Abstract
Bacillus strains are known to produce cyclic lipopeptides that are capable of providing protection against plant pathogens. Such abilities could be utilized to protect greenhouse tomatoes against diseases including bacterial canker caused by Clavibacter michiganensis subsp. michiganensis (Cmm). In the present study, Bacillus velezensis strain 1B-23 and Bacillus sp. strain 1D-12 were assessed for their potential biocontrol abilities against Cmm strain 98–1 (Cmm98–1). Both Bacillus strains interfered with growth of Cmm98–1 in vitro, as determined by agar plate assays to screen for microbial antagonism. Inoculation of Cmm98–1 infected tomato plants with B. velezenis 1B-23 or Bacillus sp. 1D-12 lead to significantly reduced disease incidence in a greenhouse setting. Liquid Chromatography coupled to Mass Spectrometry (LC-MS) of 1B-23 and 1D-12 extracts identified [Leu7]surfactin C13 (often called surfactin A), [Leu7]surfactin C14 (often called surfactin B) and [Leu7]surfactin C15 (often called surfactin C) in fractions of extracts that inhibited growth of Cmm98–1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clavibacter michiganensis subsp. michiganensis (Cmm) causes bacterial canker of tomato. This aerobic Gram-positive bacterium infects host plants vertically via seed or laterally through wounds or natural openings. It proliferates in xylem vessels, allowing progression to the rest of the plant. Gradual degradation of vascular tissue leads to wilting, and sometimes to cankers due to epidermal necrosis. Fruit quality and yield are often reduced (Nandi et al. 2018; Sen et al. 2015; Eichenlaub and Gartemann 2011).
Controlling Cmm is accomplished mainly through seed testing and hygienic practices such as sterilization of equipment and tools (Nandi et al. 2018). While various chemical pesticides can contribute to disease management (Werner et al. 2002), their cost, potential to encourage resistance, and the possibility of residues left on food (Baysal and Tör 2014) has prompted calls for alternative means of controlling disease in agriculture. Newer developments in crop disease management include using beneficial microorganisms as biocontrol agents (Baysal and Tör 2014; Chen et al. 2017).
A few microbes have already been investigated for their potential to control Cmm. These include various pseudomonads (Amkraz et al. 2010) which can mediate their effects via direct antagonism (Deng et al. 2017), possibly involving the metabolites 2,4-diacetylphloroglucinol (DAPG) and hydrogen cyanide (Lanteigne et al. 2012), or via salicyclic acid (SA)-dependent induced systemic resistance (Takishita et al. 2018), which hypersensitizes the plant to pathogenic threats. Various strains of Streptomyces can directly antagonize Cmmin vitro (Zhang et al. 2010), while the fungus Pseudozyma aphidis mediates a SA-independent induced resistance in plants (Barda et al. 2015)
Additionally, Cmm has been shown to be antagonised by strains of Bacillus subtilis both in vitro and in vivo. B. subtilis strain DJM-51, its culture supernatant, and its butanol-extracted compounds are capable of producing zones of inhibition on nutrient-broth yeast extract agar plates containing Cmm (Jung et al. 2014). Under greenhouse conditions, Bacillus subtilis strains GBO3 (Girish and Umesha 2007), Quadra 136, and Quadra 137 (Utkhede and Koch 2004) significantly reduce disease incidence of bacterial canker caused by Cmm.
Bacillus strains are known to produce biologically active compounds including cyclic lipopeptides (Dunlap et al. 2011; Li et al. 2012; Zhao et al. 2017), which can be further classified into fengycins, surfactins, and iturins (Ongena and Jacques 2007; Mora et al. 2015). Surfactins are a group of compounds typically containing the heptapeptide ELLVDLL linked via lactone bond to a beta-hydroxy fatty acid. The various surfactins can differ in the identity of amino acids at the second, fourth, and seventh positions in the peptide, in the number of carbon atoms in the fatty acid chain, or in structural conformation. These are broad-spectrum antibiotics that function to disrupt bacterial membranes, and their non-specific interactions may help to prevent the development of resistance (Zhao et al. 2017).
In this work, B. velezensis 1B-23 and Bacillus sp. D-12 were isolated from a remediated potato rhizosphere in Norfolk County, Ontario, Canada, and assessed for their biocontrol abilities against the common tomato plant pathogens, Cmm and Pseudomonas syringae DC3000, using antibiotic plate assays and pathogen challenge in a greenhouse setting. Liquid Chromatography coupled to Mass Spectrometry (LC-MS) was then used to identify antimicrobial compounds.
Materials and methods
Isolation and identification of strains
Soil samples were collected from Blizman potato fields in Norfolk County, Ontario, Canada in the summer of 2012. Over the previous 3 years, bio-organic fertilizer was added to the soil each spring in an effort toward natural remediation. In 2012 (the fourth year), 10.0 g of moist soil was collected, placed in 95 mL of sterile water, and shaken for 10 min. Then, 1.0 mL of this suspension was transferred for serial dilution up to 10−10, and the dilutions were plated on tryptic soy agar (TSA) for 48 h at 28 °C to attain single colonies (Grady et al. 2019).
Isolates 1B-23 and 1D-12 were identified as Bacillus species via 16 s rRNA gene sequencing. The complete genome of strain 1B-23 was subsequently sequenced (unpublished data) and deposited to the National Center for Biotechnology Information (NCBI) as Accession CP033967. To identify strain 1B-23 at the species level, the genome sequence data was uploaded to the Type (Strain) Genome Server (TYGS), available at https://tygs.dsmz.de, for a whole genome-based taxonomic analysis (Meier-Kolthoff and Göker 2019). Briefly, closely related type strains were identified by 16S rRNA gene sequence comparison with each of the 10,087 type strains available in the TYGS database. The 50 best matches were used to calculate precise genome distances using the Genome BLAST Distance Phylogeny (GBDP) approach under the algorithm ‘coverage’ and distance formula d5. Strain 1B-23 was thus identified as Bacillus velezensis.
In vitro assessment of antimicrobial activity
In order to determine the effectiveness of B. velezensis 1B-23 and Bacillus sp. 1D-12 against common tomato plant pathogens, their abilities to inhibit pathogens were initially determined in vitro. The pathogenic strains Cmm98–1 and PsDC3000 (Cuppels 1986) were obtained from Dr. Diane Cuppels, whose laboratory isolated them from diseased tomatoes. B. velezensis 1B-23, Bacillus sp. 1D-12, Cmm98–1, and PsDC3000 were suspended separately in 0.85% NaCl to an optical density (600 nm) of 1.0, as determined using a SmartSpec Plus Spectrophotometer (Bio-Rad Laboratories Inc., Hercules, California, U.S.A.). Fifty microliters of the B. velezensis 1B-23 or Bacillus sp. 1D-12 preparation were used to inoculate 5 mm discs of P8 Filter Paper (Thermo Fisher Scientific, Pittsburgh, PA, USA). These antimicrobial discs were placed on solid LB medium plated with 100 μL of either the Cmm98–1 or PsDC3000 preparation. Plates were sealed with Parafilm M (Bemis Company Inc., Oshkosh, WI, USA) and incubated for 72 h at 28 °C, after which zones of clearance around antimicrobial discs were measured.
Hydroponic tank inoculation
To begin examining the effects of B. velezensis 1B-23 and Bacillus sp. 1D-12 on pathogen infection of tomato plants, plants were grown in hydroponic medium as previously described (Nathoo et al. 2017) with some modifications. Briefly, an autoclaved, 90 mm × 90 mm stainless steel mesh square (mesh count of 30 × 30, wire diameter of 0.012) with its corners bent at 90 degrees was placed into each of 18 Petri plates containing 20 mL of liquid Murashige and Skoog (MS) medium (Murashige and Skoog 1962), such that MS medium is touching the bulk of the steel mesh square. Sterilized Terero Beefsteak Tomato seeds were placed on the mesh squares with one seed in each corner and one in the center. The Petri dishes were sealed with porous surgical tape, wrapped in aluminum foil and placed in a 4 °C refrigerator for 24 h, after which the foil was removed and plates were incubated at 24 °C with a 16 h photo period using F54 T5 fluorescent bulbs (Koninklijke Philips N.V., Amsterdam, Netherlands) with a light intensity 190umol. After 96 h, sterilized forceps were used to lift the steel mesh square with the seeds out of the MS medium and into a sterilized hydroponic cylindrical tank made with a 100 mm × 80 mm glass crystallizing dish and lid (Nathoo et al. 2017) containing 20 mL of MS liquid medium. Tanks were sealed with porous surgical tape and incubated for a further 72 h with gentle shaking at 50 rpm, allowing plants to adapt to the environment before inoculation.
For inoculation, B. velezensis 1B-23, Bacillus sp. 1D-12, or Cmm98–1 were grown on solid LB medium for 24 h and resuspended in 0.85% NaCl to an optical density (600 nm) of 1.0. One hundred and fifty microliters of B. velezensis 1B-23 or Bacillus sp. 1D-12, and/or Cmm98–1 were added to the 20 ml of MS medium in the hydroponic tanks. After seven additional days, the entire plant (including roots) was separated from the bacterial suspension of the hydroponic system by lifting the mesh plate.
Greenhouse trials
Torero Beefsteak Tomatoes were grown from seed (Paramount Seeds Inc., Stuart, FL, USA; Lot 102,683,913/0161510486 94% O2/17 s/c 170,525/lb. untreated seeds) under greenhouse conditions (18 h light period with 170 μmol/m2 s1, 26 °C day,18 °C night, 65% relative humidity). Germination occurred in 15 cm × 15 cm × 5 cm plastic potting trays filled with 2/3 ProMix BX Mycorrhizae (Premier Tech Ltd., Rivière-du-Loup, Québec, Canada) and 1/3 Fine Vermiculite (Therm-O-Rock East Inc., New Eagle, PA, USA), and covered with a plastic dome until sprouting. Tomato plants were then transferred to 4-in. plastic pots.
Plants were inoculated with bacteria using either protocol 1 or protocol 2. For protocol 1, 54 tomato plants were divided into 9 groups with 6 plants per group: one group contained plants that were not inoculated with any bacteria, while the remaining groups were exposed to B. velezensis 1B-23, Bacillus sp. 1D-12, Cmm98–1, PsDC3000, or the combinations B. velezensis 1B-23 + Cmm98–1, B. velezensis 1B-23 + PsDC3000, Bacillus sp. 1D-12 + Cmm98–1, and Bacillus sp. 1D-12 + PsDC3000. Liquid cultures of each bacterial strain were grown in 4 mL of LB broth and placed on a TC-7 Tissue Culture Roller (New Brunswick Scientific Co. Inc., Enfield, CT, USA) at 28 °C incubator for 24 h. The 4 mL liquid cultures were then transferred into 250 mL of LB broth for 72 h. Liquid cultures were then centrifuged at 6000 rpm for 10 min, and the pellet was resuspended in 0.85% NaCl to an optical density (600 nm) of 1.0. Thirty-five day old tomato plants were inoculated with B. velezensis 1B-23 or Bacillus sp. 1D-12 as a 5 mL drench to the roots. After 5 days to allow for plant-microbe interaction, a 5 mL root drench of Cmm98–1 or PsDC3000 was added to the appropriate plants. Two weeks after pathogen inoculation, disease incidence was determined for each plant as the percent of leaves with visible lesions.
Protocol 2 was similar to protocol 1 except that the 5 mL Bacillus root drench was applied at an OD600 of 0.7 to 32-day old tomato plants (n = 7 per group), pathogens were applied 3 days later, and observations were made 30 days after pathogen application.
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
Chemical separation of metabolites
To determine and isolate the active compounds in B. velezensis 1B-23 and Bacillus sp. 1D-12, the strains were separately cultured in LB broth for 72 h at 28 °C and washed twice with an equal volume of 100% ethyl acetate in a separatory funnel. The upper, organic phase was collected and dried using 99% anhydrous sodium sulfate. The liquid phase of the crude metabolite solution was boiled off in a round-bottom flask using an IKA® RV10™ rotary evaporator running at 960 mBar and 160 rpm. Using the same round bottom flask, the dried metabolite was resuspended using 5 mL of 100% ethyl acetate. Next, the resulting suspension was transferred to a 20 mL scintillation vial and dried under nitrogen gas to form a precipitate. The precipitate was resuspended in 300 ml 100% acetonitrile to create a crude biologically-active metabolite solution to test for antibacterial activity.
The crude solution was used to infuse antimicrobial discs to verify activity against Cmm98–1, while acetonitrile was used for control discs. The crude solution was also subject to reverse-phase High Performance Liquid Chromatography on a 1260 Infinity Series (Agilent Technologies, Santa Clara, CA, USA) with a C18 silica gel stationary phase column eluted using an acetonitrile-water gradient with 0.1% formic acid. Separated fractions were dried, weighed, and re-dissolved in methanol to a concentration of 100 mg/mL, then tested again for activity against Cmm98–1 using methanol as a negative control. The fractions displaying antibacterial activity were selected for further chemical analysis.
Identification of antibacterial compounds
Fractions from HPLC showing antibacterial activity were subjected to Ultraviolet-visible spectroscopy at 210 nm in order to identify active compounds. The separated peaks from UV-vis spectroscopy were then tested again against Cmm98–1 to confirm antibacterial activity. Isolated peaks displaying antibacterial activity were then subject to analysis using the 1260 Infinity Series (Agilent Technologies, Santa Clara, CA, USA) LC system coupled with a Q-Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer (MS) run with ESI positive mode (Thermo Fisher Scientific, Waltham, MA, USA). The LC mobile phase consisted of acetonitrile water with 0.1% formic acid and was passed through a C18 silica gel stationary phase column over a 6-min gradient period. Upon completion of LC-MS analysis using XCalibur software (Thermo Fisher Scientific, Waltham, MA, USA), specific compounds responsible for antibacterial activity were identified by comparing formulae against the AntiBase 2012 Natural Compound Identifier database (Laatsch 2012).
Following identification, surfactin B was isolated and used at various concentrations to inoculate 0.5 mm discs of P8 Filter Paper (Thermo Fisher Scientific, Pittsburgh, PA, USA), which were then placed on LB agar plated with 100 μL of a 0.1 OD suspension of either the Cmm98–1 or PsDC3000. Plates were sealed with Parafilm M (Bemis Company Inc., Oshkosh, WI, USA) and incubated for 72 h at 28 °C.
Results
Antagonism toward Cmmin vitro
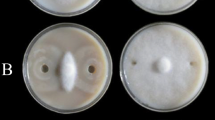
To determine the effectiveness of B. velezensis 1B-23 and Bacillus sp. 1D-12 against phytopathogens, filter discs inoculated with these strains were placed onto LB agar plates cultured with either Cmm98–1 or PsDC3000 (Fig. 1a). Clear zones of inhibition were observed against Cmm98–1, with a larger zone for B. velezensis 1B-23 compared to Bacillus sp. 1D-12. The experiment was replicated using B. velezensis 1B-23 (Fig. 1b), showing zones of inhibition against Cmm. Neither B. velezensis 1B-23 nor Bacillus sp. 1D-12 inhibited PsDC3000.
Bacillus velezensis1B-23 andBacillussp. 1D-12in vitroassessment against phytopathogens. Pathogens were suspended in 0.85% NaCl and 100 μL was spread onto each LB plate. a Filter discs containing 50 μL of B. velezensis 1B-23 (left side of each plate) and Bacillus sp. 1D-12 (right side of each plate) were placed onto the pathogenic plates and incubated for 72 h. b Filter discs containing 50 μL of B. velezensis 1B-23 were placed onto the pathogenic plates and incubated for 72 h
Protection against Cmm98–1 in a hydroponic tank
The effects of B. velezensis 1B-23 and Bacillus sp. 1D-12 on Cmm98–1 inoculated tomato plants were assessed qualitatively following co-cultivation in hydroponic tanks. Plants that were co-cultivated with Cmm98–1 and either B. velezensis 1B-23 or Bacillus sp. 1D-12, appeared healthier than plants co-cultivated with Cmm98–1 alone, similar to controls (Fig. 2).
Reduction of Cmm98–1 disease incidence in greenhouse tomatoes
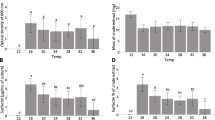
Disease symptoms were assessed for tomato plants grown under greenhouse conditions using two protocols for inoculation with B. velezensis 1B-23 or Bacillus sp. 1D-12 and the phytopathogens Cmm98–1 and PsDC3000. Using either protocol 1 or protocol 2, plants treated with both Cmm98–1 and B. velezensis 1B-23, or both Cmm98–1 and Bacillus sp. 1D-12, showed a significant decrease in disease incidence (P < 0.01 or P < 0.001) compared to Cmm98–1 alone (Fig. 3). The difference in disease incidence between plants treated with both Bacillus and PsDC3000 versus PsDC3000 alone was not significant (P > 0.05; Fig. 3).
Disease Incidence of tomato plants after pathogen inoculation. Mean disease incidence ± S.D. using Protocol 1, n = 6 (left panel). Mean disease incidence ± S.D. using Protocol 2, n = 7 (right panel). Data analysis involved one-way ANOVA followed by Tukey’s multiple comparisons test (**, P < 0.01; ***, P < 0.001)
Production of antimicrobial compounds [Leu7]surfactins C13, C14 and C15
Crude extracts from B. velezensis 1B-23 and Bacillus sp. 1D-12 were analyzed using LC-MS, revealing the presence of [Leu7]surfactin C13 (often called surfactin A), [Leu7]surfactin C14 (often called surfactin B) and [Leu7]surfactin C15 (often called surfactin C) in the antibacterial fractions (Fig. 4). Surfactin B and surfactin C were present in greater quantities than surfactin A for both strains.
Liquid chromatography coupled to mass spectrometry analysis of crudeB. velezensis1B-23 andBacillussp. 1D-12 surfactin abundance. Crude solutions of B. velezensis 1B-23 and Bacillus sp. 1D-12 were subject to liquid chromatography and mass spectrometry, indicating the presence of surfactin A, B, and C
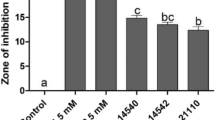
Filter discs inoculated with surfactin B produced zones of inhibition on Cmm98–1 plates at all tested concentrations, from 1 mg/mL. Zones of inhibition were not observed for PsDC3000 at concentrations up to 10 mg/mL of surfactin B (Fig. 5).
Surfactin B inhibitsCmm98–1in vitro. Cmm98–1 or PsDC3000 was suspended in 0.85% NaCl to an OD600 = 1.0 and plated on LB plates. Surfactin B was used to inoculate filter discs at the indicated concentrations (clockwise from top left: 10 mg/mL, 5 mg/mL, 2.5 mg/mL, and 1 mg/mL), which were placed on the plates and incubated for 48 h at 28 °C
Discussion
This study characterized B. velezensis 1B-23 and Bacillus sp. 1D-12 as potential biocontrol agents for tomato diseases. The in vitro assessment revealed clear zones of inhibition around Cmm98–1 in response to B. velezensis 1B-23 and Bacillus sp. 1D-12, indicating that these Bacillus strains secrete antibacterial compounds with activity against Cmm. This finding is consistent with a similar experiment using CmmATCC 7429 and B. subtilis DJM-51 (Jung et al. 2014). For Cmm98–1 inhibition by B. velezensis 1B-23, more prominent zones of clearing were observed on some plates compared to others. Because the replicate plates were run on different dates, this varying degree of inhibition could be due to some difference in the growth stage of either inoculant (1B-23 or Cmm98–1). In support of this explanation, a recent study found that the age of cultures of B. subtilis strain A18 and of Heterobasidion spp. (fungi) affected the ability of the former to inhibit the latter in vitro (Azeem et al. 2019).
To identify the potential antibacterial compounds, LC-MS analysis was performed on fractions of B. velezensis 1B-23 and Bacillus sp. 1D-12 extracts that inhibited growth of Cmm, revealing the presence of [Leu7]surfactin C13 (often called surfactin A), [Leu7]surfactin C14 (often called surfactin B) and [Leu7]surfactin C15 (often called surfactin C). A previous study also implicates the presence of [Leu7]surfactins C14 and C15 in inhibition of Cmm by Bacillus velezensis 9D-6 (Grady et al. 2019), suggesting that one or both of these surfactins may be relevant in a variety of Bacillus strains.
Despite the non-specific, broad-spectrum antibacterial properties of surfactin, neither B. velezensis 1B-23 (this study) nor B. velezensis 9D-6 (Grady et al. 2019) is able to appreciably inhibit in vitro growth of PsDC3000. This may be due to the presence of resistance mechanisms in Pseudomonas that occur because this genus commonly produces its own biosurfactants (Raaijmakers et al. 2010), with at least one strain shown to produce surfactin itself (Xia et al. 2014). Still, some studies suggest that surfactin produced by B. subtilis can inhibit Pseudomonas syringae at relatively high concentrations (25 μg on an antibacterial disc or 25 μg/mL in liquid) (Bais et al. 2004).
In addition to their antibiotic properties, various surfactins play a role in biofilm formation, which may in turn contribute to enhanced biocontrol in vivo. Surfactin-deficient Bacillus display reduced biofilm and reduced root colonization, which coincides with poorer biocontrol against phytopathogens (Bais et al. 2004; Zeriouh et al. 2014; Aleti et al. 2016).
The effects of B. velezensis 1B-23 and Bacillus sp. 1D-12 on Cmm-infected, greenhouse-grown tomatoes indicate that these strains can reduce disease symptoms in vivo and in a commercially relevant setting. Exposing tomato plants to B. velezensis 1B-23 or Bacillus sp. 1D-12 three to five days prior to Cmm98–1 exposure significantly reduced disease symptoms versus exposure to Cmm98–1 alone. Similarly, Cmm disease incidence in tomato is reduced by three bacilli, B. subtilis GBO3, Bacillus amyloliquefaciens IN937a, and Brevibacillus brevis IPC11 (Girish and Umesha 2007). While that study attributed the reduction to induced host resistance, it did not seek to characterize any antimicrobial compounds (Girish and Umesha 2007). In contrast, exposure to B. velezensis 1B-23 or Bacillus sp. 1D-12 did not reduce tomato disease symptoms due to PsDC3000. It is worth noting that the potting mix used in our study contains mycorrhizal fungus, which may enhance the level of disease control of Cmm by bacilli through an unexplored synergistic mechanism.
Our results add to a limited knowledge base on biocontrol of the tomato pathogen Cmm. They suggest B. velezensis 1B-23, Bacillus sp. 1D-12, or other surfactin-producing microbes as potential biocontrol agents against this important crop pathogen.
References
Aleti G, Lehner S, Bacher M, Compant S, Nikolic B, Plesko M, Schuhmacher R, Sessitsch A, Brader G (2016) Surfactin variants mediate species-specific biofilm formation and root colonization in Bacillus. Environ Microbiol 18:2634–2645
Amkraz N, Boudyach EH, Boubaker H, Bouizgarne B, Ait Ben Aoumar A (2010) Screening for fluorescent pseudomonades, isolated from the rhizosphere of tomato, for antagonistic activity toward Clavibacter michiganensis subsp. Michiganensis. World J Microb Biot 26:1059–1065
Azeem M, Barba-Aliaga M, BorgKarlson AK, Terenius O, Broberg A, Rajarao GK (2019) Heterobasidion-growth inhibiting Bacillus subtilis A18 exhibits medium- and age-dependent production of lipopeptides. Microbiol Res 223-225:129–136
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Barda O, Shalev O, Alster S, Buxdorf K, Gafni A, Levy M (2015) Pseudozyma aphidis induces salicylic-acid-independent resistance to Clavibacter michiganensis in tomato plants. Plant Dis 99:621–626
Baysal Ö, Tör M (2014) Smart biologics for crop protection in agricultural systems. Turk J Agric For 38:723–731
Chen J, Wu Q, Hua Y, Chen J, Zhang H, Wang H (2017) Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl Microbiol Biotechnol 101:8309–8319
Cuppels DA (1986) Generation and characterization of Tn5 insertion mutations in Pseudomonas syringae pv. tomato. Appl Environ Microbiol 51:323–327
Deng Q, Wang W, Sun L, Wang Y, Liao J, Xu D, Liu Y, Ye R, Gooneratne R (2017) A sensitive method for simultaneous quantitative determination of surfactin and iturin by LC-MS/MS. Anal Bioanal Chem 409:179–191
Dunlap CA, Schisler DA, Price NP, Vaughn SF (2011) Cyclic lipopeptide profile of three Bacillus subtilis strains: antagonists of Fusarium head blight. J Microbiol 49:603–609
Eichenlaub R, Gartemann K (2011) The Clavibacter michiganensis subspecies: molecular investigation of gram-positive bacterial plant pathogens. Annu Rev Phytopathol 49:445–464
Girish N, Umesha S (2007) Effect of plant growth promoting rhizobacteria on bacterial canker of tomato. Arch Phytopath Plant Protec 5408:235–243
Grady EN, MacDonald J, Ho MT, Weselowski B, McDowell T, Solomon O, Renaud J, Yuan ZC (2019) Characterization and complete genome analysis of the surfactin-producing, plant-protecting bacterium Bacillus velezensis 9D-6. BMC Microbiol 19:5
Jung WJ, Mabood F, Souleimanov A, Whyte LG, Niederberger TD, Smith DL (2014) Microbial pathogenesis antibacterial activity of antagonistic bacterium Bacillus subtilis DJM-51 against phytopathogenic Clavibacter michiganense subsp. michiganense ATCC 7429 in vitro. Microb Pathog 77:13–16
Laatsch H (2012) AntiBase 2012 upgrade: the natural compound identifier. Wiley-VCH, Weinheim
Lanteigne C, Gadkar VJ, Wallon T, Novinscak A, Filion M (2012) Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology 102:967–973
Li X, Mao Z, Wang Y, Wu Y, He Y (2012) ESI LC-MS and MS/MS characterization of antifungal cyclic lipopeptides produced by Bacillus subtilis XF-1. J Mol Microbiol Biotechnol 22:83–93
Meier-Kolthoff JP, Göker M (2019) TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10:2182
Mora I, Cabrefiga J, Montesinos E (2015) Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS One 10:e0127738
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nandi M, MacDonald J, Liu P, Weselowski B, Yuan ZC (2018) Clavibacter michiganensis subsp. michiganensis: bacterial canker of tomato, molecular interactions and disease management. Mol Plant Pathol 19:2036–2050
Nathoo N, Bernards MA, MacDonald J, Yuan ZC (2017) A hydroponic co-cultivation system for simultaneous and systematic analysis of plant/microbe molecular interactions and signaling. J Vis Exp 125:e55955
Ongena M, Jacques (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062
Sen Y, van der Wolf J, Visser RGF, van Heusden S (2015) Bacterial canker of tomato: current knowledge of detection, management, resistance, and interactions. Plant Dis 99:4–13
Takishita Y, Charron JB, Smith DL (2018) Biocontrol rhizobacterium Pseudomonas sp. 23S induces systemic resistance in tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front Microbiol 9:2119
Utkhede R, Koch C (2004) Biological treatments to control bacterial canker of greenhouse tomatoes. BioControl 49:305–313
Werner NA, Fulbright DW, Podolsky R, Bell J, Hausbeck MK (2002) Limiting populations and spread of Clavibacter michiganensis subsp. michiganensis on seedling tomatoes in the greenhouse. Plant Dis 86:535–542
Xia W, Du Z, Cui Q, Dong H, Wang F, He P, Tang Y (2014) Biosurfactant produced by novel Pseudomonas sp. WJ6 with biodegradation of n-alkanes and polycyclic aromatic hydrocarbons. J Hazard Mater 276:489–498
Zeriouh H, de Vicente A, Pérez-García A, Romero D (2014) Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol 16:2196–2211
Zhang W, Yang W, Meng Q, Li Y, Liu D (2010) Screening and identification of antagonistic Streptomyces spp. against Clavibacter michiganensis subsp. michiganensis from tomato rhizosphere. Front Agric China 4:159–164
Zhao H, Shao D, Jiang C, Shi J, Li Q, Huang Q, Rajoka MSR, Yang H, Jin M (2017) Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol 101:5951–5960
Funding
This study was funded by Agriculture and Agri-Food Canada (Growing Forward-II project J-001332 and project J-001589) awarded to Z-C Yuan; Natural Sciences and Engineering Research Council of Canada (NSERC) (Discovery grant RGPIN-2015-06052) awarded to Z-C Yuan; Ontario Greenhouse Vegetable Growers (OGVG) and Mitacs-Accelerate (Fund IT10293-Yuan_OGVG) awarded to Z-C Yuan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laird, M., Piccoli, D., Weselowski, B. et al. Surfactin-producing Bacillus velezensis 1B-23 and Bacillus sp. 1D-12 protect tomato against bacterial canker caused by Clavibacter michiganensis subsp. michiganensis. J Plant Pathol 102, 451–458 (2020). https://doi.org/10.1007/s42161-019-00461-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00461-w