Abstract
Pythium species causes seedling damping-off on numerous plants, resulting in severe economic losses to growers worldwide. The present study is to evaluate the control of cucumber damping-off by biosurfactants produced by Bacillus mycoides. Experiment has demonstrated the ability to produce biosurfactants by B. mycoides, as well as the potential use as a biocontrol agent for controlling cucumber seedling damping-off caused by Pythium aphanidermatum. A B. mycoides strain isolated from rice rhizosphere was capable of producing biosurfactants, one of which was identified as a novel compound closely resemble surfactin A based on HPLC coupling with electrospray ionization-mass spectrometer analysis. The presence of yeast extract suppressed the accumulation of biosurfactants, whereas peptone or glucose increased the accumulation of biosurfactants. The addition of plant oils into medium greatly reduced surface tension, but increased the production of biosurfactants. Co-application of B. mycoides culture with P. aphanidermatum suppressed the release and caused lysis of Pythium zoospores. Application of B. mycoides culture completely suppressed the formation of water-soaked lesions on cucumber leaves and reduced Pythium damping-off by 35% in greenhouse. This study provides valuable information in determining the importance and practicability of utilizing B. mycoides as a biocontrol means in controlling plant diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pythium damping-off commonly occurring on nursery bed, greenhouse flats, and fields is a very destructive soil-borne disease. The genus Pythium belonging to oomycete consists of more than 130 species that can infect a wide range of plants, resulting in severe yield losses worldwide (Dick 1990). Similar to Phytophthora, Peronospraceae, and other oomycetes, Pythium spp. produce asexual zoospores, which account for the occurrence and spread of diseases. Zoospores lacking cell wall are single-cell, motile, and flagellate spores. Zoospores are produced and released from sporangia or vesicles of plant pathogens upon exposure to free water (rain, dew, or irrigation water) (Wright et al. 2003). The infection and mortality rate of plants by zoospore-producing pathogens are much greater in saturated soils than unsaturated soils (Kuan and Erwin 1980; Yanar et al. 1997). Zoospores are often attracted by compounds produced by their host plants, swim toward to, and become encysted around root rhizosphere, which are the determinant step of diseases caused by oomycete pathogens (Raftoyannis and Dick 2006).
As with many plant diseases, application of pesticides is often required to control Pythium damping-off in order to maintain crop yields. However, the excessive and inappropriate use of chemicals has raised concerns about food safety and environment. Developing and exploring the potential use of rhizosphere-associated biological control agents, including Bacillus spp. and Pseudomonas spp., is a promising strategy for controlling soil-borne diseases (Chen et al. 2012; Földes et al. 2000; Shafi et al. 2017; Whipps 1997).
Many Bacillus spp. are capable of producing surface-active compounds, collectively called biosurfactants. The production of lipopeptides including surfactins, iturins, fengycins by B. subtilis and B. amyloliqufaciens has been demonstrated to be required for biocontrol because they show strong toxic activities against a wide variety of plant pathogens (Ongena and Jacques 2008). B. subtillus DFH08 producing fengycin and bacillomycin D has also been reported to exhibit toxicity against Fusarium graminearum (Ramarathnam et al. 2007). Analysis through matrix-assisted laser desorption ionization—time of flight—mass spectroscopy (MALDI-TOF-MS) confirmed the production of fengycin and surfactin by three Bacillus spp., including B. mycoides (Athukorala et al. 2009). Genes involved in the biosynthesis of fengycin were identified by PCR using gene-specific primers. Similar studies conducted by Alvarez et al. (2012) also reported that lipopeptides (fengycin and surfactin) produced by B. amyloliqufaciens were toxic to the plant pathogen Sclerotinia sclerotiorum. Surfactin produced by B. subtilis has been shown to be a powerful surface-active compound with foaming, emulsifying, and dispersing properties and widely used in agriculture, petroleum industries, and oil recovery (Liu et al. 2015; Sachdev and Cameotra 2013).
The culture conditions, nutrient elements, and fermentation processes used for the production of surfactin by B. subtilis have been intensively studied and optimized (Peypoux et al. 1999). Because different Bacillus spp., even different isolates of the same species could produce different types of biosurfactants, the roles of these secondary metabolites produced by a newly isolated strain on biocontrol for plant diseases shall be examined through in vitro and in vivo trials.
Bacillus mycoides Flügge is a gram-positive, saprophytic bacterium that is capable of producing endospores and commonly present in soils and plant rhizosphere environment (Buyer 1995; Lewis 1932). The biggest difference between B. mycoides and other Bacillus spp. is the formation of characteristic rhizoidal (root-like) colonies on nutrient agar plates (Di Franco et al. 2002; Gause 1939). The spreading colonies produced by B. mycoides resembling fungal filamentous growth show a repeating spiral pattern with either clockwise or counter clockwise curving. The direction of the curving pattern is genetically regulated in B. mycoides (Di Franco et al. 2002). B. mycoides has been used as an eco-friendly agent to solubilize brown coal and synthesize biodegradable plastics and chemicals such as 2,4,6-trinitrotoluene (Lin et al. 2013; Narayanan and Ramana 2012; Romanowska et al. 2015). In agriculture, B. mycoides is often used as a biocontrol agent. B. mycoides has been reported to control tobacco brown spot disease caused by Alternaria alternata (Fravel and Spurr 1977). Co-application of a B. mycoides B16 strain with a Pichia guilermondii Y2 strain reduced the development of strawberry gray mold disease caused by Botrytis cinerea (Guetsky et al. 2001). Application of a B. mycoides Bac J strain on sugar beet, presumably due to the induction of systemic resistance, reduced Cercospora leaf spot incidence caused by C. beticola by 38–91% in greenhouse and field experiments (Bargabus et al. 2002).
Biological control using Bacillus spp. has been reported with increasing success on the various vegetable crops and diseases (Shafi et al. 2017). However, little is known about biosurfactant compounds produced by B. mycoides and their roles in biocontrol efficacy. The objectives of this study are to examine whether or not B. mycoides isolated from plant root rhizosphere in Taiwan will produce biosurfactants and to assess their antimicrobial activity against Pythium aphanidermatum and control of cucumber seedling damping-off in greenhouse.
Materials and methods
Bacterial cultures and growth conditions
Bacillus mycoides strains (BM02, CHR003, NP02, and WT15) used in the current study were isolated from plant rhizosphere in central or southern Taiwan (Table 1). Plant roots and soils were collected and boiled in 100 ml water for 5 min. The resultant solutions, after a tenfold dilution, were plated on nutrient agar (NA) (Difco, Sparks, MD, USA), and the plates incubated at 30 °C for single-colony formation. Bacterial strains were identified as B. mycoides based on unique characteristics of filamentous and rhizoidal (root-like) colonies formed on agar medium (Buyer 1995; Di Franco et al. 2002; Nicholson et al. 2000). Both BM02 and WT15 strains produced bundle filaments by linking bacillary cells to form counterclockwise curving, whereas CHR003 and NP02 strains produced filamentous colonies with clockwise curving. The identity of B. mycoides was further confirmed by sequence analysis of the 16S ribosomal internal transcribed spacers (ITS rDNA). Bacterial DNA fragment was amplified with colony PCR as described by Fukui and Sawabe (2007) using a PCR Master Mix Kit (GeneMark, Taichung, Taiwan). Bacterial ITS rDNA was amplified by PCR with the primers ITS-U1F (5′-CTYAAAKRAATTGRCGGRRRSSCS-3′) and ITS-U1R (5′-CGGGCGGTGTGTRCAARRSSC-3′) as described by Rivas et al. (2004). PCR fragments were directly sequenced. Sequences were searched against the databases at the National Center for Biotechnology Information (NCBI) using the BLAST network service to determine the similarity of the amplified fragments. Sequence data reported in this article have been deposited in the EMBL/GenBank Data Libraries under accession number NR_036880.1. Bacterial cells were washed off with sterile water from NA plates, and their concentrations were adjusted to 2 × 108 colony-forming unit (cfu)/ml. For long-term storage, bacteria were placed in a 20% glycerol solution (Sigma-Aldrich, St. Louis, MO, USA) and stored at −80 °C.
To prepare fresh bacterial cultures, 5 ml of B. mycoides suspensions (2 × 108 cfu/ml) was added into a 500-ml flask containing 100-mL YPS basal broth (5 g/l yeast extract, 10 g/l peptone, and 10 g/l sodium chloride, pH 7.0) and incubated at 30 °C for 12 h on a rotary shaker set at 200 rpm. For biosurfactant production, freshly prepared cultures (10%, v/v) were added into YPS basal broth or other media and incubated for additional 5 days. All media used in the current study were modified from YPS by altering the amount of peptone or yeast extract and by adding various amount of glucose. Corn oil, peanut oil, sunflower seed oil, and soybean oil were purchased from local markets (Taichung, Taiwan) and added, each at 5 g/l, into PSG medium containing no yeast extract (Table 2). All media used in the present study were sterilized by autoclaving (121 °C, 15 psi, and 15 min.).
Pythium cultures and growth conditions
Pythium aphanidermatum (Edson) Fitzp. isolates (Kpa 5 and Potap1) were single colony obtained from diseased cucumber seedlings showing damping-off symptoms in central Taiwan (Table 1). Pythium isolates were cultured on V8 juice agar (each liter containing 100 ml V8 juice, 2 g CaCO3, and 20 g agar). For zoospore formation, Pythium isolates were cultured on V8 agar plates under a 12-h light cycle at 30 °C for 3–4 days. Ten agar disks (5 mm in dia.) covered with mycelium were cut, transferred to a petri dish (9 cm in dia.), flooded with sterile water (20 ml), and incubated at room temperature (~25 °C). Zoospores were released from sporangia after incubating for 6 to 8 h, and the concentration was adjusted to approximately 1 × 105 zoospores/ml. Release of zoospores from sporangia was quantitatively assessed on sterile 96-well polystyrene culture plates. P. aphanidermatum isolates were first cultured in 96-well plates for 3 days. Mycelial sheets, after being rinsed 3 times with water, were placed onto a petri dish (3 cm in dia.), covered with 2-ml water, commercial surfactant, or B. mycoides suspension culture, and incubated for 2–6 h at room temperature. Release of zoospores was examined by placing 2 µl suspensions under an optical microscope (40×s magnification). Each treatment contained three replicates, and experiment was repeated three times. Surfactin was purchased from Wako Chemicals (Osaka, Japan).
Surface tension measurement
Surface tension of medium was determined by the Du Noüy ring method (Khoshdast et al. 2012) using a Du Noüy’s surface tensiometer (Ito Seisakusho 514-B, Tokyo, Japan). Before measuring samples, platinum ring was washed three times with de-ion water and ethanol and dried by passing it briefly through a flame. After cooling, the platinum ring was placed back to the tensiometer and the scale was adjusted to zero. Five-day-old cultures of B. mycoides strains grown in YPS or other media were centrifuged (Sigma Lab GmbH, Osterode am Harz, Germany) at 12,000 rpm. The resultant supernatants (2 ml) were placed on the sample station of the tensiometer, and surface tension was measured.
HPLC and mass spectrometer analyses
Biosurfactants produced by B. mycoides strains were purified via the salt-assisted homogeneous liquid–liquid microextraction (SHLLME) method (Gupta et al. 2011). Briefly, 5-day-old cultures of B. mycoides grown in YPS or other media (1 ml) were centrifuged at 12,000 rpm, and each supernatant was mixed with 300 μl of acetonitrile. After adding 0.8 g of ammonium sulfate to each, samples were centrifuged at 3200 rpm for 3 min. The organic extracts (upper layer) were collected and analyzed by a Model PU-980 high-performance liquid chromatography (HPLC) (Jasco, Tokyo, Japan). Biosurfactants and surfactin standard fractions A-F (Sigma-Aldrich) were separated in a Hypersil BDS C18 column (Thermo Scientific, Sweden) at 30 °C using 3.8 mM trifluoroacetic acid and acetonitrile (1:4) as a mobile phase with flow rate set at 1 ml/min. Biosurfactants were detected by a UV detector (Jasco UV-975) wavelength set at 205 nm. Quantification of biosurfactants was made using a regression line generated from the surfactin (fraction A) standard (Sigma-Aldrich). For semi-preparative HPLC analysis, biosurfactants were separated using the above-mentioned procedure except flow rate was set at 5 ml/min and analyzed by the electrospray ionization (ESI)-mass spectrometer. The ESI-MS product-ion spectra were acquired with LTQ linear ion trap tandem mass spectrometer (Thermo Electron, San Jose, CA, USA). The spectra of this library were recorded using normalized collision energies. ESI-MS was performed using standard parameters: spray voltage set at 4 kV, capillary temperature set at 300 °C, sheath gas flow rate set at five arbitrary units in the full-scan mass spectra in the positive ion mode as described (Tang et al. 2010).
Inoculation and disease control
Cucumber (Cucumis sativus cv. Pretty Swallow) seeds were purchased from Known-You Seed Company (Kaohsiung, Taiwan). Seeds were antisepticised by soaking in 0.5% NaClO solution (Clorox, Oakland, CA, USA) for 30 min, rinsed three times with sterile H2O, immersed in bacterial suspensions (5 × 106 cfu/ml) for 12 h, and transferred to a petri dish. Seeds immersed in sterile water were used as a control. After incubation at 30 °C for 2 days, treated seeds were placed in a Sondermischung potting mix containing peat substrates (Gramoflor Gmbh & Co. KG, Vechta, Germany) and maintained in a greenhouse (30 ± 3 °C) located on the National Chung Hsing University campus (Taichung, Taiwan). Two-week-old cucumber seedlings were inoculated by placing 5 ml zoospore suspensions (5 × 104 zoospores/ml) of P. aphanidermatum into seedling rhizosphere. Each treatment contained four replicates, and each replicate had five seedlings. Experiments were conducted twice. The inoculated cucumber plants were maintained in a greenhouse for the development of Pythium damping-off. Disease incidence was recorded 7 days after inoculation.
The effect of B. mycoides culture on P. aphanidermatum was also determined on detached cucumber leaves. The first leaf of cucumber seedlings (2 week old) was harvested and placed on a moist filter paper in a glass petri dish. Zoospores of P. aphanidermatum (5 μl containing 250–300 zoospores) were mixed at equal ratio (1:1, v/v) with B. mycoides strain NP02 grown in corn oil-amended PSG broth and inoculated by dropping onto detached cucumber leaves (4 spots per leaf). The experiment was used 3 leaves per treatment and repeated two times. Zoospores mixed with sterile water were inoculated as a control. The treated leaves were kept in a growth chamber for 12–48 h with 12-h photoperiod at 30 °C for lesion development.
Statistical analysis
All experiments were conducted three times, and data were analyzed using the SAS program (version 6.12). The significance of treatments was determined by analysis of variance (ANOVA) and treatment mean ± SD separated by Tukey’s test (p ≤ 0.05).
Results
Bacillus mycoides reduces cucumber seedling damping-off
Four bacterial strains, designated BM02, NP02, CHR003, and WT15, were isolated from boiled samples (soils and plant roots) collected from rhizosphere of tomato, cowpea, or rice (Table 1). They were identified as B. mycoides based on unique characteristics of filamentous and rhizoid colonies formed on agar medium. Their identity was confirmed further based on ITS rDNA sequence, showing up to 99% identity to those of B. mycoides in the database. Bacterial culture was tested for their ability to reduce cucumber seedling damping-off caused by P. aphanidermatum under greenhouse conditions. Zoospores prepared from two different P. aphanidermatum strains (Kpa 5 and Potap1) after being mixed with or without bacterial suspensions were independently inoculated by draining spore suspensions (5 ml) into each seedling. It was demonstrated that co-inoculation of zoospores with bacterial suspension culture prepared from BM02 and NP02 strains of B. mycoides reduced damping-off incidence by 30–35% (Fig. 1). However, compared to the water controls, co-inoculation of zoospores with suspension culture prepared from CHR003 and WT15 strains did not reduce damping-off incidence.
Severity of cucumber seedling damping-off caused by the isolates (Kpa 5 and Potap1) of Pythium aphanidermatum treated with water (CK) or culture suspension prepared from four different Bacillus mycoides isolates (BM02, NP02, CHR003, and WT15) 7 days postinoculation. The asterisk indicates that the significance of treatments was determined by analysis of variance (ANOVA) and treatment mean ± SD separated by Tukey’s test (p ≤ 0.05)
Production of biosurfactants by B. mycoides
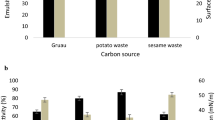
It was observed that both BM02 and NP02 isolates of B. mycoides produced foaming bubbles cultured in nutrient broth and a clear zone on blood-containing agar medium (data not shown), indicative of the production of biosurfactants. Surface tension of suspension culture prepared from the NP02 strain cultured in PSG broth gradually reduced over time (Fig. 2), indicative of the production of surface-active compounds. The addition of plant oil (coil oil, peanut oil, sunflower oil, or soybean oil) reduced surface tension drastically, confirming further the presence of biosurfactants in the suspension culture of B. mycoides.
HPLC analysis of samples prepared from the NP02 strain cultured in PSG broth identified a distinct peak with a retention time of 14.58 min (Fig. 3). HPLC analysis of commercially available surfactins (Sigma-Aldrich) identified five different peaks with the retention time of 12.04 min (fraction A), 16.18 min (fraction B), 17.59 min (fraction C), 21.48 min (fraction D), and 21.99 min (fraction E). HPLC analysis of samples prepared from the NP02 strain cultured in PSG broth amended with or without oils revealed that plant oils, particularly corn oil and soybean oil, increased the accumulation of biosurfactants by the NP02 strain of B. mycoides (Fig. 4). Time-course studies of biosurfactant accumulation by the NP02 strain cultured in PSG broth amended with 5 g/l corn oil revealed that B. mycoides exponentially increased the production of biosurfactants 80 h after incubation (Fig. 5).
The accumulation of biosurfactant by Bacillus mycoides strain NP02 cultured in PSG (peptone/sodium chloride/glucose) amended with oils for 120 h at 30 °C. PSG broth without oil was used as a control (CK). Biosurfactants were detected by HPLC and quantified using a regression line generated from the surfactin (fraction A) standard (Sigma-Aldrich)
The production of biosurfactants by the NP02 strain was investigated further by culturing the NP02 strain in different media and analyzed by HPLC (Table 2). It was demonstrated that B. mycoides cultured in YPS basal medium without glucose accumulated biosurfactants, reaching 33.17 mg/l. The addition of glucose at 10 or 20 g/l increased the production of biosurfactants; however, the addition of glucose at 40 g/l had no effects. The NP02 strain grown in the yeast extract-minus medium (PSG) accumulated high amounts of biosurfactants reaching 71.27 mg/l. The YSG medium containing no peptone slightly decreased the production of biosurfactants (26.36 mg/l).
Suspension culture of Bacillus mycoides suppresses the release of Pythium zoospores
Both P. aphanidermatum strains produced a large amount of zoospores when they were incubated in sterile water (Table 3). Addition of 5 g/l corn oil into water did not impact zoospore production because oil stayed on the top of water (data not shown). Pythium strains failed to release zoospores after being treated with culture suspensions prepared from the NP02 strain grown in PSG medium amended with 5 g/l corn oil (designated PSG-C) 5–6 h after incubation (hai). It was observed that Pythium zoospores treated with NP02 culture suspensions became encysted or directly germinated, but failed to produce zoospores after being treated with PSG (without corn oil) or PSG-C (with corn oil) medium only (Table 3). Biosurfactants (≥50 ppm) effectively suppressed zoospore production (Table 4). The commercially available surfactin (Wako Chemicals) also suppressed zoospore production.
Biosurfactants produced by B. mycoides suppress lesion formation on cucumber leaves
Inoculation of zoospore suspensions of P. aphaniermatum onto detached cucumber leaves resulted in water-soaked lesions 2 days postinoculation (dpi) (Fig. 6a). In contrast, co-inoculation of zoospore suspensions with suspension culture of B. mycoides NP02 grown in PSG medium amended with 5 g/l corn oil (PSG-C) completely suppressed lesion formation. Microscopy analysis revealed that zoospores after being treated with suspension culture of B. mycoides became stationary, producing balloon-like cells which eventually disintegrated (Fig. 6b, far right).
a Development of water-soaked lesions on detached cucumber leaves inoculated with Pythium aphanidermatum zoospores mixed with PSG-C medium (check) or culture suspension of Bacillus mycoides strain NP02 grown in PSG-C for 4 days. b Formation of a balloon-like swelling cell and lysis of a zoospore after treating with biosurfactants extracted from suspension culture of Bacillus mycoides NP02 grown in PSG-C
Identification of biosurfactants by ESI-mass spectrometer
To identify the major peak (retention time at 14.58 min) obtained from HPLC (Fig. 3b), mass spectrometry was utilized. ESI-MS analysis revealed that the peak identified from the NP02 strain of B. mycoides cultured in PSG broth had mass spectra closely resembling those of surfactin A (Fig. 7). Full-scan mass spectra of commercially available surfactin A showed intense precursor ions [M + H]+ at m/z 1008 with several minor signals in the positive ion mode. The sample prepared from B. mycoides culture also had a distinctive intense precursor ions [M + H]+ at m/z 1007.8 with numerous fragmentation routes in the mass spectra. Of them, many showed similar m/z with those of pure surfactin A.
Discussion
Significantly, the current study has demonstrated the ability to produce antimicrobial biosurfactants by B. mycoides, as well as the potential use as a biocontrol agent for controlling plant diseases. The chemical properties and the biological activities of biosurfactants produced by B. mycoides were characterized to be inhibitory to damping-off caused by Pythium spp.
Four bacterial strains, designated BM02, NP02, CHR003, and WT15, were collected from plant rhizospheres after being heated at 100 °C for 5 min. The bacteria formed filamentous and rhizoid colonies on nutrient agar medium and were identified as B. mycoides based on sequence analysis of ITS rDNA. Greenhouse trials have demonstrated that two of the isolates (BM02 and NP02) were capable of reducing cucumber seedling damping-off caused by P. aphanidermarum. BM02 and NP02 grown in nutrient broth produced foaming bubbles, suggestive of the production of surface-active materials (biosurfactants). Surface tension of culture filtrates prepared from the NP02 strain gradually decreased in a span of 120 h. The addition of plant oil into medium greatly reduced surface tension, but increased the accumulation of biosurfactants as assessed by HPLC analysis.
It is hypothesized that biosurfactants produced by B. mycoides play a role in the reduction of Pythium damping-off. Nutritional parameters influencing the accumulation of biosurfactants were also investigated. It was found that the composition of the medium can impact the amount of biosurfactants produced by B. mycoides. The presence of yeast extract appears to suppress the accumulation of biosurfactants, whereas peptone is required for the accumulation of biosurfactants. Glucose was found to promote the production of biosurfactants at the concentrations ranging from 10 to 20 g/l, consistent with the finding by Najafi et al. (2010). Interestingly, B. subtillis grown in a minimal medium produces a higher amount of surfactin than grown in a nutrient medium (Peypoux et al. 1999).
It was found that PSG-C medium (PSG amended with corn oil) alone induced sporangium become enclosed and/or directly germinated on agar medium. However, PSG-C alone failed to suppress water-soaked lesion formation caused by P. aphanidermarum. The results demonstrate further that biosurfactants produced by B. mycoides play a pivotal role in the reduction of damping-off on cucumber seedling. The addition of plant oils has also been reported to increase the production of rhamnolipid by Pseudomonas SWP-4 and surfactin by Bacillus sp. (Lan et al. 2015; Makkar et al. 2011).
The chemical properties and the biological activities of biosurfactants produced by B. mycoides were characterized to be inhibitory to Pythium sp., an oomycete plant pathogen that causes yield losses in numerous economically important plants. It was demonstrated that biosurfactants produced by B. mycoides suppressed the release and lysis of Pythium zoospores and reduced damping-off incidence. Pythium sporangium is unable to release zoospores after being treated with suspension culture of B. mycoides. Similarly, sporangium treated with commercially available surfactin fails to release zoospores. Microscopy observation reveals that zoospores become disintegrated upon exposure to suspension culture of B. mycoides. B. mycoides-derived biosurfactants apparently suppress zoospore release at lower concentration than pure surfactin, indicating the presence of unknown substances other than surfactin in the suspension culture. Mass spectrometric analysis of lipopeptide biosurfactants prepared from 54 different Bacillus strains identified three major categories: kurstakins (850–950 m/z), surfactins and iturins (1000–1100 m/z), and fengycins, polymyxins, and bacitracins (1450–1550 m/z) (Price et al. 2007). In the present study, ESI-mass spectrometer analysis reveals that ionized B. mycoides-derived biosurfactants have masses ranging from 800 to 1200 m/z. The results indicate the presence of different types of biosurfactants, likely including kurstakins, surfactins, and iturins in the suspension culture of B. mycoides. The true natures of these surfactants require further investigation. However, comparison of ESI-mass profiles reveals close similarity between surfactin A and B. mycoides-derived biosurfactants. Oils have been suggested to serve as aggregators that bind surfactin, kurstakins, iturin, and fengycin and form a complex, enhancing, or suppressing the toxicity of biosurfactants depending on the type of biosurfactants and oils used (Boyette et al. 2007; Jee et al. 2009). However, in the present study, the addition of corn oil, peanut oil, sunflower seed oil, or soybean oil during bacterial growth, all increases the accumulation of biosurfactants by B. mycoides.
Bacillus mycoides strains have been known for their ability to promote plant growth and induce defense resistance (Bargabus et al. 2002). However, it remains uncertain that B. mycoides is capable of producing toxic secondary metabolites and their roles in biocontrol. B. mycoides produces barely detectable biosurfactants grown in nutrient medium. In the present study, we have streamlined the production of biosurfactants by adding plant oils into medium used to culture B. mycoides. The addition of corn oil, soybean oil, or peanut oil increases biosurfactant accumulation substantially, and the addition of sunflower oil is less effective. Most importantly, co-inoculation of P. aphanidermarum with suspension culture prepared from B. mycoides grown in an oil-containing medium completely suppresses the development of water-soaked lesions on cucumber leaves. This suppression is very likely due to the toxic effect of biosurfactants that directly cause zoospore lysis. Massetolide, a cyclic lipopeptide biosurfactant produced by Pseudomonas fluorescens SS01, has been reported to suppress oomycete pathogens in a similar manner (De Souza et al. 2003; Raaijmakers et al. 2010). Biological control using synthetic surfactants has been proposed to a wide range of economically important zoosporic plant pathogens in the hydroponic system (Stanghellini and Miller 1997). Application of two lipopeptides, mycosubtilin and surfactin, has been shown to successfully control downy mildew on lettuce (Deravel et al. 2014). Biosurfactants, due to their ability to reduce surface tension, may damage the permeability of the plasma membrane and result in a cell leakage and rapid lysis of zoospores as observed in the present study.
In conclusion, the results derived from the current study provide valuable information in determining the importance and practicability of utilizing B. mycoides as a biocontrol means in controlling plant diseases.
References
Alvarez F, Castro M, Príncipe A, Borioli G, Fischer S, Mori G, Jofré E (2012) The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol 112(1):159–174
Athukorala SNP, Fernando WGD, Rashid KY (2009) Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can J Microbiol 55(9):1021–1032
Bargabus RL, Zidack NK, Sherwood JE, Jacobsen BJ (2002) Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol Mol Plant Pathol 61(5):289–298
Boyette CD, Hoagland RE, Weaver MA (2007) Biocontrol efficacy of Colletotrichum truncatum for hemp sesbania (Sesbania exaltata) is enhanced with unrefined corn oil and surfactant. Weed Biol Manag 7(1):70–76
Buyer JS (1995) A Soil and rhizosphere microorganism isolation and enumeration medium that inhibits Bacillus mycoides. Appl Environ Microbiol 61(5):1839–1842
Chen MH, Jack ALH, McGuire IC, Nelson EB (2012) Seed-colonizing bacterial communities associated with the suppression of Pythium seedling disease in a municipal biosolids compost. Phytopathology 102(5):478–489
De Souza JT, De Boer M, De Waard P, Van Beek TA, Raaijmakers JM (2003) Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl Environ Microbiol 69(12):7161–7172
Deravel J, Lemière S, Coutte F, Krier F, Hese N, Béchet M et al (2014) Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl Microbiol Biotechnol 98(14):6255–6264
Di Franco C, Beccari E, Santini T, Pisaneschi G, Tecce G (2002) Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiol 2(1):33
Dick MW (1990) Keys to Pythium. University of Reading Press, Reading
Földes T, Bánhegyi I, Herpai Z, Varga L, Szigeti J (2000) Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage micro-organisms. J Appl Microbiol 89(5):840–846
Fravel DR, Spurr HWJ (1977) Biocontrol of tobacco brown-spot disease by Bacillus cereus subsp. mycoides in a controlled environment. Phytopathology 67(7):930–932
Fukui Y, Sawabe T (2007) Improved one-step colony PCR detection of Vibrio harveyi. Microbes Environ 22(1):1–10
Gause G (1939) Some physiological properties of dextral and of sinistral forms in Bacillus mycoides flügge. Biol Bull 76(3):448–465
Guetsky R, Shtienberg D, Elad Y, Dinoor A (2001) Combining biocontrol agents to reduce the variability of biological control. Phytopathology 91(7):621–627
Gupta M, Pillai AKKV, Singh A, Jain A, Verma KK (2011) Salt-assisted liquid–liquid microextraction for the determination of iodine in table salt by high-performance liquid chromatography-diode array detection. Food Chem 124(4):1741–1746
Jee H-J, Shim C-K, Ryu K-Y, Park J-H, Lee B-M, Choi D-H et al (2009) Control of powdery and downy mildews of cucumber by using cooking oils and yolk mixture. Plant Pathol J 25(3):280–285
Khoshdast H, Abbasi H, Sam A, Noghabi KA (2012) Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem Eng J 60:127–134
Kuan T, Erwin D (1980) Predisposition effect of water saturation of soil on Phytophthora root rot of alfalfa. Phytopathology 70(10):981–986
Lan G, Fan Q, Liu Y, Chen C, Li G, Liu Y et al (2015) Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem Eng J 101:44–54
Lewis IM (1932) Dissociation and life cycle of Bacillus mycoides. J Bacteriol 24(5):381–421
Lin H-Y, Yu C-P, Chen Z-L (2013) Aerobic and anaerobic biodegradation of TNT by newly isolated Bacillus mycoides. Ecol Eng 52:270–277
Liu Q, Lin J, Wang W, Huang H, Li S (2015) Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem Eng J 93:31–37
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1(5):19
Najafi AR, Rahimpour MR, Jahanmiri AH, Roostaazad R, Arabian D, Ghobadi Z (2010) Enhancing biosurfactant production from an indigenous strain of Bacillus mycoides by optimizing the growth conditions using a response surface methodology. Chem Eng J 163(3):188–194
Narayanan A, Ramana KV (2012) Polyhydroxybutyrate production in Bacillus mycoides DFC1 using response surface optimization for physico-chemical process parameters. 3 Biotech 2(4):287–296
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64(3):548–572
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Peypoux F, Bonmatin JM, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51(5):553–563
Price NPJ, Rooney AP, Swezey JL, Perry E, Cohan FM (2007) Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. FEMS Microbiol Lett 271(1):83–89
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34(6):1037–1062
Raftoyannis Y, Dick MW (2006) Effect of oomycete and plant variation on zoospore cover and disease severity. J Plant Pathol 88(1):95–101
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuewen G, de Kievit T (2007) Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53(7):901–911
Rivas R, Velázquez E, Zurdo-Piñeiro JL, Mateos PF, Martínez Molina E (2004) Identification of microorganisms by PCR amplification and sequencing of a universal amplified ribosomal region present in both prokaryotes and eukaryotes. J Microbiol Methods 56(3):413–426
Romanowska I, Strzelecki B, Bielecki S (2015) Biosolubilization of Polish brown coal by Gordonia alkanivorans S7 and Bacillus mycoides NS1020. Fuel Process Technol 131:430–436
Sachdev DP, Cameotra SS (2013) Biosurfactants in agriculture. Appl Microbiol Biotechnol 97(3):1005–1016
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31(3):446–459
Stanghellini ME, Miller RM (1997) Biosurfactants: their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis 81(1):4–12
Tang J-S, Zhao F, Gao H, Dai Y, Yao Z-H, Hong K et al (2010) Characterization and online detection of surfactin isomers based on HPLC-MSN analyses and their inhibitory effects on the overproduction of nitric oxide and the release of TNF-α and IL-6 in LPS-induced macrophages. Mar Drugs 8(10):2605–2618
Whipps JM (1997) Developments in the biological control of soil-borne plant pathogens. In: Callow JA (ed) Advances in botanical research, vol 26. Academic Press, Cambridge, pp 1–134
Wright B, Rowse HR, Whipps JM (2003) Application of beneficial microorganisms to seeds during drum priming. Biocontrol Sci Tech 13(6):599–614
Yanar Y, Lipps PE, Deep IW (1997) Effect of soil saturation duration and soil water content on root rot of maize caused by Pythium arrhenomanes. Plant Dis 81(5):475–480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, YH., Chou, YJ., Liu, YC. et al. Inhibition of cucumber Pythium damping-off pathogen with zoosporicidal biosurfactants produced by Bacillus mycoides . J Plant Dis Prot 124, 481–491 (2017). https://doi.org/10.1007/s41348-017-0110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0110-z