Abstract
The current work deals with efficient removal of acetaminophen (AC) from hospital wastewater using electro-Fenton (EF) process. The degradation yield of 99.5% was obtained under optimal experimental conditions, namely 5.75 mg L−1 initial AC concentration, 2.75 pH solution, 3-cm inter-electrode distance, 100 mg L−1 KCl electrolyte, 122.5 µL L−1 H2O2, 8 mA cm−2 current density at equilibrium time of 8 min. Analysis of variance (ANOVA) suggested that the effect of mentioned operating parameters was statistically significant on the AC removal. The low probability amount of P value (P < 0.0001), the Fisher’s F-value of 65.91, and correlation coefficient of the model (R2 = 0.9545) revealed a satisfactory correlation between the experimental and the predicted values of AC removal. The predicted removal efficiency of 99.4% was in satisfactory agreement with the obtained experimental removal efficiency of 98.7%. The AC degradation during the EF followed a first-order kinetic model with rate constants (Kapp) of 0.6718 min−1. Using the ordinary radical scavengers revealed that main mechanism of AC degradation controlled by the hydroxyl free radicals produced throughout the EF process. The excess amount of iron (II) scavenged the active radicals and diminished the concentration of ·OH available to react with AC. The optimum molar ratio of H2O2 to Fe2+ was found to be 2.5. The developed EF process as a promising technique applied for treatment of real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural and industrial activities, as well as urban community’s sewage disposal, led to a severe pollution of water sources during the last two decades. It caused a worldwide concern due to significant effect on the environment and human health. Emerging contaminants (ECs) as the newly generated chemical products including pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) reported as the serious environmental pollutants which increasingly used and found up to ppm level in water sources even drinking water and soil (Farré et al. 2012; Klamerth et al. 2013).
Among them, acetaminophen (N-(4-hydroxyphenyl) acetamide) as one of the most heavy medicines is used even without medical prescription and nearly 58–68% of acetaminophen (AC) is excreted from the body and eventually discharged into municipal wastewater systems. The presence of AC leads to diseases and problem such as critical liver injury and hepatocyte necrosis. Moreover, protein denaturation, lipid peroxidation and DNA damage, and even mortality by AC overdose are reported due to its toxicity. Therefore, its removal from water sources as emerging contaminant greatly desired (Abdullah et al. 2016; Bedner and MacCrehan 2006).
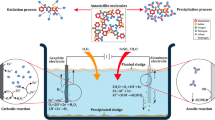
The literature reveals that wastewater treatments such as filtration, coagulation, and adsorption provide satisfactory removal of pharmaceuticals from wastewater (Coimbra et al. 2016; Groeschke et al. 2016; Jung et al. 2015). However, the generation of sludge and the necessity of adsorbent regeneration are important drawbacks for the application of such treatments. Lately, the advanced oxidation process (AOP) as a cost-effective technique with reasonable efficiency received great attention among environmental scientists who interested on wastewater treatment process. Throughout the AOP, the hydroxyl radicals react with the organic pollutants and through producing intermediates generates H2O, CO2, and other innocuous materials finally (Panizza and Oturan 2011). To generate the hydroxyl radical, various methods such as chemical, electrochemical, and photochemical methods are applied. Among them, EF technique which produces the hydroxyl radical electrocatalytically through Fenton’s reaction (Eq. 1) is subjected to investigated in the current work (Yahya et al. 2014) (Fig. 1).
Besides the operative degradation of organic-based contaminants such as dyes (Guivarch et al. 2003), antibiotics (Yahya et al. 2014), and pesticides (Abdessalem et al. 2010) for complete decomposition of harmful materials, its fast and cost-effective feature without using any harmful chemical makes it a good candidate for wastewaters treatment. Besides the current study in the field of electrochemistry, using electrochemical sensors as a promising device which provides a certain type of response that is directly related to the quantity of a specific chemical species showed a rapidly growing scientific field in the environmental monitoring research and practiced by the current research group recently (Ahmadzadeh et al. 2015a, c; Fouladgar and Ahmadzadeh 2016; Kassim et al. 2011; Pardakhty et al. 2016; Rezayi et al. 2014; Soltani et al. 2016).
To the best of our knowledge, although several studies are carried out for degradation of acetaminophen using electro-Fenton process there is no report on the removal of acetaminophen from hospital wastewater using response surface methodology (RSM) (de Luna et al. 2012; Sirés et al. 2004; Su et al. 2012). In the current work, a great effort was made to improve the treatment process of hospital wastewater in terms of energy consumption and removal efficiency. Most of the reported EF investigations for wastewater treatment are based on one factor at the time method. Herein, RSM is used to evaluate the effect of main parameters and their simultaneous interaction to obtain the optimal operating condition for the developed EF process. The applied experimental design methodology benefited the current work by reducing the number of experiments and providing a reasonable and fast result interpretation by developing a polynomial treatment model. Moreover, kinetic models of electro-Fenton degradation studied in the optimum operating condition.
Materials and methods
Chemicals
Acetaminophen (C8H9NO2, code number: A7085, grade: BioXtra, ≥ 99.0%), hydrogen peroxide solution (H2O2, code number: H1009, grade: 30% w/w in H2O, contains stabilizer), sodium phosphate (Na3PO4, code number: 342483, grade: 96.0%), and methanol (CH3OH, code number: 494291, grade: HPLC grade: ≥ 99.9%) were purchased from Sigma-Aldrich. Potassium permanganate (KMnO4, code number: 105082, grade: 99.9%), sodium chloride (NaCl, code number: 106404, grade: ≥ 99.5%), sodium carbonate (Na2CO3, code number: 106395, grade: ≥ 99.5%), potassium chloride (KCl, code number: 104936, grade: ≥ 99.5%), sodium sulfate (Na2SO4, code number: 106649, grade: ≥ 99.0%), sodium hydrogen carbonate (Na3PO4, code number: 106329, grade: ≥ 99.7%), sodium hydroxide (NaOH, code number: 106462, grade: ≥ 99.0%), hydrochloric acid fuming (HCl, code number: 100317, grade: 37%), sulfuric acid (H2SO4, code number: 100731, grade: 95–97% %), sodium nitrate (NaNO3, code number: 106535, grade: ≥ 99.0%), and tert-butanol (C4H10O, code number: 109629, grade: ≥ 99.5%) were obtained from company Merck. All the solutions were prepared with chemicals of at least analytical grade using tap water.
Apparatus and experimental procedures
All experiments were carried out in a cylindrical glass as an electrochemical cell containing 250 mL of solution at room temperature. The reactor setup was equipped with two iron plate electrodes located in the center of the EF cell with immersed dimensions of 3 × 1×0.2 cm. Electrolysis processes were carried out under the current-controlled conditions using DC power supply (Megatone Electronics Corp., Taiwan). The pH adjustment was performed by HCl and NaOH solutions using a Metrohm pH meter model 827 pH laboratory (Metrohm AG, Switzerland).
Heidolph magnetic stirrer model Hei-Standard (Heidolph Company, Germany) was used to run all the experiments under the agitation of 200 rpm. All treated solution samples were analyzed for quantitative determination of residual AC using a KNAUER Smartline HPLC (KNAUER Wissenschaftliche Geräte GmbH, Germany). The Fe2+ concentration of the treated solution was measured using Shimadzu atomic absorption spectrophotometer model AA-670 (Shimadzu Corporation, Japan) set at 248 nm. The experiments related to the investigation of the electrolyte type and dosage effect, the Fe2+ concentration of the treated solution and first-order kinetic model were performed in triplicate and the error bars corresponding to the deviation shown in all cases. The removal efficiency of AC was calculated by the following equation (Kamaraj and Vasudevan 2016; Venkatkarthick et al. 2016):
where C0 and C t (mg L−1) denote the concentration of AC before and after the EF process, respectively.
Hospital wastewater preparation and analysis
The real samples collected from Kerman Hospital. According to the hospital report, the features of the raw wastewater included the total suspended solids (TSS) of 104 ± 18 mg L−1, total dissolved solids (TDS) of 564 ± 43 mg L−1, temperature of 21.3 ± 1.4 °C, chemical oxygen demand (COD) of 576.7 ± 53 mg L−1, biochemical oxygen demand (BOD5) of 284.60 ± 41 mg L−1, pH of 7.7 ± 0.4, and electrical conductivity (EC) of 1039.71 ± 68 µmho cm−1.
KNAUER Smartline HPLC was used for quantitative analysis of residual AC using C18 column; 250 × 4.6 × 5 mm with a UV detector at a wavelength of 242 nm. The mobile phase was a mixture of MeOH and H2O with a ratio of 30/70 (vol. %) with an injection flow rate of 1 mL/min. Sampling (5 mL of AC-treated solution) was performed at regular time intervals and filtered (0.2 µm) before each analysis. In the first step, the hospital wastewater samples of 500 mL were filtered using a 1.2-μm glass fiber membrane filter and the pH of the sample solutions was adjusted to 2 by H2SO4. It is important to state that owing to the presence of high concentration of organic-based contaminants in hospital wastewater it is recommended to use low volume samples for avoiding the blockage of the cartridges. The SPE cartridges are conditioned by 3 mL of each solvent (acetone, methanol, and deionized water with the pH of 2) at the flow rate of 3 mL min−1. Vacuum manifold system with the flow rate of 15 mL min−1 was applied to percolate the hospital wastewater samples. The loaded cartridges were rinsed by 3 mL of methanol/water mixture (5:95 v/v) and n-hexane at the flow rate of 1 mL min−1. Finally, the obtained combination of aliquots was evaporated by a mild nitrogen flow and the remaining precipitate was dissolved in 0.2 mL of methanol. The injected volume of 20 μL in each run was filtered using a 0.45-μm nylon filter before injection into the HPLC system (Camacho-Muñoz et al. 2009).
Experimental design
According to the scientific reports, to optimize the operating parameters two approaches were used, namely one factor at a time (OFAT) and RSM. RSM offers certain advantages over OFAT method and can consider the interactions among the variables. RSM as a statistics-based method involves simultaneous adjustment and optimization of operating parameters. The central composite design (CCD) was used to analyze the obtained results by fitting them into the developed model. To conduct a successful study, RSM is applied to find a suitable approximation for the relation between independent main variables and response as expressed below (Ahmadzadeh et al. 2017; Yoosefian et al. 2017):
where β0, β j , β jj , and β ij denote the regression coefficients for the intercept, linearity, quadratics, and interaction terms, respectively. Y, X i , and X j denote the estimated response and the coded independent variables (i, j).
Electrochemical investigation and the process optimization
Hence, the nature of electrolyte salt plays a significant role in the defecation steps (diffusion, adsorption, and fixation) and the distance between two electrodes affected the removal efficiency and energy consumption. Therefore, in the preliminary studies, the effect of electrolyte type and dosage and inter-electrodes distance on the removal efficiency of AC was investigated. Moreover, the effect of four independent process variables initial AC concentration (X1), pH (X2), H2O2 concentration (X3) and current density (X4) was investigated to optimize the removal efficiency of AC from hospital wastewater over 30 experiments with six replicates at center points. The numerical values and levels of the variables including (− α, − 1, 0, + 1, + α) are presented in Table 1. The regression analysis was applied to fit the response, and the ANOVA was performed to test the statistical significance of the response surface quadratic model. To evaluate the percentage effect of each operating factor on removal efficiency of AC, Pareto analyses were conducted according to the following relation (Yoosefian et al. 2017):
The kinetics of AC removal process was investigated using first-order and second-order kinetic models. In the first-order kinetic studies, ln (C0/C t ) was plotted against time t, where C t is the concentration of the AC during EF process for time interval t and C0 is its initial concentration (Fulazzaky et al. 2017). To investigate the second-order kinetic model, 1/C t was plotted against time t.
Results and discussion
Setting the initial operating parameters
The effect of electrolyte type on the removal efficiency of AC was investigated, and the obtained results are presented in Fig. 2. The best removal efficiency was attained using KCl as an electrolyte at equilibrium time of 10 min. Moreover, it was found that the degradation rate of AC contaminant was significantly higher and up to 92.10 and 89.78 percent when KCl and NaCl were used as electrolytic supports at equilibrium time of 10 min, respectively. However, the degradations rates of 86.31 and 82.46% are found under the same conditions for Na2SO4 and Na2CO3, respectively.
Probably the obtained higher degradation rates could be attributed to the addition of KCl as a proper electrolyte with high mobility to the EF reactor. It demonstrated a significant improvement in efficiency of the process by providing a proper passage for the electric current and increasing the conductivity of the contaminated water. It affects the Faradic yield, cell voltage, and energy consumption in the EF reactor. Besides, using KCl has other benefits such as reducing the adverse effects of other anions, for instance HCO− 3 and SO42−. Since the presence of CO32− anions may result in the precipitation of Ca2+ cations that form an insulating layer on the surface of the cathode and lead to increase in the ohmic resistance of the EF reactor (Khandegar and Saroha 2013; Yoosefian et al. 2017).
Additionally, the presence of chlorine ions (Cl−) might result in producing in situ hypochlorous acid (HOCl) according to reactions expressed below and enhances the degradation of AC (Kamaraj and Vasudevan 2016).
Besides, chlorine ions can prevent the formation of the passivation layer on the surface of the electrode and enrich the anodic dissolution which results in producing more catalysts and, consequently, higher levels of ·OH generation. The reaction between hydroxyl radical and CO32− led to electron transfer to the hydroxyl radical and rendering it which resulted in reducing the removal efficiency of EF process (Kamaraj and Vasudevan 2016).
The removal efficiency of AC was assessed as a function of KCl concentration ranging from 40 to 400 mg L−1 at an initial pH 3.0, H2O2 concentration 60 µL L−1, initial AC concentration 5 mg L−1, distance between the electrodes 3 cm, and current density 10 mA cm−2. As shown in Fig. 3, by increasing the KCl concentration, an improvement in the removal efficiency was observed which is in accordance with the characteristics of the AOPs. It is noteworthy to mention, in the constant value of main variables including initial AC concentration, pH solution, inter-electrode distance, H2O2 dosage, and current density, a certain amount of hydroxyl radical as the active intermediate generated. By increasing the concentration of KCl to the values more than 100 mg L−1, a significant reduction in removal efficiency was observed due to lowering the number of hydroxyl radicals (see Fig. 3). The observed decrease in the number of hydroxyl radicals attributed to the excess amount of the KCl which acts as a radical scavenger.
Moreover, several experiments were conducted at AC concentration of 5 mg L−1, the H2O2 dosage of 70 µL L−1, pH of 3, the current density of 10 mA cm−2, KCl dosage of 100 mg L−1, and the electrolysis time of 8 min by changing the electrodes distance between 1 and 5 cm. However, for the too short distance such as 1 cm, ferrous ions which are regenerated via the reduction of the ferric ion at the cathode and act as an inducer of Fenton chain reaction could be easily oxidized to ferric ion at the anode and inhibit the efficiency of Fenton chain reactions. Besides, too long distance such as 5 cm limits the transfer of ferric ions to the cathode surface that controls the ferrous ion regeneration (Babuponnusami and Muthukumar 2012). The optimum electrode distance of 3 cm with lower energy consumption of 0.501 (kWh m−3) was designated for further studies (Table 2).
Assessment of the developed quadratic model performance
As given in Table 3, according to the Fisher’s F-value (65.91) with a low probability value of P < 0.0001, the quadratic model of ANOVA suggested that the model is highly significant. Moreover, the correlation coefficient (R2) between the experimental and the predicted value of the model was checked to confirm the goodness of model fitting. The high amount of R2 value of 0.9545 indicates the regression model is statistically significant and only 4.55% of the total variation is not explained by the model. Furthermore, the predicted correlation coefficient (pred. R2) of 0.8754 demonstrates satisfactory agreement with the adjusted correlation coefficient (adj. R2) of 0.9400. It can be added that the coefficient of variance of 3.29% suggests better precision and reliability of the data obtained by performing the experiments, while a non-significant lack of fit value (> 0.05) implies the validity of the quadratic model (Doltabadi et al. 2016; Yoosefian et al. 2017). The ANOVA indicates the applicability of the model for efficient removal of AC through EF process within the limits of the experimental factors.
The designed operating conditions by RSM and the obtained values of AC removal efficiency as the response are presented in Table 4. The software suggested a quadratic model for analyzing the obtained data and the regression equation as the response in terms of coded factors is expressed as below:
where X1, X2, X3, and X4 denote initial AC concentration (mg L−1), solution pH, H2O2 dosage (µL L−1), and X4 current density (mA cm−2), respectively. The removal efficiency of AC significantly was influenced by the solution pH with its considerable negative effect. Moreover, the initial AC concentration as the second most significant factor revealed the negative effect on the removal efficiency of AC through EF process.
The Pareto plot provides remarkable information to interpret the main variables and their interaction effects on removal efficiency of AC. The solution pH and initial AC concentration are the most determining factors which synergistically affect the removal efficiency of AC up to 72%. The contributions of the quadratic effects of b 22 , b 24 , and b 23 on the AC removal efficiency are 10.51, 8.74, and 5.01%, respectively (Fig. 4).
At the center points of the operational parameters, the removal efficiency of AC decreased from 96.27 to 86.62% by increasing its concentration from 5.75 to 15.25 mg L−1. Decreasing the AC concentration resulted in rapid increase in removal efficiency which is in accordance with the characteristics of AOPs. Since a particular amount of active intermediates was produced along with the increase in AC concentration, whereas the amount of generated hydroxyl radical in optimized condition was constant, the removal efficiency decreased (Estrada et al. 2012). The effect of wastewater pH ranging from 2 to 5 examined the performance of EF process at the center point of the operational parameters. As shown in Fig. 5, the removal efficiency of AC decreased from 96.59% at a wastewater pH of 2.75 to its minimum level of 78.46% when the pH of wastewater increased to 4.25.
The literature surveys revealed that Fenton reaction was generally conducted in acidic solution with pH values ranging from 2 to 4. It is probably due to the precipitation of iron species as ferric hydroxides at higher pH values. Moreover, the formation of stable complexes between iron species and H2O2 at lower pH values results in deactivation of catalysts, accordingly decreasing the oxidation efficiency. The optimal pH value obtained in the current work was found to be 2.75. It is important to note that theoretically lower pH is more favorable for producing H2O2 since the conversion of dissolved oxygen to hydrogen peroxide consumes protons in acidic solution. Furthermore, at pH lower than 3, H2O2 would remain steady according to the formation of oxonium ion as expressed below (Zhao et al. 2012):
Figure 6 shows that at the center point of the operational parameters the removal efficiency of AC decreases from 87.48 to 90% by increasing H2O2 dosage from 67.5 to 122.5 µg L−1. The increase in the removal efficiency was due to the increase in ·OH radical concentration as a result of the H2O2 addition. However, a decrease in removal efficiency was observed at the high dosage of H2O2, due to scavenging effect of the hydroxyl radical (see Eqs. 9 and 10) and the recombination of the hydroxyl radical to form H2O2 (see Eq. 12) (Nidheesh and Gandhimathi 2012; Wang et al. 2016).
The current density dependence of AC removal efficiency was investigated over a wide range of 2–10.0 mA cm−2. The effects of current density and initial AC concentration on removal efficiency depicted in 3D plot are presented in Fig. 7.
As shown in Fig. 7, the removal efficiency of AC was increased by increasing the current density and the maximum removal efficiency of 95.5% was obtained at 6.55 mA cm−2. The obtained results revealed that by increasing the current intensity value from 4 to 8 mA cm−2, the removal efficiency improved from 85.91 to 89.83%. The effect of current density as a significant operational parameter can be explained based on the mole ratio of the added H2O2 into the EF reactor to the amount of released iron (II) ions into the solution (H2O2/Fe(II)). Figure 8 shows that, by increasing the current density, the number of iron (II) ions released into the solution increased (sacrificial anode). It should be considered that since the amount of 95 µL L−1 H2O2 added into the EF reactor was kept constant at all experiments, an increase in iron (II) ions released into the solution results in decreasing the mole ratio of H2O2 to Fe and consequently increases the removal efficiency. According to Eq. 13, the higher amount of iron (II) ions results in consuming the higher amount of H2O2. Consequently, a higher amount of ·OH generated results in removal efficiency improvement (see Fig. 8) (Moussavi et al. 2012).
As shown in Fig. 8, the optimum mole ratio of H2O2 to Fe2+ was observed approximately at 2.5. However, decrease in AC removal efficiency at higher reaction time (10 min) can be attributed to higher anode scarification and producing an excess concentration of iron (II), which resulted in decreasing the mole ratio of H2O2 to Fe(II) to below the optimum level of 2.5. The excess iron (II) scavenged the ·OH as expressed by Eq. 14, diminishing the concentration of ·OH available to react with the organic compounds (Moussavi and Aghanejad 2014).
To achieve the best removal efficiency, the main parameters including initial concentration, H2O2 dosage, current density, and pH was set to ‘in range’ option and the removal efficiency as the response was set to ‘maximize’ option in Design Expert software. The developed model predicted the maximum removal efficiency of 99.4% with the desirability of 0.997 under the optimal condition including AC initial concentration of 5.75 mg L−1, the H2O2 dosage of 122.5 µL L−1, pH of 2.75, electrolyte dose of 100 mg L−1 KCl, the current density of 8 mA cm−2, and 3 cm inter-electrodes distance. The predicted removal efficiency by the developed quadratic model was in satisfactory agreement with the obtained experimental removal efficiency of 98.7%.
Analysis of removal kinetic model
According to Fig. 9, a straight line with the slope of 0.6718 (R2 = 0.9941) was observed which corresponds to the apparent rate constant (Kapp) value for AC (Ganesan et al. 2013; Kamaraj et al. 2016). The first-order kinetic model can satisfactorily describe the current experimental obtained results. Some other thermodynamic investigations were conducted by the current research group who qualified our studies to a great extent and can extend to further investigation (Ahmadzadeh et al. 2011a, b; 2015b; Rounaghi et al. 2009).
Since carboxylic acids which were formed by oxidative ring-opening reactions are less reactive toward hydroxyl radicals compared to the aromatics, the mineralization kinetics is fast at the initial stages of treatment but becomes much slower at a longer time. The kinetic equilibriums were calculated and are summarized in Table 5.
Oxidation mechanism of AC removal
The removal efficiency of the AC was evaluated in the presence of the ordinary radical scavengers including tert-butanol (TB), sulfate, carbonate, nitrate, phosphate, and chloride to investigate the importance of ·OH radical generation on removal efficiency. The obtained results revealed that the removal efficiencies of the EF process under the optimized condition and in the presence of KCl, Na2SO4, NaNO3, Na3PO4, NaHCO3, and TB were found to be 99.40 ± 0.67, 81.26 ± 0.82, 69.21 ± 0.93, 57.55 ± 0.95, 45.45 ± 1.05, 31.99 ± 0.94, and 13.46 ± .080, respectively. According to the obtained results, using TB as strong radical scavenger resulted in significant decrease in AC removal efficiency which confirmed the necessity of ·OH presence for the effective treatment process (Babuponnusami and Muthukumar 2012).
Control experiments have been carried out to investigate the contribution of electrocoagulation process (running the treatment process without the addition of H2O2) and Fenton process (running the treatment process without applying electric current). The obtained results revealed that electrocoagulation and Fenton processes are involved in the treatment process of AC removal with numerical values of 1.05 ± 0.41 and 0.0%, respectively.
Hospital wastewater sample
According to the described procedure, the hospital wastewater samples were prepared for quantitative analysis of residual AC after treatment process using HPLC technique. The obtained results revealed that the AC concentration of 1.35 ± 0.23 mg L−1 in the examined hospital wastewater samples reached zero after applying EF process under optimal condition.
Conclusions
The obtained results revealed that the developed EF process as an environmentally friendly and emerging technique could be applied for efficient treatment of hospital wastewater sample. According to the predicted model, the pH was the most significant operating parameter which affected the degradation rate of AC contaminant. The obtained results demonstrated a satisfactory correlation between the experimental removal efficiency of 98.7% and predicted removal efficiency of 99.4% with the correlation coefficient of 0.9545. The molar ratio of H2O2 to Fe2+ was optimized. The EF process kinetics successfully fitted to the first-order model with the rate constant (Kapp) of 0.6718 min−1. The AC concentration of the treated real samples reached zero after applying EF process under optimal condition.
References
Abdessalem AK, Bellakhal N, Oturan N, Dachraoui M, Oturan MA (2010) Treatment of a mixture of three pesticides by photo-and electro-Fenton processes. Desalination 250:450–455
Abdullah N, Fulazzaky MA, Yong EL, Yuzir A, Sallis P (2016) Assessing the treatment of acetaminophen-contaminated brewery wastewater by an anaerobic packed-bed reactor. J Environ Manag 168:273–279
Ahmadzadeh S, Kassim A, Rezayi M, Abdollahi Y, Hossein G (2011a) A conductometric study of complexation reaction between meso-octamethylcalix [4] pyrrole with titanium cation in acetonitrile-ethanol binary mixtures. Int J Electrochem Sci 6:4749–4759
Ahmadzadeh S, Kassim A, Rezayi M, Rounaghi GH (2011b) Thermodynamic study of the complexation of p-Isopropylcalix [6] arene with Cs + cation in dimethylsulfoxide-acetonitrile binary media. Molecules 16:8130–8142
Ahmadzadeh S, Rezayi M, Faghih-Mirzaei E, Yoosefian M, Kassim A (2015a) Highly selective detection of titanium (III) in Industrial waste water samples using meso-octamethylcalix [4] pyrrole-doped PVC membrane ion-selective electrode. Electrochim Acta 178:580–589
Ahmadzadeh S, Rezayi M, Karimi-Maleh H, Alias Y (2015b) Conductometric measurements of complexation study between 4-Isopropylcalix [4] arene and Cr 3 + cation in THF-DMSO binary solvents. Measurement 70:214–224
Ahmadzadeh S, Rezayi M, Kassim A, Aghasi M (2015c) Cesium selective polymeric membrane sensor based on p-isopropylcalix [6] arene and its application in environmental samples RSC. Advances 5:39209–39217
Ahmadzadeh S, Asadipour A, Pournamdari M, Behnam B, Rahimi HR, Dolatabadi M (2017) Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: optimization and modelling through response surface methodology. Process Saf Environ Prot 109:538–547. https://doi.org/10.1016/j.psep.2017.04.026
Babuponnusami A, Muthukumar K (2012) Advanced oxidation of phenol: a comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 183:1–9
Bedner M, MacCrehan WA (2006) Transformation of acetaminophen by chlorination produces the toxicants 1, 4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ Sci Technol 40:516–522
Camacho-Muñoz D, Martín J, Santos JL, Aparicio I, Alonso E (2009) An affordable method for the simultaneous determination of the most studied pharmaceutical compounds as wastewater and surface water pollutants. J Sep Sci 32:3064–3073
Coimbra R, Escapa C, Paniagua S, Otero M (2016) Adsorptive removal of diclofenac from ultrapure and wastewater: a comparative assessment on the performance of a polymeric resin and activated carbons. Desalin Water Treat 57:27914–27923
de Luna MDG, Veciana ML, Su C-C, Lu M-C (2012) Acetaminophen degradation by electro-Fenton and photoelectro-Fenton using a double cathode electrochemical cell. J Hazard Mater 217:200–207
Doltabadi M, Alidadi H, Davoudi M (2016) Comparative study of cationic and anionic dye removal from aqueous solutions using sawdust-based adsorbent. Environ Prog Sustain Energy 35:1078–1090
Estrada AL, Li Y-Y, Wang A (2012) Biodegradability enhancement of wastewater containing cefalexin by means of the electro-Fenton oxidation process. J Hazard Mater 227:41–48
Farré M, Kantiani L, Petrovic M, Pérez S, Barceló D (2012) Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J Chromatogr A 1259:86–99
Fouladgar M, Ahmadzadeh S (2016) Application of a nanostructured sensor based on NiO nanoparticles modified carbon paste electrode for determination of methyldopa in the presence of folic acid. Appl Surf Sci 379:150–155
Fulazzaky MA, Majidnia Z, Idris A (2017) Mass transfer kinetics of Cd (II) ions adsorption by titania polyvinylalcohol-alginate beads from aqueous solution. Chem Eng J 308:700–709
Ganesan P, Kamaraj R, Vasudevan S (2013) Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on graphene from aqueous solution. J Taiwan Inst Chem Eng 44:808–814
Groeschke M, Kumar P, Winkler A, Grützmacher G, Schneider M (2016) The role of agricultural activity for ammonium contamination at a riverbank filtration site in central Delhi (India). Environ Earth Sci 75:129
Guivarch E, Trevin S, Lahitte C, Oturan MA (2003) Degradation of azo dyes in water by electro-Fenton process. Environ Chem Lett 1:38–44
Jung C, Oh J, Yoon Y (2015) Removal of acetaminophen and naproxen by combined coagulation and adsorption using biochar: influence of combined sewer overflow components. Environ Sci Pollut Res 22:10058–10069
Kamaraj R, Vasudevan S (2016) Facile one-pot synthesis of nano-zinc hydroxide by electro-dissolution of zinc as a sacrificial anode and the application for adsorption of Th4+, U4+, and Ce4 + from aqueous solution. Res Chem Intermed 42:4077–4095
Kamaraj R, Pandiarajan A, Jayakiruba S, Naushad M, Vasudevan S (2016) Kinetics, thermodynamics and isotherm modeling for removal of nitrate from liquids by facile one-pot electrosynthesized nano zinc hydroxide. J Mol Liq 215:204–211
Kassim A et al (2011) A Novel ion selective polymeric membrane sensor for determining thallium (I) with high selectivity. In: IOP conference series: materials science and engineering. IOP Publishing, p 012010
Khandegar V, Saroha AK (2013) Electrocoagulation for the treatment of textile industry effluent—a review. J Environ Manag 128:949–963
Klamerth N, Malato S, Agüera A, Fernández-Alba A (2013) Photo-Fenton and modified photo-Fenton at neutral pH for the treatment of emerging contaminants in wastewater treatment plant effluents: a comparison. Water Res 47:833–840
Moussavi G, Aghanejad M (2014) The performance of electrochemical peroxidation process for COD reduction and biodegradability improvement of the wastewater from a paper recycling plant. Sep Purif Technol 132:182–186
Moussavi G, Bagheri A, Khavanin A (2012) The investigation of degradation and mineralization of high concentrations of formaldehyde in an electro-Fenton process combined with the biodegradation. J Hazard Mater 237:147–152
Nidheesh P, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15
Panizza M, Oturan MA (2011) Degradation of Alizarin Red by electro-Fenton process using a graphite-felt cathode. Electrochim Acta 56:7084–7087
Pardakhty A, Ahmadzadeh S, Avazpour S, Gupta VK (2016) Highly sensitive and efficient voltammetric determination of ascorbic acid in food and pharmaceutical samples from aqueous solutions based on nanostructure carbon paste electrode as a sensor. J Mol Liq 216:387–391
Rezayi M, Karazhian R, Abdollahi Y, Narimani L, Sany SBT, Ahmadzadeh S, Alias Y (2014) Titanium (III) cation selective electrode based on synthesized tris (2pyridyl) methylamine ionophore and its application in water samples. Sci Rep 4:0000–0025
Rounaghi G, Mohajeri M, Ahmadzadeh S, Tarahomi S (2009) A thermodynamic study of interaction of Na + cation with benzo-15-crown-5 in binary mixed non-aqueous solvents. J Incl Phenom Macrocycl Chem 63:365–372
Sirés I, Arias C, Cabot PL, Centellas F, Rodríguez RM, Garrido JA, Brillas E (2004) Paracetamol mineralization by advanced electrochemical oxidation processes for wastewater treatment. Environ Chem 1:26–28
Soltani H, Pardakhty A, Ahmadzadeh S (2016) Determination of hydroquinone in food and pharmaceutical samples using a voltammetric based sensor employing NiO nanoparticle and ionic liquids. J Mol Liq 219:63–67
Su C-C, Chang A-T, Bellotindos LM, Lu M-C (2012) Degradation of acetaminophen by Fenton and electro-Fenton processes in aerator reactor Separation and purification technology 99:8–13
Venkatkarthick R, Kiruthika GVM, Davidson DJ, Ravichandran S, Sozhan G, Vasudevan S (2016) New insight into understand the enhanced photoconductivity properties of Ti (O2) plate spurted with Al2O3 for water oxidation. Chem Select 1:5037–5041
Wang N, Zheng T, Zhang G, Wang P (2016) A review on Fenton-like processes for organic wastewater treatment Journal of Environmental. Chem Eng 4:762–787
Yahya MS, Oturan N, El Kacemi K, El Karbane M, Aravindakumar C, Oturan MA (2014) Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-fenton process: kinetics and oxidation products. Chemosphere 117:447–454
Yoosefian M, Ahmadzadeh S, Aghasi M, Dolatabadi M (2017) Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption. J Mol Liq 225:544–553. https://doi.org/10.1016/j.molliq.2016.11.093
Zhao X, Zhang B, Liu H, Chen F, Li A, Qu J (2012) Transformation characteristics of refractory pollutants in plugboard wastewater by an optimal electrocoagulation and electro-Fenton process. Chemosphere 87:631–636
Acknowledgements
The authors express their appreciation to Neuroscience Research Center and Pharmaceutics Research Center both affiliated to Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran, for supporting the current work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadzadeh, S., Dolatabadi, M. Removal of acetaminophen from hospital wastewater using electro-Fenton process. Environ Earth Sci 77, 53 (2018). https://doi.org/10.1007/s12665-017-7203-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-7203-7