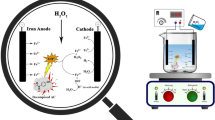
Abstract
The removal efficiency of the emerging antibiotic waste, amoxicillin, from aqueous media was investigated using the electro-Fenton method with an aluminum anode through the one-factor-at-a-time method, and all the experiments were performed in a useful volume of 750 ml. While the optimum conditions were achieved, the removal kinetic of the contaminant from the environment was investigated. Optimal values of the process parameters including the concentration of the contaminant, process time, initial pH of the samples, electrolyte concentration, electrode distance, and the applied current density were determined as 100 mg.l−1, 90 min, neutral pH, 0.02 M Na2SO4, 5.5 cm, and 5.5 mA.cm−2, respectively. In order to find the optimum conditions, in addition to pollutant removal efficiency, energy consumption was also analyzed. The application of aluminum anodes was found to enhance the efficiency of the process in neutral pH (roughly 95% removal rate), which may be introduced as a potential solution for limitations of the conventional electro-Fenton process in degrading recalcitrant compounds such as amoxicillin. Finally, the obtained results were presented and discussed in detail.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical compounds, including amoxicillin, are considered to be the emerging contaminants. Most of the time, they are produced in discontinuous processes, which is culminating in the entrance of these chemicals into the wastewater [1, 2]. It is estimated that almost half of the world’s pharmaceutical wastewater discharges into the environment without any special treatment owing to their chemical and biological stability [3]. Besides, these materials can cause chronic toxicity to various environments and living organisms, such as human’s body. Among the pharmaceutical contaminants, amoxicillin is of great importance on the grounds that 80 to 90% of it could be excreted unchanged [4, 5]. Hence, it is crucial to find ways to eliminate these substances from the wastewater before entering the environment.
So far, no efficient technology has been developed to remove this chemical compound from the wastewater. Hence, efforts are currently focused on the development of the potentially effective removal methods to degrade the contaminant. Among several proposed techniques, advanced oxidation methods (AOPs) have shown promising outcomes in removing the amoxicillin contaminants. Particularly, electro-Fenton which is known as one of the most effective methods in removing emerging substances from aquatic environments has been attended by the researchers through last decade [6].
As an alternative method to the conventional Fenton process, the electro-Fenton presents a combination of advantages such as decreased overall costs, high removal efficiency, and relatively lower toxicity [7, 8]. However, the method is encountered by challenges such as high power consumption. Recently, it has been found that using different anode and cathode materials may enhance the efficiency of the system. In this regard, the high removal efficiency in a broader range of pH has been achieved [9, 10]. Consequently, various types of organic pollutants (including dyes, pharmaceuticals, aniline contaminants, leachate, and herbicides) have been successfully removed from the aqueous environments using the mentioned method [11].
Radwan et al. investigated the effect of different anode electrodes on the degradation efficiency of phenol. They used stainless steel (SS) and nickel as anodes and achieved the removal efficiency of 95% in 90 min and 72% in 120 min for each metal, respectively [12]. The removal of contaminants using the combined process of electro-Fenton and electro-coagulation with iron electrodes and DSA has been reported by Ding et al. They concluded that the leachate degradation process is more effective in combined process, compared to each of the electro-Fenton and electro-coagulation processes [13]. In another study, Dindas et al. analyzed the mineralization of pharmaceutical wastewater using both of the electro-Fenton and electro-coagulation with Fe electrodes and achieved less than 30% total organic carbon (TOC) removal [14]. Hamdi et al. investigated the performance of an electro-Fenton-like process with a Cu-Ni-Al alloy as the anode in removal of the organic pollutants. The efficiency of the process and TOC removal were measured as 87 and 79%, respectively [15].
Whereas most of the reported research works confirm the critical role of Fe in the electro-Fenton process, aluminum ion seems to be helpful support to achieve higher efficiency, as it provides wider applicable pH range. In other words, aluminum not only can serve as a highly electrical conductive anode through the electro-Fenton process but also enriches the system with Al3+ ions to form flocs at different pHs, where the conventional electro-Fenton process has limited efficiency. Such a strategy can potentially result in enhanced process efficiency. The graphite cathode also increases the removal efficiency during the reaction.
The electro-Fenton process which includes iron ions and hydrogen peroxide initially leads to the formation of strong hydroxyl radicals (OH°) through Eq. 1. The graphite cathode promotes the hydrogen peroxide evolution in the system through Eq. 2 and consequently enhances the formation of hydroxyl radicals. The graphite cathode also contributes the system efficiency via converting the ferric (Fe3+) to ferrous ions (Fe2+) through Eq. 3 [16, 17]:
Besides acceleration the electro-Fenton process through the mentioned reactions, the aluminum anode may potentially facilitate the formation of aluminum-based flocs, according to the following reactions:
Therefore, such a modified degradation process can be briefly illustrated based on the oxidation process by the general Fenton reaction (Eq. 1), which is followed by the flocculation process via aluminum ions (Eq. 4). The flocculation process, itself, is supported by the synthesis of hydroxide ions at the cathode (Eq. 5). Besides, the potential sludge generated by the aluminum anode contains nutrients that can be utilized in soil fertilization; the level of heavy metals in the aluminum anode is also significantly lower than other type of sludge, and they barely consider as a threaten for the environment. Although large amount of sludge disposed into landfill could be a concern, aluminum sludge can be recovered and used as pollutant removal agent (heavy metals, synthetic dyes, phosphorous, etc.) and used again in the water and wastewater treatment as coagulants as well as co-conditioner substances [18].
This study deals with using aluminum anode to investigate the abovementioned potential in the modification of the electro-Fenton process. Aluminum anode also may decrease the acid consumption through the process, as it can activate the electro-Fenton process in a wider range of pH. To the best of our understanding, this is the first time that such a strategy is followed to remove amoxicillin from wastewater.
Experimental
Setup Design
The electro-Fenton process was carried out via a test pilot, including a beaker container (110 mm in diameter, 200 mm in height, and applicable volume of 750 ml), test electrodes, and the power supply. The setup of the test pilot is schematically illustrated in Fig. 1.
Graphite and commercially pure aluminum electrodes (the effective surface of 90 cm2) were used as cathode and anode, respectively. Before each experiment, the surface of the electrodes was polished via SiC sandpapers up to 1500 grade and immersed in a dilute solution of nitric acid for 1 h. They, finally, were washed with distilled water to remove any possible trace of acid on their surfaces [19]. A direct current (DC) power supply equipped with voltage and amperage regulators was connected to the anode and cathode electrodes, as shown in Fig. 1. The whole solution sample was continuously stirred via a magnetic stirrer (150 rpm).
Materials and Methods
The experimental procedure of this study was designed based on one-factor-at-a-time (OFAT) method, according to APHA standard instructions [20]. Initially, the pharmaceutical wastewater was prepared using amoxicillin pollutant. Sodium sulfate powder was added to the solution as the electrolyte. Ferrous sulfate powder was also used as the source of iron cations (the main catalytic compound), provided solution with a constant concentration of Fe2+ (0.1 mM). Thereafter, vertically arranged electrodes were connected to the power supply and adjusted to a variable distance. The other process parameters such as applied current density and pH of the solution were considered according to data presented in Table 1. The changes in concentration of the solution were monitored through a method in which samples (5 ml) were extracted from the solution every 30 min and then centrifuged (5000 rpm) for 20 min in order to flocs separation. The concentration of the samples was recorded using a spectrophotometer (maximum absorption wavelength of 273 nm for amoxicillin) and then used for removal rate calculations [21, 22].
Energy consumption during the electrochemical process (E, kW.h.kg−1) was calculated according to Eq. 6 [17, 23]:
in which U, I, t, and V show the applied voltage (in V), electrical current (in A), retention time (in hours), and volume of the wastewater (m3), respectively. The initial concentration of the contaminant, C, is expressed in kg.m−3, and the instantaneous fraction of removed contaminant is indicated by X.
The studied pollutant, amoxicillin (C16H19N3O5S), was purchased from Farabi Co., Iran. The material was consisted of a penam ring that includes two methyl and one amide groups. In order to facilitate the current flow in amoxicillin solution, Na2SO4 (Merck, Germany) was used, and to supply the required iron cations, FeSO4.7H2O (Merck) was used. Nitric acid 1N (Merck) was used to wash the electrodes after sanding.
The main equipment used in this research included a Hach spectrophotometer model DR 4000, a Mettler digital scale model PJ300 with the accuracy of 0.001 g, a Megatek power supply model PM-3005D, an IKA magnetic stirrer model RH-Bassic2, a Metrohm pH meter model 691, and a centrifuge machine manufactured by Sigma Model 101. All the experimental steps were carried out at the normal room temperature. The quantified results were achieved through the averaging of at least three acceptable recordings (error < 5%). In order to evaluate the efficiency of the aluminum electrode, after every 50 tests, a test was conducted in the same condition as the first test, and the difference in removal percentage was less than 5%, which showed the stability of aluminum anodes.
Results and Discussion
Optimal Concentration of the Amoxicillin
The results of the contaminant removal at different initial concentrations and times are given in Fig. 2a. As can be seen, the maximum removal of amoxicillin occurred in 90 min. As time passes, the degradation rate is approximately constant. At this specific time, the removal efficiencies for concentrations of 10, 30, 50, 75, 100, and 120 mg.l−1 were 100, 99.6, 97.1, 93.2, 91.8, and 69.5%, respectively. Because the other parameters, including the amount of iron ion and hydrogen peroxide added, the electric current intensity, and the distance between the electrodes, are constant, the amount of produced hydroxyl radicals is constant. Consequently, with the increase in the initial concentration of wastewater, the probability of hydroxyl radicals colliding with pollutant particles is diminished, leading to a decrease in process efficiency [24]. In addition, in the aluminum anode, the efficiency decreases by increasing the pollutant concentration and keeping other effective parameters constant. By not changing the initial parameters, according to Faraday law, the amount of metal cations remains constant, and this causes insufficient metal hydroxides formed in the system, which seems to be inadequate to precipitate pollutants [25]. In electrochemical systems, energy consumption is another important parameter that must be considered. In order to find the optimum energy consumed to remove the pollutant, the amount of energy consumed in all concentrations until 80% of pollutant removal was calculated through Eq. 6 (Fig. 2b). The lowest energy consumption for removal of amoxicillin happened in the initial concentration of 100 mg.l−1. This happens due to the fact that two main parameters highly affected the energy consumption, i.e., amoxicillin concentration and time, and the gap between the concentration values outweighs the gap between times, and the concentration of 100 mg.l−1 possessed the lowest amount of energy consumption, which was around 153 kWh.kg−1, in which case the removal percentage was 91.8%, selecting as the optimal initial concentration.
Optimal pH
In this section, the amoxicillin removal efficiency at different pHs was investigated, the result of which is shown in Fig. 3a. As can be observed, the removal efficiency of amoxicillin at pHs of 3, 5, neutral (7.6), 9, and 11 with an optimal time of 90 min is 94.2, 93.3, 91.5, 79, and 50.5, respectively. pH affects the type of iron ion producing in the solution (ferric or ferrous). Therefore, it plays an important function in the catalytic role of iron and the decomposition of hydrogen peroxide. As the pH increases, the rate of hydrogen peroxide conversion to water and oxygen increases, and iron hydroxide sediments are formed, culminating in the decrease in removal efficiency [12, 26].
Besides, as the pH value rises to above 8, the hydroxyl radicals created by the hydrogen peroxide produced at the cathode are converted to oxygen radicals, which have less oxidizing power than the hydroxyl radicals. Also, the production of hydroxyl radicals in the solution decreases, which is due to the emergence of ferric hydroxides in these pH values. At low pH values, the oxidation capacity of hydroxyl radicals increases, which promote the oxidation process efficiency. Another critical point is that at very low pH values (range less than 3), the removal efficiency in the oxidation process decreases. It happens on the grounds that in this low pH range, hydrogen ions scavenge the hydroxyl radicals and due to the production of unnecessary iron complex species, culminating in the decline in the removal rate and removal efficiency of this process decreases [27].
Apart from the mentioned reasons, amoxicillin possesses three different pKs of 2.68, 7.49, and 9.63, which belong to the carboxyl, amine, and phenol hydroxide group, respectively [28]. At neutral pH, the carboxyl group is converted to the COO group, while the amine and hydroxide groups remain unchanged. Consequently, at this pH, amoxicillin can be decomposed with the ability to neutralize the charge [29]. It is noteworthy mentioning that aluminum ions have the lowest solubility at neutral pH values, which makes the function of the aluminum anode suitable for forming flocs in the neutral range. This happens because, in this case, the predominant mechanism of coagulation is sweep coagulation, which has high efficiency in trapping pollutants and increasing the removal efficiency [30]. Therefore, it is likely that the equal efficiency in the neutral and acidic conditions (Eqs. 7 and 8) is the predominance of flocculation in the aluminum electrode and tertiary treatment process. As the pH rises and enters the alkaline range, the degradation efficiency decreases because in this range of pH, the Al3+ ions are converted to soluble types of Al(OH)4− (Eq. 9), which does not help to increase the process efficiency [31].
The energy consumption at different pHs is shown in Fig. 3b. As can be seen, the highly acidic condition has the lowest energy consumption. Since the difference in pollutant removal efficiency between the acidic and neutral conditions of wastewater is negligible, and in order to prevent pH adjustment as well as adding chemicals, the research was continued at neutral pH.
The change in initial pH over time is shown in Fig. 4. As can be seen, the final wastewater pH, after 90 min, is 8.73, which is an acceptable value for being discharged into the environment. The main reason for this increase seems to be the generation of OH− through Eq. 1. Besides, the production of hydrogen ions at the cathode can cause the oversaturation of CO2 in the solution and the release of this compound from the wastewater, which leads to the pH increment [32]. As the pH increases according to Eq. 10, the Al(OH)4− species is formed in the solution, and the presence of this ion gives the solution a buffering property which leads to the stabilization and even reduction of the alkalinity of the solution [25, 26, 33, and].
Optimal [H2O2]/[Fe]
Another parameter affecting the process efficiency is the molar ratio of hydrogen peroxide to iron ions, the results of which are presented in Fig. 5. If the optimal ratio of these two parameters is not considered, the removal efficiency will be significantly reduced. An excessive amount of these two chemical substances, also, makes the process less economic and also allows the presence of excessive iron ions in the effluent. Thus, it may cause the iron concentration of treated wastewater to exceed the standard allowable level. As shown in Fig. 5a, the removal efficiencies in the ratios of hydrogen peroxide to iron 5, 10, 20, and 50 are 85, 91.5, 80, and 60%, respectively. The reason for the decrease in removal efficiency with the increase in the ratio of hydrogen peroxide to iron is the production of oxygen scavenging species originating from the excessive amount of hydrogen peroxide. Also, the high amounts of hydrogen peroxide react with hydroxyl radicals and produce less powerful radicals according to Eq. 11. In addition, if the ratio of hydrogen peroxide to iron sets too low, other scavenging reactions occur between the hydrogen peroxide and the ferrous ion, which also reduces the removal efficiency (Eq. 12). Other than, at high concentrations of hydrogen peroxide, the process of the spontaneous decomposition of hydrogen peroxide and its conversion to water and oxygen takes place according to Eq. 13 [34]:
In order to find the optimal condition of [H2O2]/[Fe], the electrical energy consumption of the four conditions with a ratio of 5, 10, 20, and 50 in 80% pollutants removal was compared with each other. As can be seen in Fig. 5b, the ratio of hydrogen peroxide to iron 10 has the lowest energy consumption, and as a result, this condition was used as the optimal value in other experiments.
Optimal Electrolyte Concentration
Another important parameter that affects the process is the concentration of electrolyte. The results of removal efficiency in different electrolyte concentration are shown in Fig. 6. According to Fig. 6, the removal efficiency of amoxicillin at electrolyte concentrations of 0.01, 0.02, and 0.03 M is equal to 84.4, 91.8, and 80.9%, respectively. The presence of the electrolyte facilitates the movement of electrons between the cathode and the anode. The increase in the electrolyte concentration reduces the ohmic resistance of the wastewater, which increases the electrical conductivity and also reduces the retention time of the treatment process. Another advantage of increasing the electrical conductivity is lowering the voltage required to generate a constant current intensity, which reduces the required electrical energy consumption and aluminum producing by anode [35]. However, the excessive increase in electrolyte concentration leads to the formation of scavenging species such as chlorine and sulfate. Therefore, the concentration of ferrous ions decreases, weakens the performance of the oxidation process, and reduces the removal efficiency [36]. In another study, the formation of complexes with aluminum and passivation of this electrode surface due to high sulfate ion concentration has been reported. The study reveals that the presence of this layer leads to an increase in the potential difference and thus reduces the efficiency of pollutant degradation [37].
According to the results, the maximum removal efficiency reached at 0.02 M, and the minimum energy consumption was also obtained at the mentioned concentration. As the electrolyte concentration increases, the ions encounter less resistant from solution and float more freely in solution. This may be the reason which explicates the small gap between the energy consumption in these two states, despite the significant difference in removal efficiencies.
Optimal Distance Between Electrodes
The distance between electrodes is another important parameter analyzed in this study, and the result of which is given in Fig. 7. According to Fig. 7, the removal efficiencies of amoxicillin at 3.5, 5.5, and 7.5 cm distances were 89.2, 92, and 85.1%, respectively. Reducing the distance between the electrodes leads to a falloff in the electrical resistance of the wastewater. Therefore, at a constant current intensity, a less electrical voltage is required, which reduces the energy consumption [38]. Other than, as the distance decreases, the electrostatic attraction between the electrodes increases, and the produced metal ions collide with each other and precipitate, reducing their effectiveness in pollutant separation.
It should be mentioned that excessive increase in the electrodes distance will also reduce the removal efficiency of the process. This may be due to increase in the ions migration time between the two electrodes, leading to the decrease in the electrostatic attraction. Hence, the number of coagulant ions reduces and makes the process less effective [39, 40]. Besides, the energy consumption at 5.5 cm was slightly less than 3.5 cm. As mentioned, by reducing the distance between the electrodes to some extent, the passage of ions in the solution is facilitated. Hence, in order to create a constant current intensity, less electrical voltage is required to be applied. Therefore, the reason for the similarity of the results between these two modes can be the decrease in voltage due to the reduction of distance. However, because of the better efficiency of process in 5.5 cm condition, it was selected as the optimal value.
Optimal Current Density
The changes in the concentration of amoxicillin over time in different current densities are shown in Fig. 8a. Experiments were performed at five conditions with the values of 1, 2.25, 4, 5.5, and 7.25 mA.cm−2, and the removal efficiencies at these levels were obtained 66, 68.5, 77, 86.1, and 86%, respectively. As the results show, the increase in the current density and potential difference lead to the promotion of removal efficiency. It also leads to an augment in the production of ferrous ions and ultimately to an increase in hydroxyl radicals which is produced, raising the efficiency of the oxidation process [41]. It should be mentioned that too much raising in the current density can lead to hydrogen peroxide overproduction at the carbon cathode. This uncontrolled increase can also be the reason for radicals’ combination, which cut down the oxidation power of the process. By doing so, the scavenging phenomenon occurs and reduces the concentration of effective radicals in the solution [29]. Besides, current density not only plays an important role in the amount, shapes, and dimensions of the coagulants produced in the anode but also helps to produce more hydrogen bubbles in smaller sizes at the cathode, accelerating the floatation process. Increasing the current density also could cause an increase in metal hydroxide species, reducing the repulsive forces between the suspended particles to such an extent that the van der Waals gravitational forces dominate the repulsion process, and the particles stick to each other [34]. However, excessive current intensity can give rise to a change in the electrical charge of the flocs and change them to the same charge particles, which eventually causes them to be redistributed [4]. Furthermore, this increase leads to an increase in solution temperature and also, according to Faraday correlation, increases the corrosion rate of aluminum and causes additional costs (more energy and aluminum consumption) [20]. Finally, due to a small difference in removal efficiency (5%) in current densities of 5.5 and 7.25 mA.cm−2, the amount of energy consumption was investigated (Fig. 8b). As can be seen, the difference between electrical energy consumption is about 30 kWh.kg−1, and 5.5 mA.cm−2 is considered optimum condition.
Reaction Kinetics
The kinetic models that were analyzed in this study were zero-order, pseudo-first-order, and pseudo-second-order models. Table 2 shows the values of the reaction in the optimum conditions at different kinetic orders. As can be observed, the reaction follows the pseudo-first-order kinetic due to having a higher R2 ratio in this model. The kinetic correlation is shown in Eq. 14.
In this reaction, c and c0 show the concentration of amoxicillin at times 0 and t, respectively, and k is the pseudo-first-order kinetic constant-coefficient [42].
In comparison between present study kinetic and other researches in the field of amoxicillin removal using the electro-Fenton method, Kaur et al. investigated the removal of amoxicillin using Ti/RuO2 anode. According to this study, pseudo-first order was the kinetic model that had the best overlap with the removal rate data (k = 0.0063 (min−1)); the energy consumption was also 404 kWh.kg−1 to remove 2 g of TOC [24]. Kalantary et al., in another study, investigated the kinetic of amoxicillin removal from water using the electro-Fenton process in the presence of Fe3O4 nanoparticles. The results show that the removal process follows the first-order kinetic with a coefficient of 0.0579 (min−1) [43]. Comparing the kinetic coefficient of the present study and the studies mentioned above, we find out that the rate of amoxicillin removal with aluminum anode was faster than Ti/RuO2 anode and anode with Fe3O4 nanoparticles. Energy consumption for the present study also was about 150 kWh.kg−1, which is less than the energy consumed in the above two studies.
Investigating all the parameters affecting the proposed electro-Fenton process showed that this method was able to degrade amoxicillin pollutant efficiently without any needs for tertiary treatment (roughly 95% degradation rate). The results indicated that this system not only provides the process with a high and fast degradation rate in a wider range of pH, which makes it more practical, but also performs as a more economical method to eliminate amoxicillin from aqueous media. The method can be applied easily since there is no need to add extra acid or base compounds in order to adjust the pH, and the anode is also a commercially available aluminum plate.
Conclusion
In this study, the performance of the electro-Fenton system for wastewater treatment containing the emerging substance amoxicillin was investigated. The electrodes used in this series were carbon and aluminum for the cathode and anode, respectively; various process parameters were optimized using OFAT system. At first, different concentrations of the pharmaceutical compound were evaluated, and after calculating the removal efficiency and electrical energy consumption, 100 mg.l−1 was selected as the optimal concentration. Subsequently, the experiment at neutral pH was the best condition with the lowest energy consumption, and finally, the output pH was within the safe range for being released into the aqueous matrices. The optimal [H2O2]/[Fe] was 10, and the concentration of sodium sulfate electrolyte solution showed a maximum removal efficiency of 0.02 M for amoxicillin removal. The optimal distance between the electrodes was 5.5 cm, and the current density of 5.5 mA.cm−2 showed the optimum condition. Finally, the removal kinetic was investigated, and the laboratory data were in good agreement with the pseudo-first-order kinetics.
References
Weng X, Cai W, Lin S, Chen Z (2017) Degradation mechanism of amoxicillin using clay supported nanoscale zero-valent iron. Appl Clay Sci 147:137–142. https://doi.org/10.1016/j.clay.2017.07.023
Singh V, Pandey B, Suthar S (2018) Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 201:492–502. https://doi.org/10.1016/j.chemosphere.2018.03.010
Kıdak R, Doğan Ş (2018) Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason Sonochem 40:131–139. https://doi.org/10.1016/j.ultsonch.2017.01.033
Ensano BMB, Borea L, Naddeo V, Belgiorno V, de Luna MDG, Balakrishnan M, Ballesteros FC Jr (2019) Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J Hazard Mater 361:367–373. https://doi.org/10.1016/j.jhazmat.2018.07.093
Ayodele OB, Lim JK, Hameed BH (2012) Pillared montmorillonite supported ferric oxalate as heterogeneous photo-Fenton catalyst for degradation of amoxicillin. Appl Catal A-Gen 413:301–309. https://doi.org/10.1016/j.apcata.2011.11.023
Jafari K, Heidari M, Rahmanian O (2018) Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason Sonochem 45:248–256. https://doi.org/10.1016/j.ultsonch.2018.03.018
Ahmadzadeh S, Asadipour A, Pournamdari M, Behnam B, Rahimi HR, Dolatabadi M (2017) Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: optimization and modelling through response surface methodology. Process Saf Environ 109:538–547. https://doi.org/10.1016/j.psep.2017.04.026
Wang S (2008) A comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigments 76(3):714–720. https://doi.org/10.1016/j.dyepig.2007.01.012
Ren G, Zhou M, Su P, Liang L, Yang W, Mousset E (2018) Highly energy-efficient removal of acrylonitrile by peroxi-coagulation with modified graphite felt cathode: Influence factors, possible mechanism. Chem Eng J 343:467–476. https://doi.org/10.1016/j.cej.2018.02.115
Garcia-Segura S, Eiband MMS, de Melo JV, Martínez-Huitle CA (2017) Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem 801:267–299. https://doi.org/10.1016/j.jelechem.2017.07.047
Nidheesh PV (2018) Removal of organic pollutants by peroxi coagulation. Environ Chem Lett 16(4):1283–1292. https://doi.org/10.1007/s10311-018-0752-5
Radwan M, Alalm MG, Eletriby H (2018) Optimization and modeling of electro-Fenton process for treatment of phenolic wastewater using nickel and sacrificial stainless steel anodes. J Water Process Eng 22:155–162. https://doi.org/10.1016/j.jwpe.2018.02.003
Ding J, Jiang M, Zhao G, Wei L, Wang S, Zhao Q (2020) Treatment of leachate concentrate by electrocoagulation coupled with electro-Fenton-like process: efficacy and mechanisms. Sep Purif Technol 255:17668. https://doi.org/10.1016/j.seppur.2020.117668
Dindas GB, Çalışkan Y, Çelebi EE, Tekbaş M, Bektaş N, Yatmaz HC (2020) Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-Fenton and photocatalytic oxidation processes. J Environ Chem Eng 8(3):103777. https://doi.org/10.1016/j.jece.2020.103777
Hamdi N, Proietto F, Ben Amor H, Galia A, Inguanta R, Ammar S, Gadri A, Scialdone O (2020) Effective removal and mineralization of 8-hydroxyquinoline-5-sulfonic acid through a pressurized electro-Fenton-like process with Ni− Cu− Al layered double hydroxide. ChemElectroChem 7(11):2457–2465. https://doi.org/10.1002/celc.202000463
Guvenc SY, Dincer K, Varank G (2019) Performance of electrocoagulation and electro-Fenton processes for treatment of nanofiltration concentrate of biologically stabilized landfill leachate. J Water Process Eng 31:100863. https://doi.org/10.1016/j.jwpe.2019.100863
Ghalebizade M, Ayati B (2019) Acid orange 7 treatment and fate by electro-peroxone process using novel electrode arrangement. Chemosphere 235:1007–1014. https://doi.org/10.1016/j.chemosphere.2019.06.211
Dassanayake KB, Jayasinghe GY, Surapaneni A, Hetherington C (2015) A review on alum sludge reuse with special reference to agricultural applications and future challenges. Waste Manag 38:321–335. https://doi.org/10.1016/j.wasman.2014.11.025
Sopaj F, Oturan N, Pinson J, Podvorica FI, Oturan MA (2019) Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem Eng J 384:123249. https://doi.org/10.1016/j.cej.2019.123249
APHA, A.W.W.A. 2020. WEF (2020). Standard methods for the examination of water and wastewater, 22.
Kong FX, Lin XF, Sun GD, Chen JF, Guo CM, Xie YF (2019) Enhanced organic removal for shale gas fracturing flowback water by electrocoagulation and simultaneous electro-peroxone process. Chemosphere 218:252–258. https://doi.org/10.1016/j.chemosphere.2018.11.055
Limousy L, Ghouma I, Ouederni A, Jeguirim M (2017) Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Pollut Res 24(11):9993–10004. https://doi.org/10.1007/s11356-016-7404-8
Suhan MBK, Shuchi SB, Anis A, Haque Z, Islam MS (2020) Comparative degradation study of remazol black B dye using electro-coagulation and electro-Fenton process: kinetics and cost analysis. Environ Nanotechnol Monit Manag 14:100335. https://doi.org/10.1016/j.enmm.2020.100335
Kaur R, Kushwaha JP, Singh N (2019) Amoxicillin electro-catalytic oxidation using Ti/RuO2 anode: mechanism, oxidation products and degradation pathway. Electrochim Acta 296:856–866. https://doi.org/10.1016/j.electacta.2018.11.114
Naje AS, Chelliapan S, Zakaria Z, Ajeel MA, Alaba PA (2017) A review of electrocoagulation technology for the treatment of textile wastewater. Rev Chem Eng 33(3):263–292. https://doi.org/10.1515/revce-2016-0019
Núñez J, Yeber M, Cisternas N, Thibaut R, Medina P, Carrasco C (2019) Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry. J Hazard Mater 371:705–711. https://doi.org/10.1016/j.jhazmat.2019.03.030
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572. https://doi.org/10.1016/j.jece.2013.10.011
Li H, Hu J, Wang C, Wang X (2017) Removal of amoxicillin in aqueous solution by a novel chicken feather carbon: kinetic and equilibrium studies. Water Air Soil Pollut 228(6):201. https://doi.org/10.1007/s11270-017-3385-6
Wang B, Xu X, Tang H, Mao Y, Chen H, Ji F (2020) Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon. Appl Surf Sci 528:147048. https://doi.org/10.1016/j.apsusc.2020.147048
Ghernaout D, Alghamdi A, Ghernaout B (2019) Electrocoagulation process: a mechanistic review at the dawn of its modeling. J Environ Sci Allied Res 2:51–67
Bensadok KS, Benammar S, Lapicque F, Nezzal G (2008) Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. J Hazard Mater 152(1):423–430. https://doi.org/10.1016/j.jhazmat.2007.06.121
Izadi A, Hosseini M, Darzi GN, Bidhendi GN, Shariati FP (2018) Treatment of paper-recycling wastewater by electrocoagulation using aluminum and iron electrodes. J Environ Health Sci Eng 16(2):257–264. https://doi.org/10.1007/s40201-018-0314-6
Nidheesh PV, Singh TA (2017) Arsenic removal by electrocoagulation process: recent trends and removal mechanism. Chemosphere 181:418–432. https://doi.org/10.1016/j.chemosphere.2017.04.082
Davarnejad R, Nikseresht M (2016) Dairy wastewater treatment using an electrochemical method: Experimental and statistical study. J Electroanal Chem 775(15):364–373. https://doi.org/10.1016/j.jelechem.2016.06.016
Kim T, Kim TK, Zoh KD (2020) Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J Water Process Eng 33:101109. https://doi.org/10.1016/j.jwpe.2019.101109
Chezeau B, Boudriche L, Vial C, Boudjemaa A (2020) Treatment of dairy wastewater by electrocoagulation process: advantages of combined iron/aluminum electrodes. Sep Sci Technol 55(14):2510–2527. https://doi.org/10.1080/01496395.2019.1638935
Keyikoglu R, Can OT, Aygun A, Tek A (2019) Comparison of the effects of various supporting electrolytes on the treatment of a dye solution by electrocoagulation process. Colloids Interface Sci Commun 33:100210. https://doi.org/10.1016/j.colcom.2019.100210
Tahreen A, Jami MS, Ali F (2020) Role of electrocoagulation in wastewater treatment: a developmental review. J Water Process Eng 37:101440. https://doi.org/10.1016/j.jwpe.2020.101440
Ghosh S, Debsarkar A, Dutta A (2019) Technology alternatives for decontamination of arsenic-rich groundwater—a critical review. Environ Technol Innov 13:277–303. https://doi.org/10.1016/j.eti.2018.12.003
Tiwari A, Sahu O (2017) Treatment of food-agro (sugar) industry wastewater with copper metal and salt: chemical oxidation and electro-oxidation combined study in batch mode. Water Resour Ind 17:19–25. https://doi.org/10.1016/j.wri.2016.12.001
Hakizimana JN, Gourich B, Chafi M, Stiriba Y, Vial C, Drogui P, Naja J (2017) Electrocoagulation process in water treatment: a review of electrocoagulation modeling approaches. Desalination 404:1–21. https://doi.org/10.1016/j.desal.2016.10.011
Zhang Y, Luo G, Wang Q, Zhang Y, Zhou M (2020) Kinetic study of the degradation of rhodamine B using a flow-through UV/electro-Fenton process with the presence of ethylenediaminetetraacetic acid. Chemosphere 240:124929. https://doi.org/10.1016/j.chemosphere.2019.124929
Kalantary RR, Farzadkia M, Kermani M, Rahmatinia M (2018) Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: process optimization using response surface methodology. J Environ Chem Eng 6(4):4644–4652. https://doi.org/10.1016/j.jece.2018.06.043
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- A commercially available aluminum plate was used as the anode for the electro-Fenton process.
- High decomposition rate of amoxicillin was achieved via relatively low-cost materials.
- A remarkable removal rate in neutral pH ranges was measured using the Al plate.
- Less energy was consumed compared to similar studies.
Rights and permissions
About this article
Cite this article
Nayebi, B., Ayati, B. Degradation of Emerging Amoxicillin Compound from Water Using the Electro-Fenton Process with an Aluminum Anode. Water Conserv Sci Eng 6, 45–54 (2021). https://doi.org/10.1007/s41101-021-00101-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-021-00101-4