocarbon Measurements (United States Atomic Energy Commission, Technical Information Service, Washington, 1956)
P.M. Dighe, D.N. Prasad, K.R. Prasad, S.K. Kataria, S.N. Athavale, A.L. Pappachan, A.K. Grover, Boron-lined proportional counters with improved neutron sensitivity. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 496, 154–161 (2003)
P.M. Dighe, D. Das, D.N. Prasad, L.P. Kamble, C.P. Nagaraj, R.K. Kaushik, S. Sarkar, S.S. Taliyan, P.P. Selvam, K. Binoy, Design and development of high temperature 10B coated proportional counters for PFBR. BARC Newsl. 2015, 22–32 (2015)
P.M. Dighe, D. Das, Performance studies of boron lined proportional counters for reactor applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 770, 29–35 (2015)
C. Guo-Yun, W. Zhi-Yong, X. Yong, L. Sheng-Jie, H. Fu-Cheng, H. San-Bo, Z. Li, Z. Jing-Wu, M. Jia-Yi, A boron-lined proportional counter (BLPC) used in a mixed field of reactors. Chin. Phys. C. 36, 434 (2012)
K.S. McKinny, T.R. Anderson, N.H. Johnson, Optimization of coating in boron-10 lined proportional counters. IEEE Trans. Nucl. Sci. 60, 860–863 (2012)
G.F. Knoll, Radiation Detection and Measurement (Wiley, New York, 1979)
M. Fares, A. Messai, S. Begaa, M. Messaoudi, K. Negara, M.Y. Debili, Design and study of the characteristics of a versatile ionization chamber for gamma-ray dosimetry. J. Radioanal. Nucl. Chem. 326, 1405–1411 (2020). https://doi.org/10.1007/s10967-020-07446-5
P.M. Dighe, D.N. Prasad, K.V. Rao, C.K. Pithawa, R.K. Patil, Anode wire mounting technique for high temperature boron-10 lined proportional counter. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 621, 713–715 (2010)
Acknowledgements
This work has been carried out at Es-Salam Research reactor. The authors would like to thank the Director General of CRNB and the staff of the DEDIN Division; also we would like to thank all the engineers and technicians of the Detection and Measurement Department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fares, M., Debili, M.Y., Messaoudi, M. et al. Boron-10 lined proportional counter development for thermal neutron detection. Radiat Detect Technol Methods 5, 110–116 (2021). https://doi.org/10.1007/s41605-020-00226-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41605-020-00226-5