Abstract
Peroxicoagulation is an electrochemical advanced oxidation processes in which both ferrous ions and hydrogen peroxide are generated in the cell. Organic pollutants are thus removed by degradation and coagulation. The peroxicoagulation process is a combination of electro-Fenton and electrocoagulation processes. The peroxicoagulation process is very efficient for the removal of aniline and herbicides from water and for the treatment of landfill leachate and textile wastewaters. Under acidic conditions, electro-Fenton is the predominant removal means, whereas electrocoagulation is the main removal means under neutral and alkaline conditions. As a consequence, pH regulation to acidic conditions is essential for the mineralization of organic pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The invention of the electro-Fenton method by the Oturan group (Oturan and Pinson 1995; Oturan 2000; Oturan et al. 2000) and the Brillas group (Brillas et al. 1996, 2000) in the starting of this century has attracted several investigators. Electro-Fenton process is an indirect electrochemical advanced oxidation process and also an extended Fenton process (Nidheesh et al. 2013). Electro-Fenton process rectifies one of the major problems of conventional Fenton process, i.e., the usage of hydrogen peroxide for the generation of hydroxyl radicals. This is accomplished by the usage of externally supplied oxygen for the generation of hydrogen peroxide in the electrolytic cell (Brillas et al. 2009; Nidheesh and Gandhimathi 2012; Oturan and Aaron 2014; Nidheesh 2015). In the acidic medium, dissolved oxygen present in the water medium undergoes cathodic reduction and generates hydrogen peroxide as in Eq. (1).
Carbonaceous electrodes are found to be very efficient for the production of hydrogen peroxide in the water medium (Oturan et al. 2000; Brillas et al. 2009; Nidheesh and Gandhimathi 2012). Thus, oxygen supply near the cathodic surface generates hydrogen peroxide in the electrolytic system continuously by two electron reduction of oxygen. This reaction utilizes in electro-Fenton process for the generation of hydroxyl radical. External addition of ferrous ions in the electrolytic system reacts with in situ generated hydrogen peroxide under acidic conditions and produces highly potent oxidant, hydroxyl radicals as in Eq. (2). Hydroxyl radicals are the second most powerful oxidizing agent and are capable of degrading organic pollutants present in water medium via hydroxylation, dehydrogenation and redox reactions (Oturan 2000; Oturan et al. 2000).
Another important feature of electro-Fenton process which attracts lots of researchers is the lesser optimal iron concentration compared to the conventional Fenton process. The optimal concentration of iron in electro-Fenton process is in the range of mg/L, while that of conventional Fenton process is in g/L (Nidheesh et al. 2013). Higher amount of catalyst in conventional Fenton process results in the scavenging reactions as given in Eqs. (3) and (4) (Brillas et al. 2009; Nidheesh et al. 2018). Higher hydrogen peroxide and ferrous ion concentrations results in elevated hydroxyl radical generation in the water medium. At the same time, in situ generated hydroxyl radicals react with excess ferrous ions and hydrogen peroxide.
Acidic pH requirement of conventional Fenton process is also a drawback which retards its full-scale application (Nidheesh 2015). In conventional Fenton process, hydroxide ions generate after the Fenton’s reactions increase the solution pH significantly. This results in the formation of insoluble iron hydroxides in the water medium. Regeneration of ferrous ions from ferric ions is also a problem in Fenton process. Ferric ions generated by Fenton’s reaction react with hydrogen peroxide and produce ferrous ions in the water medium (Eqs. 5 and 6). But, this reaction is very slow compared with Fenton reaction and overall, most of the ferric ions generated in the system convert to its hydroxide forms. Rate constants for the reaction 5 and 6 were reported as 0.001–0.01 and 1.2 × 106 M−1 s−1, respectively, while that of Fenton reaction is 70 M−1 s−1 (Neyens and Baeyens 2003). This slower ferrous ion regeneration rate is also the main reason behind the higher iron dosage requirement in conventional Fenton process. Cathodic reduction of ferric ions and subsequent generation of ferrous ions in electro-Fenton process (Eq. 7) is the reason behind lesser optimal iron dosage requirement. This reaction is much faster than the ferrous ion regeneration reaction in conventional Fenton process.
Insignificant change in pH during the electrolysis also helps very much for the effective degradation of pollutants in electro-Fenton process (George et al. 2014; Nidheesh et al. 2014; Jinisha et al. 2018). Hydroxyl ions generated from Fenton reactions are counterbalanced by the water oxidation reaction (Eq. 8) reaction occurs in the electrolytic cell (El-Desoky et al. 2010). The iron dosage requirement in electro-Fenton process is also not affected by the type of pollutant and the medium. For example, the amount of iron dosage required for the complete removal of dyes from synthetic medium and real wastewater is almost same (Nidheesh and Gandhimathi 2014a, b, 2015a, b; Nidheesh et al. 2014).
Enhancement in biodegradability of wastewater is one of the advantages of advanced oxidation processes over conventional wastewater treatment methods (Nidheesh and Gandhimathi 2015a). Therefore, coupling of electro-Fenton process and biological process, known as bio-electro-Fenton process, reduces the longer electrolysis time required for the complete mineralization of pollutants (Olvera-Vargas et al. 2016a, b; Roshini et al. 2017).
Even there are several advantages for electro-Fenton process, one of the main drawbacks of this process is the longer electrolysis time requirement for the complete mineralization of pollutants (Dirany et al. 2011; George et al. 2014; Oturan and Aaron 2014; Le et al. 2015). One of the main reasons behind this is the generation of short-chain carboxylic acids as by-products, which are resistant to the attack of hydroxyl radicals (Oturan et al. 2008). Also, the effective collision between hydroxyl radical and the pollutant decreases with increase in electrolysis time (Nidheesh et al. 2014). Thus, electrolysis time required for the electro-Fenton process is very high to accomplish complete mineralization.
Peroxicoagulation process is the modified version of electro-Fenton process, which combines the advantages of electro-Fenton and electrocoagulation processes. Electrocoagulation process is also an electrolytic process and also known as electrochemical coagulation process in which contaminants are removing from water medium via separation mechanisms like sorption and complexation. Researches in this field are revealed that the process is very efficient for the decontamination of water containing heavy metals, fluoride, nitrate, phosphate and arsenic, and are capable of treating textile wastewater, oil wastewater, landfill leachate, pulp and paper industry effluent, bilge water, tannery wastewater, dairy wastewater and paint manufacturing wastewater. This process uses metal electrode like iron, aluminum as the anode, and it generates insoluble metal hydroxides during electrolysis. These hydroxides are responsible for the coagulation and subsequent removal of contaminants.
In peroxicoagulation process, iron anode is used for supplying ferrous ion required for the Fenton’s reaction. The cathode is usually a gas diffusion electrode as in the case of electro-Fenton process. Air supply near the cathode surface generates hydrogen peroxide, and anode generates ferrous ions in the solution during electrolysis. Thus, external addition of any chemicals is not required in this process. In situ generated Fenton reagents produce hydroxyl radicals and cause the mineralization of organic pollutants, as explained earlier. Additional ferrous ion generated in the system is responsible for the removal of pollutants by making its insoluble hydroxides. Thus, peroxicoagulation process is a combination of degradation and separation process. Comparison of electrocoagulation, electro-Fenton and peroxicoagulation process is given in Table 1.
Mechanism of pollutant removal by peroxicoagulation
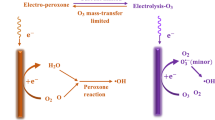
Peroxicoagulation is a combination of electro-Fenton and electrocoagulation processes, as stated earlier. To my best knowledge, it is first reported by Brillas et al. (1997) for the removal of aniline from water medium. Organic pollutant present in the water medium will be removed in two ways as shown in Fig. 1. First one is the degradation pathway as in the case of electro-Fenton process, and second is the separation route as in electrocoagulation process. Ferrous ion generated from the iron or stainless steel anode reacts with in situ generated hydrogen peroxide and produce hydroxyl radicals. These radicals attack organic contaminant until the complete mineralization. At the same time, iron converts from its divalent form into its trivalent form in the presence of air. Ferric ion formed by the aeration forms its hydroxides and results in coagulation with contaminants.
To find the organic pollutant removal contribution of electro-Fenton and electrocoagulation processes in peroxicoagulation process, Venu et al. (2016) carried out experiments in various pH conditions and the result obtained are shown in Fig. 2. The authors analyzed the soluble chemical oxygen demand (COD) removal capacity of these electrolytic processes from stabilized landfill leachate at different pH conditions. Electro-Fenton process was performed with an optimal ferrous concentration of 15 mg/L, and graphite electrodes were used as anode and cathode. Stainless steel was used for supplying ferrous ions in peroxicoagulation and electrocoagulation processes. From the results obtained, the authors concluded that electro-Fenton process is the predominant pollutant process of peroxicoagulation process at lower pH conditions, especially below 3 and electrocoagulation process is the dominant process in higher pH conditions. This indicates that pollutants are removed by degradation in lower pH conditions, while are separated from the aqueous phase at higher pH conditions. Instability of hydrogen peroxide generated at the cathode surface is one of the reasons behind the lower contribution of electro-Fenton process in peroxicoagulation process at higher pH conditions.
Contribution of electro-Fenton and electrocoagulation processes in peroxicoagulation process for the landfill leachate treatment at various pH conditions (Venu et al. 2016), where COD is chemical oxygen demand, EF is electro-Fenton process, EC is electrocoagulation process and PC is peroxicoagulation process. Electro-Fenton process predominates as the main pollutant removal mechanism of peroxicoagulation process at pH below 4. At the same time, pollutant removal efficiency of electrocoagulation process increases with increase in solution pH and predominates as the main pollutant removal mechanism of peroxicoagulation process at pH above 5.
Speciation of ferrous and ferric ions at different pH conditions also affects the performance of peroxicoagulation process and the dominant pollutant removal mechanism. Ferrous ions are in its original state below pH 10, and it will be in its hydroxide forms like Fe(OH)2, Fe(OH)+ and Fe(OH) −3 in other pH conditions. At higher pH conditions, these hydroxides are in the form of Fe(OH)+, Fe(OH)2 and Fe(OH) −3 . Thus, most probably ferrous ions generated electrolytically are in its original divalent state. Oxidation of ferrous ions to ferric ions also occurs only above pH 4 as in Eq. (9) (Pignatello et al. 2006; Magario et al. 2012). Thus, below pH 4, all the electrolytically generated ferrous ions undergo Fenton’s reactions, and this is the main reason behind the predominant degradation mechanism of peroxicoagulation process at lower pH conditions as observed by Venu et al. (2016). Ferric ions generated by the aeration undergo hydrolysis reaction and form its insoluble hydroxides like Fe(OH)2+, Fe(OH) +2 , Fe(OH)3 and Fe(OH) −4 and are predominant at pH above 4. Ferrous hydroxides precipitation also occurs in the electrolytic system and it starts at pH 5 and is maximum at pH 12.
Nidheesh and Gandhimathi (2014a) explained the same mechanism by characterizing the sludge generated at various pH conditions (pH 3, 6 and 9). Scanning electron microscopy images of the sludge produced at these pH conditions are shown in Fig. 3. Sludge produced at the acidic condition has minerals with different sizes but are highly ordered. X-ray diffraction analysis of the sludge is shown that the material is crystalline. Sludge generated at pH 6 and 9 has different materials with different sizes and is arranged irregularly. Amorphous nature of the sludge increases with increase in solution pH. These results indicate that the sludge generated at acidic conditions contains very less amount of organic pollutants and the concentration of these pollutants is very high at higher pH conditions. Thus, pollutants are degraded at the acidic conditions while are separated in pH > 3.
Scanning electron microscopy images of sludge generated after textile wastewater treatment by peroxicoagulation process at various pH conditions (Nidheesh and Gandhimathi 2014a). Note the highly ordered minerals produced at pH 3. This has been disturbed with increase in solution pH. Sludge produced at pH 9 is a mixture of particles having various sizes.
Solution pH plays a major role in the pollutant removal mechanism of peroxicoagulation process, as described above. An increase in pH of solution with an increase in electrolysis time is a feature of peroxicoagulation process. Nidheesh and Gandhimathi (2014a) examined the performance of peroxicoagulation process with initial pH conditions 3, 6 and 9. All the experiments were operated in batch mode. Solution pH increased to 6 from 3 and 9.8 from 6, within an electrolysis time of 30 min. In the case of pH 9, solution pH increased at the early stages of electrolysis and then decreased. This drop in pH with later stages of electrolysis is mainly attributed to the anodic oxidation Fe(OH) −4 , which is the dominant form of ferric ion at elevated pH conditions. Researchers controlled the solution pH to 3 by adding acid at a regular interval to maintain the degradation mechanism as the dominant pollutant removal mechanism of peroxicoagulation process (Nidheesh and Gandhimathi 2014a, c; Venu et al. 2014).
Applications of the peroxicoagulation process
Peroxicoagulation process is very efficient to remove various non-biodegradable pollutants from water medium. Various articles reported on peroxicoagulation are summarized in Table 2. Uses of peroxicoagulation process, comparison of peroxicoagulation process performance with other methods and necessary actions taken for further improvement on peroxicoagulation process performance are explained below.
Aniline
Aniline is a type of volatile organic compound used for the production of various herbicides, drugs, dyes, resins and inks, and the effluents generated from coal, paper, petroleum and other chemical industries generally contain aniline (Brillas and Casado 2002). As per Environmental Protection Agency, it is group B2 carcinogen and is responsible for bladder and spleen tumors.
Brillas et al. (1997) analyzed the performance of peroxicoagulation process for the removal of aniline from water medium. The authors tested the mineralization efficiency of the process in the presence of iron anode and carbon–polytetrafluoroethylene cathode under pH-regulated condition. Aniline solution with initial concentrations 100 and 1000 mg/L was considered, and electrolysis was carried out at constant applied currents of 100, 300, and 450 mA. Mineralization efficiency of the process increased with increase in applied current, and the maximum efficiency was observed at 450 mA. Total organic carbon (TOC) removal efficiency of the process for aniline solution of initial concentrations 100 and 1000 mg/L was found as 95 and 91%, respectively, after 30 min of electrolysis.
Based on the results of the above study, Brillas group (Brillas and Casado 2002) examined the aniline degradation performance of peroxicoagulation process in a pilot flow reactor. The solution was recirculated in the reactor within the flow rate of 200 to 900 L/h. Mineralization efficiency of the process is indirectly proportional to the solution volume and directly proportional to the applied current. More than 61% of mineralization efficiency was observed after 1 h electrolysis of 30 L of 1000 mg/L aniline solution in the flow reactor with a recirculation rate of 900 L/h.
The authors (Brillas and Casado 2002) compared the performance of peroxicoagulation process with electro-Fenton process, concerning energy cost and treatment efficiency. Treatment efficiency was calculated as a ratio of experimental TOC removal with respect to theoretical TOC removal and expressed in percentage. The authors considered the requirement of 28 electrons for the complete mineralization of 1 mole of aniline for theoretical calculations. Treatment efficiency of both processes was very high at initial stages of electrolysis and decreased with electrolysis time. In all the cases, treatment efficiency of peroxicoagulation process is quite greater than that of electro-Fenton process.
Energy requirement of peroxicoagulation process is less than electro-Fenton process. For example, energy requirement for electro-Fenton process using Ti/Pt anode was 45 kWh/m3 while that of peroxicoagulation process was 28 kWh/m3, without any changes in mineralization efficiency. This indicates the higher oxidation power of peroxicoagulation process compared to electro-Fenton process. Another study by Brillas group (Brillas et al. 1997) reported that aniline mineralization efficiency of peroxicoagulation process is much higher than electro-Fenton and photo-electro-Fenton processes.
Stabilized landfill leachate
Stabilized landfill leachate is non-biodegradable wastewater and is hard to treat by conventional wastewater treatment processes. Venu et al. (2014) analyzed the performance of peroxicoagulation process on stabilized landfill leachate treatment. The authors found that performance of stainless steel is better than iron as an anode material, and the efficiency is very high at pH-regulated conditions (The experiments were carried out at pH 3).
Sludge generation rate in the presence of iron anode is greater than that in the presence of stainless steel anode (Venu et al. 2014). The amount of sludge generated by iron and stainless steel anodes was observed as 89 and 40 mL/L, respectively, after 120 min of electrolysis. This higher sludge generation in the presence of iron anode is due to the release of higher ferrous ions than that from stainless steel, and this higher ferrous concentration leads to pollutant removal via separation mechanism than via degradation process. This is the main reason behind higher sludge generation in the presence of iron anode. At the optimal operating conditions (pH 3, 10 V, inner electrode spacing of 4 cm, stainless steel anode and graphite cathode of area 25 cm2 each), peroxicoagulation process is capable of removing 93% of COD from stabilized landfill leachate.
External addition of 5.25 mM hydrogen peroxide also increased the performance of peroxicoagulation process significantly at the initial stages of electrolysis and a slight increase in final removal efficiency was noted (Venu et al. 2014). Leachate treatment efficiency of peroxicoagulation process is much higher than electro-Fenton and electrocoagulation process as reported by Venu et al. (2016). For example, soluble COD removal efficiencies of electrocoagulation, electro-Fenton and peroxicoagulation processes at pH 3 were found as 10, 67 and 84%, respectively.
Dyes
Synthetic dyes and effluents generated from textile industry create a lot of problem to the environment like reduction in light penetration to water bodies, deceleration of photosynthetic activity and biota growth inhibition, and are discussed in several articles (Gupta et al. 2013; Khandegar and Saroha 2013; Nidheesh et al. 2013).
Peroxicoagulation process is very efficient for the removal of synthetic dyes from water medium and can treat textile wastewater effectively (Zarei et al. 2009, 2010a, b; Ghanbari and Moradi 2015). Peroxicoagulation process was found effective to remove synthetic dyes like rhodamine B (Nidheesh and Gandhimathi 2014c), basic yellow 2 (Salari et al. 2009), basic blue 3 (Zarei et al. 2010b), malachite green (Zarei et al. 2010b) and basic red 46 (Zarei et al. 2010b) from water medium. Absolute dye removal increased with increase in initial dye concentration, even the removal efficiency of peroxicoagulation process decreased with increase in initial dye concentration (Nidheesh and Gandhimathi 2014c). After 180 min of electrolysis, the efficiency of peroxicoagulation process decreased from 95 to 40% with the increase in dye concentration from 10 to 50 mg/L.
Salari et al. (2009) used gas diffusion electrode and iron as cathode and anode for the in situ generation of hydrogen peroxide and ferrous ion and subsequent removal of basic yellow 2 from water medium. This system is efficient to remove 90% dye within 30 min and 81% TOC within 6 h of electrolysis. The authors compared the dye removal efficiency of peroxicoagulation process with that of electro-Fenton process and electrocoagulation process at pH 3 and found that the efficiency of peroxicoagulation process is significantly higher than that of other two electrolytic processes.
Zarei et al. (2009) checked the performance of peroxicoagulation process in the presence of carbon–polytetrafluoroethylene and carbon nanotube-polytetrafluoroethylene cathodes by considering basic yellow 2 as a model pollutant. The authors found that carbon nanotube–polytetrafluoroethylene electrode is more efficient than carbon–polytetrafluoroethylene electrode. Current efficiencies of carbon–polytetrafluoroethylene and carbon nanotube–polytetrafluoroethylene were observed as 16–45% and 40–61%, respectively. This higher current efficiency of carbon nanotube–polytetrafluoroethylene results in higher electrochemical hydrogen peroxide.
Based on the above results, Zarei et al. (2010b) used iron–carbon nanotube–polytetrafluoroethylene electrolytic system for the removal four dyes namely basic blue 3, malachite green, basic red 46 and basic yellow 2 at pH 3. The authors tested the efficiency of peroxicoagulation process for removing these dyes in single system and in mixed system. Removal of dye was very rapid in single system and more than 90% dye was removed within 10 min of electrolysis. This system is capable of removing 92% of TOC from single dye system after 6 h of electrolysis. To check the efficiency of peroxicoagulation process in mixed dye system, the authors considered 20 mg/L of basic yellow 2 and 5 mg/L each of other three dyes. Almost complete removal of dye was observed within 40 min of electrolysis, and 93% mineralization efficiency was noticed after 6 h of electrolysis.
Nidheesh and Gandhimathi (2014a) carried out textile wastewater treatment by peroxicoagulation process, pH-regulated peroxicoagulation and electro-Fenton processes. Electro-Fenton, peroxicoagulation and pH-regulated peroxicoagulation process are able to remove 97, 82 and 97% of color, and 64, 75 and 70% of COD, respectively. COD removal efficiency of peroxicoagulation process is higher than that of electro-Fenton and pH-regulated peroxicoagulation process. This enhanced pollutant removal efficiency of peroxicoagulation process is mainly attributed to the higher pollutant removal by electrocoagulation than electro-Fenton process. Solution pH during the electrolysis was increased from 3 to 6, within a short period. At this condition, electrocoagulation dominates electro-Fenton process as explained earlier.
With the regulation of pH, pollutant removal efficiency of peroxicoagulation process by degradation mechanism increases (Nidheesh and Gandhimathi 2014a). This was very clear with the amount of sludge produced from peroxicoagulation and pH-regulated peroxicoagulation processes. Sludge production in peroxicoagulation process and pH-regulated peroxicoagulation process operated at pH 3 was found as 280 and 134 mL/L, respectively. Sludge production in pH-regulated peroxicoagulation process is also indicates the electrocoagulation during the process and is the main reason behind higher COD removal of pH-regulated peroxicoagulation process than electro-Fenton process.
Higher color removal efficiencies of electro-Fenton and pH-regulated peroxicoagulation processes compared to peroxicoagulation process also indicates the degradation of dyes by the attack of hydroxyl radicals generated in the system (Nidheesh and Gandhimathi 2014a). Chromophores of dyes are attacked by hydroxyl radicals and results in degradation of dye molecules. This reaction results in substantial reduction of color from the wastewater contaminated with dyes.
Ghanbari and Moradi (2015) compared the performances of peroxicoagulation, electrocoagulation, electro-Fenton and electrochemical Fenton (Electrocoagulation process with external hydrogen peroxide addition) for the treatment of real textile wastewater. The authors observed more than 75% of color and more than 64% COD removal efficiencies for all the electrolytic processes with the efficiency of order electrochemical Fenton ≥ electrocoagulation > peroxicoagulation > electro-Fenton. Energy required for the processes were found as 2.73 kWh/kg COD, 3.38 kWh/kg COD, 63.64 kWh/kg COD and 23.19 kWh/kg COD; and iron consumption were found as 139.3, 139.3, 50.3 and 626.8 mg, respectively, for electrocoagulation, electrochemical Fenton, electro-Fenton and peroxicoagulation processes. Biochemical oxygen demand (BOD)/COD ratio of textile wastewater also increased from 0.137 to 0.178, 0.341, 0.363 and 0.317, respectively, for electrocoagulation, electrochemical Fenton, electro-Fenton and peroxicoagulation processes.
Herbicide
A variety of herbicides is using in worldwide for agricultural purposes, particularly for weed control. These organic compounds are non-biodegradable and are toxic to animal and humans (Rodrigo et al. 2014). The use of water containing herbicides results in poisoning of humans and other animals. Non-biodegradable nature of herbicides leads to bioaccumulation of these compounds and subsequent health problems. Thus, removal of these compounds from water medium is a primary option to reduce its toxicity.
Brillas et al. (2003a) tested the performance of peroxicoagulation process by considering chlorophenoxy and chlorobenzoic herbicides like 4-chlorophenoxyacetic acid, 4-chloro-2-methylphenoxyacetic acid, 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid and 3,6-dichloro-2-methoxybenzoic acid as model pollutants. Peroxicoagulation process was found to be very efficient for the removal of these herbicides from water medium at pH 3. The authors observed more than 90% of dissolved organic carbon removal after 6 h of electrolysis. Treatment efficiency of the process varied with target compounds and is in the range of 1.3–5.9. Based on the herbicide concentration in the water during electrolysis, the authors also reported the first-order constants for the pollutant removal. First-order rate for herbicide removal was varied between 0.14 and 0.41 min−1, and the maximum rate was observed in the presence of chlorobenzoic herbicide, 3,6-dichloro-2-methoxybenzoic acid.
The authors (Brillas et al. 2003a) also reported the coagulated and mineralized TOC removal percentages, based on their experiments. At lower applied current, coagulated and mineralized total organic removal percentages are almost same. But, increase in current increased the pollutant removal contribution of coagulation. This is exclusively well shown for the chlorobenzoic herbicide, 3,6-dichloro-2-methoxybenzoic acid. At 100 mA, TOC removal percentages of coagulation and mineralization are 48 and 46%, respectively. Further increase in current to 300 mA, resulted in the complete pollutant removal by coagulation.
To improve the performance of peroxicoagulation process, Brillas group conducted experiments on photo-peroxicoagulation process for the removal of herbicide 4-chloro-2-methylphenoxyacetic acid (Boye et al. 2003a), 4-chlorophenoxyacetic acid (Brillas et al. 2003b) and 2,4,5-trichlorophenoxyacetic acid (Boye et al. 2003b). The introduction of UV light in peroxicoagulation process increased its efficiency significantly by the additional hydroxyl radical generation by photo-Fenton reaction. Similar results are observed by Lizama-Bahena et al. (2015) for the removal of chlorinated herbicides like atrazine, alachlor, and chlorbromuron from water medium. Complete mineralization of these pollutants was occurred within 75 min of electrolysis and in the presence of UV light. At the same time, maximum mineralization efficiency for peroxicoagulation process was observed as 97% for atrazine with an initial concentration of 62 mg/L.
Conclusion
Peroxicoagulation process is a promising technology to remove non-biodegradable organic pollutants from water medium. The process has high mineralization efficiency. Most of the organic pollutants which are not removable by the conventional process are removed easily by peroxicoagulation process. Contaminants are removed from aqueous solution by degradation (Fenton reactions) and by separation (coagulation). Apart from its higher efficiency, peroxicoagulation process received much attention due to its simplicity, absence of external chemical addition and in situ generation of hydrogen peroxide and iron.
Even though the process is highly efficient to remove the contaminants, their real-field implementation is restricted due to the passivation of electrodes. The rate of iron electrode passivation is higher in real-field conditions compared to laboratory conditions (van Genuchten et al. 2016). Formation of iron oxides over the electrodes retards further electrolytic generation of ferrous ions in the water medium. This will also affect the Fenton and coagulation reactions.
Similar way, carbonaceous electrodes, frequently used as the cathode for electrolytic generation of hydrogen peroxide also undergoes passivation. Most of the ions and salt will deposit in the pores of these electrodes and retards the hydrogen peroxide production. Nidheesh and Gandhimathi (2014c) observed this during the peroxicoagulation treatment of rhodamine B dye. The authors observed a yellowish color iron hydroxide and a white layer deposition of sodium salt over graphite electrode after the treatment. This passivation problem can be rectified quickly by regular cleaning of electrodes.
Increase in pH with an increase in electrolysis time is another problem of peroxicoagulation process. This solution pH increase reduces mineralization efficiency of the process, while coagulation efficiency increases. Thus, after some time of electrolysis, most of the pollutants are removed by coagulation, not by Fenton reactions. This can be rectified by reducing the solution pH to 3 at regular intervals.
Increase in applied current enhances the efficiency of the process significantly, as observed by various researchers. But, the increase in applied current is not the right way to improve the effectiveness of peroxicoagulation process. Increase in applied current increase the iron release from the anode. This results in rapid increase in solution pH with electrolysis time. At this condition, coagulation is the dominant pollutant removal mechanism than Fenton reaction. This problem can be solved by conducting the reactions at lower applied current. The lower current regulated the iron release from the anode and retards the rate of pH increase. Thus, most of the pollutants are removed by degradation method.
References
Boye B, Brillas E, Dieng MM (2003a) Electrochemical degradation of the herbicide 4-chloro-2-methylphenoxyacetic acid in aqueous medium by peroxi-coagulation and photoperoxi-coagulation. J Electroanal Chem 540:25–34. https://doi.org/10.1016/S0022-0728(02)01271-8
Boye B, Marième Dieng M, Brillas E (2003b) Electrochemical degradation of 2,4,5-trichlorophenoxyacetic acid in aqueous medium by peroxi-coagulation. Effect of pH and UV light. Electrochim Acta 48:781–790. https://doi.org/10.1016/S0013-4686(02)00747-8
Brillas E, Casado J (2002) Aniline degradation by Electro-Fenton and peroxi-coagulation processes using a flow reactor for wastewater treatment peroxi-coagulation processes using a flow. Chemosphere 47:241–248. https://doi.org/10.1016/S0045-6535(01)00221-1
Brillas E, Mur E, Casado J (1996) Iron(II) catalysis of the mineralization of aniline using a carbon-PTFE O2–fed cathode. J Electrochem Soc 143:L49–L53. https://doi.org/10.1149/1.1836528
Brillas E, Sauleda R, Casado J (1997) Peroxi-coagulation of aniline in acidic medium using an oxygen diffusion cathode. J Electrochem Soc 144:2374–2379. https://doi.org/10.1149/1.1837821
Brillas E, Calpe JC, Casado J (2000) Mineralization of 2,4-D by advanced electrochemical oxidation processes. Water Res 34:2253–2262. https://doi.org/10.1016/S0043-1354(99)00396-6
Brillas E, Boye B, Baños MÁ et al (2003a) Electrochemical degradation of chlorophenoxy and chlorobenzoic herbicides in acidic aqueous medium by the peroxi-coagulation method. Chemosphere 51:227–235. https://doi.org/10.1016/S0045-6535(02)00836-6
Brillas E, Boye B, Dieng MM (2003b) Peroxi-coagulation and photoperoxi-coagulation treatments of the herbicide 4-chlorophenoxyacetic acid in aqueous medium using an oxygen-diffusion cathode. J Electrochem Soc 150:E148–E154. https://doi.org/10.1149/1.1543950
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631. https://doi.org/10.1021/cr900136g
Dirany A, Efremova Aaron S, Oturan N et al (2011) Study of the toxicity of sulfamethoxazole and its degradation products in water by a bioluminescence method during application of the electro-Fenton treatment. Anal Bioanal Chem 400:353–360. https://doi.org/10.1007/s00216-010-4441-x
El-Desoky HS, Ghoneim MM, El-Sheikh R, Zidan NM (2010) Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton’s reagent. J Hazard Mater 175:858–865. https://doi.org/10.1016/j.jhazmat.2009.10.089
George SJ, Gandhimathi R, Nidheesh PV, Ramesh ST (2014) Electro-fenton oxidation of salicylic acid from aqueous solution: batch studies and degradation pathway. Clean Soil Air Water 42:1701–1711. https://doi.org/10.1002/clen.201300453
Ghanbari F, Moradi M (2015) A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: electrical energy consumption and biodegradability improvement. J Environ Chem Eng 3:499–506. https://doi.org/10.1016/j.jece.2014.12.018
Gupta VK, Kumar R, Nayak A et al (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interface Sci 193–194:24–34. https://doi.org/10.1016/j.cis.2013.03.003
Jinisha R, Gandhimathi R, Ramesh ST et al (2018) Removal of rhodamine B dye from aqueous solution by electro-Fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 200:446–454. https://doi.org/10.1016/j.chemosphere.2018.02.117
Khandegar V, Saroha AK (2013) Electrocoagulation for the treatment of textile industry effluent—a review. J Environ Manage 128:949–963. https://doi.org/10.1016/j.jenvman.2013.06.043
Le TXH, Bechelany M, Lacour S et al (2015) High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon N Y 94:1003–1011. https://doi.org/10.1016/j.carbon.2015.07.086
Lizama-Bahena C, Álvarez-Gallegos A, Hernandez JA, Silva-Martinez S (2015) Elimination of bio-refractory chlorinated herbicides like atrazine, alachlor, and chlorbromuron from aqueous effluents by Fenton, electro-Fenton, and peroxi-coagulation methods. Desalin Water Treat 55:3683–3693. https://doi.org/10.1080/19443994.2014.939858
Magario I, García Einschlag FS, Rueda EH et al (2012) Mechanisms of radical generation in the removal of phenol derivatives and pigments using different Fe-based catalytic systems. J Mol Catal A Chem 352:1–20. https://doi.org/10.1016/j.molcata.2011.10.006
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50. https://doi.org/10.1016/S0304-3894(02)00282-0
Nidheesh PV (2015) Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. RSC Adv 5:40552–40577. https://doi.org/10.1039/C5RA02023A
Nidheesh PV, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15. https://doi.org/10.1016/j.desal.2012.05.011
Nidheesh PV, Gandhimathi R (2014a) Effect of solution pH on the performance of three electrolytic advanced oxidation processes for the treatment of textile wastewater and sludge characteristics. RSC Adv 4:27946–27954. https://doi.org/10.1039/C4RA02958E
Nidheesh PV, Gandhimathi R (2014b) Comparative removal of Rhodamine B from aqueous solution by electro-Fenton and electro-Fenton-like processes. Clean Soil Air Water 42:779–784. https://doi.org/10.1002/clen.201300093
Nidheesh PV, Gandhimathi R (2014c) Electrolytic removal of Rhodamine B from aqueous solution by peroxicoagulation process. Environ Sci Pollut Res 21:8585–8594. https://doi.org/10.1007/s11356-014-2775-1
Nidheesh PV, Gandhimathi R (2015a) Textile wastewater treatment by electro-fenton process in batch and continuous modes. J Hazard Toxic Radioact Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000254
Nidheesh PV, Gandhimathi R (2015b) Electro Fenton oxidation for the removal of Rhodamine B from aqueous solution in a bubble column reactor under continuous mode. Desalin Water Treat 55:263–271. https://doi.org/10.1080/19443994.2014.913266
Nidheesh PV, Gandhimathi R, Ramesh ST (2013) Degradation of dyes from aqueous solution by Fenton processes: a review. Environ Sci Pollut Res 20:2099–2132. https://doi.org/10.1007/s11356-012-1385-z
Nidheesh PV, Gandhimathi R, Sanjini NS (2014) NaHCO3 enhanced Rhodamine B removal from aqueous solution by graphite-graphite electro Fenton system. Sep Purif Technol 132:568–573. https://doi.org/10.1016/j.seppur.2014.06.009
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227. https://doi.org/10.1016/j.chemosphere.2017.12.195
Olvera-Vargas H, Cocerva T, Oturan N et al (2016a) Bioelectro-Fenton: a sustainable integrated process for removal of organic pollutants from water: application to mineralization of metoprolol. J Hazard Mater 319:13–23. https://doi.org/10.1016/j.jhazmat.2015.12.010
Olvera-Vargas H, Oturan N, Buisson D, Oturan MA (2016b) A coupled Bio-EF process for mineralization of the pharmaceuticals furosemide and ranitidine: feasibility assessment. Chemosphere 155:606–613. https://doi.org/10.1016/j.chemosphere.2016.04.091
Oturan MA (2000) Ecologically effective water treatment technique using electrochemically generated hydroxyl radicals for in situ destruction of organic pollutants: application to herbicide 2,4-D. J Appl Electrochem 30:475–482. https://doi.org/10.1023/A:1003994428571
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. a review. Crit Rev Environ Sci Technol 44:2577–2641. https://doi.org/10.1080/10643389.2013.829765
Oturan MA, Pinson J (1995) Hydroxylation by electrochemically generated OH radicals. Mono- and polyhydroxylation of benzoic acid : products and isomers™ distribution. J Phys Chem 99:13948–13954. https://doi.org/10.1021/j100038a029
Oturan MA, Peiroten J, Chartrin P, Acher AJ (2000) Complete destruction of p-Nitrophenol in aqueous medium by electro-Fenton method. Environ Sci Technol 34:3474–3479. https://doi.org/10.1021/es990901b
Oturan MA, Pimentel M, Oturan N, Sirés I (2008) Reaction sequence for the mineralization of the short-chain carboxylic acids usually formed upon cleavage of aromatics during electrochemical Fenton treatment. Electrochim Acta 54:173–182. https://doi.org/10.1016/j.electacta.2008.08.012
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84. https://doi.org/10.1080/10643380500326564
Rodrigo MA, Oturan MA, Oturan N (2014) Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev 114:8720–8745. https://doi.org/10.1021/cr500077e
Roshini PS, Gandhimathi R, Ramesh ST, Nidheesh PV (2017) Combined electro-Fenton and biological processes for the treatment of industrial textile effluent: mineralization and toxicity analysis. J Hazard Toxic Radioact Waste 21:4017016. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000370
Salari D, Niaei A, Khataee A, Zarei M (2009) Electrochemical treatment of dye solution containing C.I. Basic yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks. J Electroanal Chem 629:117–125. https://doi.org/10.1016/j.jelechem.2009.02.002
van Genuchten CM, Bandaru SRS, Surorova E et al (2016) Formation of macroscopic surface layers on Fe(0) electrocoagulation electrodes during an extended field trial of arsenic treatment. Chemosphere 153:270–279. https://doi.org/10.1016/j.chemosphere.2016.03.027
Venu D, Gandhimathi R, Nidheesh PV, Ramesh ST (2014) Treatment of stabilized landfill leachate using peroxicoagulation process. Sep Purif Technol 129:64–70. https://doi.org/10.1016/j.seppur.2014.03.026
Venu D, Gandhimathi R, Nidheesh PV, Ramesh ST (2016) Effect of solution pH on leachate treatment mechanism of peroxicoagulation process. J Hazard Toxic Radioact Waste 20:4–7. https://doi.org/10.1061/ASCE)HZ.2153-5515.0000315
Zarei M, Salari D, Niaei A, Khataee A (2009) Peroxi-coagulation degradation of C.I. Basic yellow 2 based on carbon-PTFE and carbon nanotube-PTFE electrodes as cathode. Electrochim Acta 54:6651–6660. https://doi.org/10.1016/j.electacta.2009.06.060
Zarei M, Niaei A, Salari D, Khataee A (2010a) Application of response surface methodology for optimization of peroxi-coagulation of textile dye solution using carbon nanotube-PTFE cathode. J Hazard Mater 173:544–551. https://doi.org/10.1016/j.jhazmat.2009.08.120
Zarei M, Niaei A, Salari D, Khataee AR (2010b) Removal of four dyes from aqueous medium by the peroxi-coagulation method using carbon nanotube-PTFE cathode and neural network modeling. J Electroanal Chem 639:167–174. https://doi.org/10.1016/j.jelechem.2009.12.005
Acknowledgements
The author is thankful to the Director, CSIR-NEERI, Nagpur, India, for providing encouragement, and kind permission for publishing the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nidheesh, P.V. Removal of organic pollutants by peroxicoagulation. Environ Chem Lett 16, 1283–1292 (2018). https://doi.org/10.1007/s10311-018-0752-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0752-5