Abstract
The preparation of vanillin from lignin is one of the lignin valorization strategies. However, obtaining high vanillin yield is still a challenge. Therefore, the process of vanillin production and factors that affect yield of vanillin has attracted much attention. Here, oxidation of vanillin was performed to study its degradation behavior under lignin alkaline oxidation conditions. High-performance liquid chromatography, liquid chromatography–electrospray mass spectrometry, gas chromatography–mass spectrometer and gel permeation chromatography were employed to analyze the products including monomers and dimers. Results demonstrated that reaction temperature and time greatly affected vanillin degradation; vanillin can be completely converted in 5 h at 160 °C. At 160 °C, the main products of vanillin oxidation were small molecule acids and alcohols, other monophenols, and even condensed dimers. A possible vanillin degradation pathway was proposed. The results indicate that vanillin degradation and condensation are the main reasons for decreasing vanillin yield during lignin valorization under alkaline oxidation circumstances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulose, as a renewable carbon resource for fuel, chemicals, and materials, is gaining more attention considering environmental concern and shorten of fossil resources. Chemo- and bio-catalysis technologies for (hemi-)cellulose conversion were well developed. However, lignin utilization is still a big challenge. Though some lignin conversion methods (such as hydrogenolysis [1,2,3], oxidation [4,5,6,7], bioengineering) have been developed, these methods are still in their early stages. Consequently, the recent focus of biomass conversion is lignin valorization, which not only broadens the raw material resource but also increases economic efficiency of current biorefinery industry [8,9,10,11,12]. Lignin is an aromatics polymer, which is an idea source for producing value-added chemicals like aromatics [8, 13, 14].
Vanillin (3-methoxy-4-hydroxy benzaldehyde) is an important aroma chemical produced worldwide and widely used as flavoring agents for chocolate and ice cream, intermediate of drugs like Aldomet, l-Dopa Trimethoprim; ripeners and sunscreens, even catalysts [15,16,17]. Total annual vanillin production is about 20,000 tons, 15% came from lignin, 85% came from petroleum-based raw material guaiacol, and less than 1% was extracted from vanilla beans [15, 18, 19]. Given its sustainability, lignin has a great potential to become the primary source for vanillin in the future, compared to the exhausted petroleum resource.
Although vanillin has high economic value, the yield of vanillin from lignin is still much lower than its theoretical level. To find out the reasons for the limited yield of vanillin, effects of lignin sources [20], structures [21], pretreatment [22], catalyst [4, 23, 24], reaction conditions on lignin conversion to vanillin have been extensively studied [13]. For instance, Rodrigues et al. investigated the effect of some lignin features (including wood species, pulping process, and lignin isolation process) on its oxidative conversion to high-added-value phenolic aldehydes. The frequency of phenolic hydroxyl groups in noncondensed structures was declaimed to be one possible limiting factor of phenol yield [25]. Wu et al. used steam-explosion hardwood lignin to produce aldehydes (mainly vanillin, syringaldehyde, and hydroxybenzaldehyde) under alkaline conditions. Monophenol yields significantly increased when CuSO4 and FeCl3 were employed as catalysts. Consequently, 14.6 wt% of lignin was converted to three aldehydes with vanillin yield of 4.6 wt% [26]. Recently, the oxidative degradation of monophenols has become new research hotspot. Rodrigues and coworkers systematically investigated the kinetics of vanillin oxidation and found that vanillin oxidation is a first-order reaction under high-alkalinity conditions (pH > 12) [27]. Sultanov et al. studied the degradation of a variety of guaiacyl and syringyl lignin model compounds under alkaline oxidative conditions (1 M KOH, 0.1 MPa O2, 70 °C), and compared the activity difference between guaiacyl and syringyl model compounds so as reactivity among various 4-substituted syringols with different substituents. They found that vanillin and vanillic acid were stable under alkali oxidation conditions. Although oxidization of vanillin under more severe conditions (1.25 M NaOH, 150 °C, 1 MPa O2) was conducted, and a mixture of carboxylic acids and hydroxyl acids were obtained, nevertheless specific structural information of degradation products was not given [28]. TiO2 photocatalytic oxidation of vanillin can produce ring open intermediates like formic acid, acetic acid, and oxalic acid. Trans-ferulic acid can also open rings to produce these intermediates in photocatalytic oxidation [29]. Vanillin can also be selectively oxidized into vanillic acid under the promotion of gold nanoparticles supported on alumina and titania in alkaline aqueous media at 80 °C in the presence of pressurized oxygen, which resulted in selectivity up to 99% at conversions over 90% [16]. So, degradation of vanillin under aerobic alkaline conditions may be a key factors for the low yield of vanillin.
Here, we prepared vanillin derived from pine wood oxidation in alkaline media and investigated the degradation of vanillin under the same alkaline oxidative conditions. HSQC, GC–MS, LC–MS, and LC analysis were employed to analyze the products including dimers. We found that vanillin was not simply converted to small molecules of acids and alcohols by further oxidation but also to dimers via condensation. On the basis of our findings, a possible degradation mechanism of vanillin under alkaline oxidation conditions was proposed. This work led us to clarify limited vanillin yield during lignin alkaline oxidation from a new perspective, i.e., degradation of vanillin.
2 Methods
2.1 Materials
Pine wood was produced in South China (lignin, 23%). High purity O2 (99.99%) was obtained from Yi gas Gases Co. ltd (Guangdong, China). NaOH, chloroform, and tetrahydrofuran (THF) were purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Standards for vanillin (99%), p-hydroxybenzaldehyde (99%), vanillic acid (98%), acetic acid (98%), formic acid (98%), oxalic acid (99.9%), methanol (99.5%), and ethanol (99.5%) were also obtained from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). All chemicals were analytical reagents and used without purification.
2.2 Alkaline Oxidation
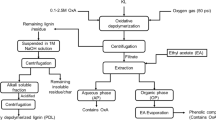
Batch experiments were performed in a 100-ml stainless-steel autoclave reactor equipped with a thermocouple and a magnetic coupling mechanical stirring rod. In a typical experiment, the reactor was loaded with 50 ml NaOH aqueous solution and 1 g pine or 0.2 g vanillin, and was sealed. Then, the reactor was purged three times and pressurized with O2 to 1 MPa. During the reaction, the reactor was stirred at a predefined speed. A heating procedure preceded as follows: first, heating from room temperature (about 30 °C) to target temperature with heating rate of 2.5 °C/min; then, heating at the target temperature for a fixed time. After the reaction, the reactor was cooled in air for about 3 h, and then was opened to collect the reaction solution.
Ten milliliters of the reaction solution was acidified to pH 2–3 with 0.5 ml concentrated hydrochloric acid (35%). Acidified liquid was diluted with 1.5 ml of deionized water, then was filtered (over a 0.45-µm Teflon filtrate pad) for quantitative analysis of formic acid, acetic acid, methanol, and ethanol by HPLC with a SH1011 column.
The acidified liquid was distilled to remove water, small acids, and HCl under vacuum at 40 °C, and then 5 ml of acetonitrile was added to dissolve the solid products; 2.5 ml acetonitrile phase was mixed with 5 ml of water (containing 0.5 g/l acetophenone) and filtered (over a 0.45-µm Teflon filtrate pad) for quantitative analysis of aromatics by HPLC with a C18 column. The rest 2.5 ml acetonitrile phase was distilled to remove solvent, and then dissolved in 2.5 ml of deionized water. This solution was applied to quantitative analysis of heavy acid (oxalic acid) by HPLC with a SH1011 column.
Ten milliliters of acidified liquid was extracted with THF until the THF layer appeared colorless. All the THF extractions were collected together. THF phase was added with a small amount of NaHCO3 to neutralize the residual acid from acidification, and then added with anhydrous Na2SO4 to absorb the residual water in the solution. The resulting anhydrous THF phase was concentrated to 2 ml and filtrated over a 0.45-µm Teflon filtrate pad.
2.3 Analysis Methods
2.3.1 High-Performance Liquid Chromatography (HPLC)
The HPLC test was performed on an HPLC instrument (WATERs e2695) equipped with a 2489 UV–Vis detector and 2414 RI Detector. Agilent ZORBAX Eclipse XDB-C18 column (4.6 mm × 150 mm, 5 μm) was used to quantitative analysis of aromatics. Eluent, 20% acetonitrile (containing 0.1% trifluoroacetic acid) and 80% water (containing 0.1% trifluoroacetic acid); flow rate, 1 ml/min; injection volume, 10 μl; detection temperature, 30 °C; wave length, 260 nm; retention time, 20 min. To determine small acids and alcohols, the HPLC test was performed by using a Shodex SUGAR SH1011 (8 mm × 300 mm, 6 μm) column at 30 °C. The eluent was 5 mmol/l H2SO4 aqueous solutions with flow rate of 0.5 ml/min; injection volume was 10 μl, and retention time was 35 min.
2.3.2 Liquid Chromatography–Electrospray Mass Spectrometry (LC–MS)
LC–MS test was preceded at Agilent 1290–6540, 1260–6420 LC–MS system using a C18 column at 30 °C. Eluent A was acetonitrile, and eluent B was water (0.1% ammonium acetate). Separation was achieved with 20% A/80% B at 0–3 min, 60% A/40% B at 3–10 min, 100% A at 10–15 min. Flow rate was 0.1 ml/min and injection volume was 10 μl.
2.3.3 Gel Permeation Chromatography (GPC)
GPC analysis was performed on an Agilent 1260 LC system equipped with a refractive index detector (RID) by using PL gel mixed-C column (7.5 mm × 300 mm, 5 μm) at 35 °C. THF was used as eluent at a flow rate of 1 ml∙min−1. The sample injection volume was 20 µl.
2.3.4 Gas Chromatography–Mass Spectrometer (GC–MS)
Before GC–MS analysis, a derivatization step was added to increase volatility of vanillin oxidation products. The derivatization experiment was conducted according to Ref. [1]. Briefly, 5-ml THF extractions were dried by vacuum distillation at 35 °C, then mixed with 0.5 ml of pyridine and 0.5 ml of N-methyl-N-(trimethylsilyl)trifluoroacetamide and sealed. The mixture was put in an oven preheated to 80 °C and heated for 30 min. For qualitative analysis, derivatives were analyzed by TRACE 1300ISQ GC/MS (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a TG-5MS column. The following operating conditions were used: injector and detector temperatures were 250 and 280 °C, respectively. The temperature program was set as follows: 60 °C, 2 min; 60–250 °C, heating rate 10 °C∙min−1; 250 °C, 15 min.
2.3.5 Heteronuclear Single Quantum Coherence (2D HSQC NMR)
To investigate the alkaline oxidation performance of vanillin, 2D HSQC NMR spectrum of THF extracts was used to record structural information of heavy products. 2D HSQC NMR analysis was run on a BRUKER AVANCE III 400-MHz spectrometer. Around 100 mg of sample was dissolved in 1.5 ml deuterated dimethyl sulfoxide (DMSO-d6). The parameters of collecting and processing are listed as follows: spectral frequency, 400.15 MHz for f1 (13C), 100.61 MHz for f2 (1H); spectral width, 22,137.686 Hz for f1 dimension, 4807.692 Hz for f2 dimension; number of the collected complex points for f2 dimension was 2048 with a recycle delay of 2 s; number of scan for f1 dimension was 72 with 256 time increments. The 1JCH used was 145 Hz. The solvent DMSO-d6 peak (δC/δH 39.5/2.49 ppm) was used as an internal reference. MestReNova software (9.0.1 version) was used for the spectra analysis.
2.3.6 Calculation Formulas
For the alkaline oxidation of lignin, yields of monophenol (Ymonophenol) and small acids and alcohols (Ysmall molecule), selectivity of vanillin [30] and monophenol theoretical yield (Ytheoretical) were calculated by Eqs. (1)–(4), respectively. Yields of small molecules were calculated on basis of pine wood weight (Eq. 2), since acids and alcohols can both from oxidation of lignin and (hemi-)cellulose.
Wmonophenol: the weight of monophenol; WPine: the weight of pine wood; Ysmall molecule: the weight of small molecule; Wv: the weight of vanillin; W(total monophenols): the weight of total monophenols. P (C–O–C): C–O–O linkages (including β-O-4, α-O-4, 4-O-5) occurrence probability in lignin structure. According to Refs. [13, 25], C–O–O linkages occurrence probability softwood lignin are: 43–50% (β-O-4), 6–8% (α-O-4), 4% (4-O-5). Thus, highest P (C–O–C) is up to 62% in total, so Ytheoretical of softwood can reach 38%.
For the alkaline oxidation of vanillin, the vanillin conversion (Cv), mole yields of products (Yproduct) and carbon balance (CB) were calculated by Eqs. (5)–(7), respectively.
Ymonophenol: the weight of monophenol; Wv: the weight of initial vanillin. Wv1: the weight of remained vanillin; nproduct: the moles of product; X: carbon numbers of product formula; nv: the moles of initial vanillin.
3 Results and Discussion
3.1 Alkaline Oxidation of Pine Lignin
In order to study the evolution of real vanillin under lignin alkaline oxidation conditions, a set of experiments of oxidative depolymerization of pine wood under different reaction temperatures and times were conducted. According to the GC–MS spectrum (Figure S1), oxidation products involve monophenols, carboxylic acids, alcohols, and hydroxyl acid, while small molecules such as formic acid, acetic acid, methanol, and ethanol were removed by the vacuum distillation and can be detected by HPLC. Monophenols include vanillin, p-hydroxybenzaldehyde, p-hydroxyacetophenone, vanillin acid, acetovanillone, ferulic acid, benzyl alcohol, and 4-ethylphenol. Vanillin is the major monophenol (selectivity ≥ 70%) owing to lignin in softwood (pine) mainly consisting of guaiacyl unit (type-G lignin), which can be converted into vanillin by a well-recognized mechanism of retro-aldol condensation [21, 31, 32].
As shown in Table 1, when the reaction time is 1 h, yields of vanillin and other monophenols increased from 120 to 160 °C and then decreased over 160 °C. The highest yield of vanillin (21%) was obtained at 160 °C, which was twice the yield of 120 °C; however, yields of small molecule compounds increased from 120 to 160 °C (Table 1, entries 1–5). At 160 °C, vanillin yield reached 12.9% in 10 min and increased to 21.1% when reaction time prolonged to 1 h, then reduced to 15.5% after reaction for 180 min. Meanwhile, the increase of reaction time led to an increase of yields of small molecule products (Table 1, entries 3, 7–9).
Type-G lignin-rich softwood (pine) was used as a raw material in alkaline oxidation experiments, but vanillin yield obtained (21%) was much lower than the theoretical value (38%, calculated on basis of ester linkages content [3]). Clarify side reactions and byproducts are of great importance for answering the question of why vanillin yield is low. Here, in addition to monophenols, by-products are small molecules, long-chain aliphatic alcohol, as well as large molecular weight lignin fragments (not listed in the table). Given that oxidation of cellulose and hemicellulose also give carboxylic acid and alcohols, analysis is very difficult in the real biomass system. Therefore, degradation of pure vanillin under alkaline oxidation conditions will be discussed below.
3.2 Alkaline Oxidation of Vanillin
According to a previous study, reaction temperature and time greatly affect vanillin yield. In order to figure out the effect of reaction temperature and time, what happened to vanillin during lignin valorization need to be clear. GC–MS analysis showed that oxidation products of vanillin are similar to the by-products of real lignin system, i.e., monophenols, carboxylic acids, alcohols, and hydroxyl acids (Figure S2). Vanillin is unstable under alkaline aerobic conditions and it will be oxidative depolymerized and re-polymerized. The conversion of vanillin and distribution of products is greatly influenced by reaction conditions. Here, degradation of vanillin under different reaction conditions will be discussed in detail.
3.2.1 Temperature
Under aerobic alkaline condition (7.5 wt% NaOH, 1 MPa O2, 400 rpm, 30 min), an increase of temperature from 50 to 160 °C resulted in vanillin conversion increased from 11.0 to 73.9% (Table 2, entries 1–3). The molar yield of acid products was higher than that of alcohols because alcohols can be further oxidized to carboxylic acids. A small amount of formic acids (1.1 mol%) and acetic acid (3.6 mol%) can be generated at 50 °C (Table 2, entry 1). Oxidative cleavage of the methoxyl group on vanillin resulted in the formation of formic acid and p-hydroxybenzaldehyde, while the benzene ring-opening reaction was attributed to the generation of both formic acid and acetic acid [29]. As the reaction temperature increased to 100 °C, trace vanillic acid (0.1 mol%) was detected (Table 2, entry 2). The formation of vanillic acid resulted from the selectivity oxidation of vanillin under high pH conditions [16]. A small amount of oxalic acid (0.7 mol%) and methanol (1.9 mol%) were formed, except formic and acetic acid. Oxalic acid may be derivatives from ring-opening of vanillin or vanillic acid [27, 29]. Vanillin decomposed significantly when the reaction temperature increased to 160 °C, with high vanillin conversion (73.9%) and total yields of identified products increased to 38.2 mol% (Table 2, entry 3). In addition, vanillic acid yield was only slightly increased because a portion of vanillic acid is prone to degrade into small molecule acids and alcohols and other substances at high temperatures (Table 2, entry 3). In Figure S2, a large number of methyl 2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)acetate (16.96 min) accompanied by some trace monophenols with carbon number over 8 (14.46, 14.79, 18.05, 19.60, 23.37, 24.52 min) and hydroxybenzaldehyde (11.67 min) were generated. p-Hydroxybenzaldehyde probably resulted from methoxylation of vanillin, while other monophenols might be the products of reaction between vanillin and small acids or alcohols. Especially, acetovanillone, p-hydroxybenzaldehyde, and ferulic acid generated in the vanillin oxidation system can also be observed in the alkaline oxidation of lignin (Figure S1), which means the production of these chemicals in lignin oxidation may come from both lignin itself and vanillin further oxidation. The formation of these monophenols implies that vanillin in the lignin oxidation system might convert to other monophenols and thus leaded to a decrease of vanillin selectivity. In addition, further degradation of monophenols by ring-opening cause the appearance of trace long-chain acids and esters.
Moreover, oxidation of vanillic acid was carried out to confirm whether small molecule products were generated from vanillic acid. All small molecule products were produced and acetic acid was the main product, indicating that partial degradation of vanillin resulted in the formation of vanillic acid and followed by decomposition of vanillic acid. It is noteworthy that oxidation of vanillic acid gave similar long-chain acids and esters with oxidation of vanillin (Figure S3). This finding leads us to conclude that vanillic acid is a crucial intermediate.
Obviously, most of the vanillin decomposed at 160 °C in only 30 min; nevertheless, alkaline oxidation of lignin to vanillin gave highest yield after reaction for 1 h, the loss of vanillin could be considerable.
3.2.2 Time
In order to study the effects of reaction time on vanillin degradation, several experiments were conducted under temperature of 160 °C with reaction time differed from 10 min to 5 h (7.5 wt% NaOH, 1 MPa O2, 400 rpm) (Table 2, entries 3–6). Vanillin conversion was 63.6 and 20.3% of vanillin was converted to small molecules of acids and alcohols in only 10 min (Table 2, entry 4), indicating that vanillin was very unstable and ring-opening reaction of vanillin could occur under high temperature [29]. After 30 min, yields of small molecule products increased to 37.7 mol%; oxalic acid yield was tripled compared to that of initial amount (2.9 mol%) (Table 2, entry 3). As the reaction time prolonged to 5 h, vanillin was almost completely degraded, long-chain acids and esters remained in the system (Figure S4); total yield of small molecule products significantly increased to 63.1 mol%, especially for formic acid and acetic acid, while yields of oxalic acid and methanol barely changed (Table 2, entries 5–6). Above results implying that vanillin is very unstable at relatively harsh condition, which could be totally converted with extend of reaction time.
3.2.3 Stirring Speed, O2 Pressure, and Alkali Concentration
Except for temperature and time, stirring speed, O2 pressure, and alkali concentration can also affect the conversion of vanillin. Reaction conditions of 7.5 wt% NaOH, 1 MPa O2, 400 rpm, 160 °C, and 30 min was chosen as contrast reaction (Table 2, entry 3). When increasing the stirring speed to 1100 rpm, conversion of vanillin was decreased from 73.9 to 42.4%; however, yields of vanillic acid and small molecule products had no noteworthy change (Table 2, entries 3 and 7). Higher O2 pressure (2 MPa) resulted in slightly increased vanillin conversion but achieved different product distribution, i.e., more formic acid and acetic acid while less oxalic acid (Table 2, entries 3 and 8). The sharp decrease of oxalic acid yield may be caused by the excessive oxidative degradation of oxalic acid by excess O2. Meanwhile, vanillic acid yield dropped to zero, probably due to the fact that vanillic acid is more unstable under harsh reaction conditions, and degraded into more stable small molecule acids and alcohols. When changing the alkali concentration from 7.5 to 15 wt%, vanillin conversion significantly decreased to 38.3% and was accompanied by an increase in vanillic acid yield (from 0.5 to 1.4 mol%), mainly due to the fact that high alkali concentrations can protect the vanillin from decomposed to small molecules but accelerate the oxidation of vanillin to vanillic acid [16, 29, 33,34,35,36,37].
3.2.4 Catalyst
To obtain higher vanillin, metal oxide catalysts are widely used to selective oxidation of lignin to vanillin [5, 15, 24, 38]. Thus, stability of vanillin under aerobic alkaline conditions in the presence of catalyst is also investigated. The employment of CuO led to a significant increase of oxalic acid and methanol yields. It is likely that the presence of CuO accelerated the ring-opening reaction of vanillin and inhibited the degradation of oxalic acid (Table 2, entry 10). Extending the reaction time to 10 h, yields of formic acid and methanol increased while the yields of acetic acid and oxalic acid decreased; ultimately yields of the four products were considerable (Table 2, entry 11). More long-chain acids and esters were generated (Figure S5).
3.3 Analysis of Unknown Products
Under severe reaction conditions, vanillin degradation not only included oxidation and ring-opening reactions in the alkali oxidation system, but also involved a complex polymerization reaction that generated large molecular weight products. Structural information of these unknown products needs to be confirmed. According to Table 2, vanillin mainly degraded to small acids and alcohols along with a small amount of vanillic acid. Vanillin was almost converted after 5 h, but mole carbon balance was only 64.1 mol%, the other 35.9 mol% was still unclear (Table 2, entry 6). Similarly, more or less unknown products existed in other reactions (Table 2). Yield of the known product was less than the conversion of the starting material, probably due to that the small molecule products in the system degraded to CO2 and water or formed macromolecular products that are difficult to detect. CO2 was not detected in the waste gas because CO2 molecules generated under alkaline conditions were fixed in the solution in the form of CO32−, which cannot enter the gas phase. To verify the formation of macromolecules, GPC analysis of heavy products was performed and the results are presented in Fig. 1.
Molecular weight distribution of heavy products. Conditions: (entries 1–3) 0.2 g vanillin, 7.5 wt% NaOH, 1 MPa O2, 400 rpm, 30 min. (Entries 4–6) 0.2 g vanillin, 7.5 wt% NaOH, 1 MPa O2, 160 °C, 400 rpm. (Entries 7) 0.2 g vanillin, 7.5 wt% NaOH, 1 MPa O2, 160 °C, 30 min. (Entry 8) 0.2 g vanillin, 50 ml 7.5 wt% NaOH, 400 rpm, 160 °C, 30 min. (Entries 9) 0.2 g vanillin, 1 MPa O2, 160 °C, 400 rpm, 30 min. (Entries 10 and 11) 0.2 g vanillin, 7.5 wt% NaOH, 1 MPa O2, 160 °C, 400 rpm. (Entry 12) 0.2 g vanillic acid, 50 ml 7.5 wt% NaOH, 1 MPa O2, 160 º C, 400 rpm, 30 min
No large molecular weight products were generated at a low temperature of 50 °C, while the remaining conditions gave large molecular weight products (Fig. 1, Table 3). In a short reaction time of 30 min, condensation aggravated with the increase of reaction temperature was not obvious (Fig. 1a). Condensation was accelerated as stirring speed, alkali concentration, or O2 pressure increased, accompanied by a widening of distribution of large molecular weight products (Fig. 1c; Table 3, entries 7–9). On the contrary, condensation became more obvious with the increase of reaction time at 160 °C. The Mw of macromolecules increased from 194 to 364 when the reaction time was extended from 30 min to 5 h (Fig. 1b). Furthermore, the addition of CuO resulted in Mw of macromolecules dramatically increased from 200 to 260 at 30 min, and reached 469 after 10-h reaction (Fig. 1d; Table 3, entries 10 and 11). In addition, vanillic acid is less stable than vanillin under the same alkaline oxidation conditions and suffered faster degradation (Table 2, entry 12) and severe condensation to give high Mw of 452 (Fig. 1d; Table 3, entry 12), which can help explain the low vanillic acid yield during vanillin oxidation (Table 2).
To shed light on the evolution of vanillin degradation in the alkali oxidation system and obtain more accurate condensation products information, LC–MS and HSQC analysis on the heavy products of reaction 3 was performed, and the results are shown in Figs. 2 and 3.
As can be seen in Fig. 2, all condensation products contained structure blocks of vanillin (m/z = 151.0405) or vanillic acid (m/z = 167.0350). The molecular weights of the products were m/z = 191.0714, 235.1368, 248.9611, and 264.9662, respectively. Correlation with structural information from HSQC NMR spectrum, seven monomers of I–VII were obtained, all of them were monophenols. Among them, III was vanillin and IV was vanillic acid, the rest of the products could be the products of ring-opening addition of vanillin.
From the HSQC NMR spectrum of heavy products, 13C–1H correlations exhibited three main regions, including aliphatic (δC/δH = 0–50/0–2.5 ppm), side-chain (δC/δH = 50–95/2.5–6.0 ppm), and aromatic region (δC/δH = 5.0–8.0/100–135 ppm). In addition, strong signals at δC/δH = 191.27/9.27–10.24 ppm beyond these three regions were caused by aldehyde groups of aromatic aldehydes.
In the side-chain region, three strong signals all resulted from methoxyl groups, the signal at δC/δH = 56.02/3.85 ppm attributed to methoxyl groups linked to aromatic ring is clearly shown in the spectrum (Fig. 3) [39, 40], while the other two correlation signals at δC/δH = 56.02/3.45 ppm and δC/δH = 59.75/3.92 ppm were caused by methoxyl groups on the Cβ of side chain on the aromatic-ring of product V and VII, respectively. Meanwhile, the strong signal at δC/δH = 66.09/4.05 ppm was signed to –CH2– in butyl group of product I, and the signal at δC/δH = 71.81/4.48 ppm was attributed to –CH2– in butenyl groups of product II. In the aromatic region, strong signals of guaiacyl (G) units clearly appeared, including correlations for C2–H2 (δC/δH = 112/7.39 ppm), C5–H5 (δC/δH = 115.54/7.00 ppm), and C6–H6 (δC/δH = 126.75/7.44 ppm), respectively [39,40,41]. Signals at δC/δH = 129.98/5.31 ppm and δC/δH = 134.46/6.64 ppm were signed to vinyl group of product II and product VII, respectively. In the aliphatic region, signals were caused by methyl or methylene in the side chain of aromatic ring. It is worth mentioning that strong signals of methylene in the diarylmethane structure (δC/δH = 29.67/1.25 ppm) were found in the spectrum [42]. Diarylmethane structure is chromophoric group, which can well explain the colorless of solution become orange-yellow after reaction (Fig. 3).
During the alkaline oxidation of vanillin, the formation of monophenols I, II, V, VI, and VII might be attributable to substitution of C5 and methoxyl groups in benzene of vanillin by another vanillin subsequently ring-opening reaction, or by fragments (aliphatic alcohols and acids, Figure S2) from degradation of vanillin. Moreover, the presence of diarylmethane structure in heavy products leads us to conclude that more complex condensation happened between two vanillin molecules or other monophenols and formed dimer.
To further confirm product evolution during alkaline oxidation of lignin, HSQC analysis of heavy product of lignin (from pine) alkaline oxidation under the same condition was performed. All products (I–VII) can be clearly found in the HSQC spectrum, which means that vanillin underwent the same degradation and condensation process (Fig. 4). From the results, we have obtained that instability of monophenols is a key factor of limited monophenols yield from lignin.
3.4 Presumable Mechanism of Vanillin Degradation
Correlation the structural information of the small molecule products and condensed products, a possible mechanism for vanillin degradation was deduced, as shown in Scheme 1. Vanillin degradation may occur through three routes. One route is form vanillic acid after direct oxidation and vanillic acid further degraded via ring-opening to form small molecules such as oxalic acid, acetic acid, formic acid, ethanol, methanol, and other fragments undetected (Route 1). In addition, the other two routes both start from condensation and end by ring opening. In Route 2, fragments re-condensed with vanillin to produce monophenols, such as I, II, V, VI, VII, and X (Figure S2, 16.96 min). For Route 3, vanillin molecules and/or other monophenols condense into dimers like VIII and IX. Subsequently, dimers decomposed to complex monophenols of II, V, VI, and VI. Then, all monophenols obtained by three routes will further degrade to long-chain acids and esters via ring opening, and subsequently decomposed to small molecules. These small molecules will be completely oxidized to CO2 and water.
4 Conclusions
In summary, vanillin is unstable under alkaline aerobic conditions, especially under high temperature. Under a temperature of 160 °C for 30 min, 73.9% vanillin was transformed into small acids, alcohols, and other monophenols, even dimers. LC–MS and HSQC were used to study the structural features of heavy products, which confirmed that vanillin condensed in the presence of NaOH and O2. Moreover, the degradation of pure vanillin was similar to those vanillin generated during the alkaline oxidation of lignin. In addition, a presumable mechanism for the vanillin degradation was provided. These results revealed that degradation of vanillin is a crucial limiting factor of low vanillin yield by lignin alkaline oxidation. Therefore, in practical application, mild reaction conditions and in situ extraction of monophenols can be a useful strategy to avoid unnecessary condensation and decomposing, and thus increase vanillin yield.
References
Van den Bosch S, Schutyser W, Vanholme R, Driessen T, Koelewijn SF, Renders T, De Meester B, Huijgen WJJ, Dehaen W, Courtin CM, Lagrain B, Boerjan W, Sels BF (2015) Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ Sci 8(6):1748–1763. https://doi.org/10.1039/c5ee00204d
Renders T, Van den Bosch S, Koelewijn S-F, Schutyser W, Sels BF (2017) Lignin-first biomass fractionation, the advent of active stabilisation strategies. Energy Environ Sci 10(7):1551–1557. https://doi.org/10.1039/C7EE01298E
Song Q, Wang F, Cai JY, Wang YH, Zhang JJ, Yu WQ, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6(3):994–1028. https://doi.org/10.1039/c2ee23741e
Deng HB, Lin L, Sun Y, Pang CS, Zhuang JP, Ouyang PK, Li JJ, Liu SJ (2009) Activity and stability of perovskite-type oxide LaCoO3 catalyst in lignin catalytic wet oxidation to aromatic aldehydes process. Energy Fuels 23:19–24. https://doi.org/10.1021/ef8005349
Zakzeski J, Dębczak A, Bruijnincx PCA, Weckhuysen BM (2011) Catalytic oxidation of aromatic oxygenates by the heterogeneous catalyst Co-ZIF-9. Appl Catal A 394(1–2):79–85. https://doi.org/10.1016/j.apcata.2010.12.026
Sales FG, Abreu CAM, Pereira JAFR (2004) Catalytic wet-air oxidation of lignin in a three-phase reactor with aromatic aldehyde production. Braz J Chem Eng 21:211–218. https://doi.org/10.1590/S0104-66322004000200010
Werhan H, Assmann N, von Rohr PR (2013) Lignin oxidation studies in a continuous two-phase flow microreactor. Chem Eng Process 73:29–37. https://doi.org/10.1016/j.cep.2013.06.015
Galkin MV, Smit AT, Subbotina E, Artemenko KA, Bergquist J, Huijgen WJ, Samec JS (2016) Hydrogen-free catalytic fractionation of woody biomass. Chemsuschem 9(23):3280–3287. https://doi.org/10.1002/cssc.201600648
Zakzeski J, Jongerius AL, Weckhuysen BM (2010) Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem 12(7):1225–1236. https://doi.org/10.1039/c001389g
Lv W, Si Z, Tian Z, Wang C, Zhang Q, Xu Y, Wang T, Ma L (2017) Synergistic effect of EtOAc/H2O biphasic solvent and Ru/C catalyst for cornstalk hydrolysis residue depolymerization. Acs Sustain Chem Eng 5(4):2981–2993. https://doi.org/10.1021/acssuschemeng.6b02535
Zhang XH, Zhang Q, Wang TJ, Ma LL, Yu YX, Chen LG (2013) Hydrodeoxygenation of lignin-derived phenolic compounds to hydrocarbons over Ni/SiO2–ZrO2 catalysts. Bioresour Technol 134:73–80. https://doi.org/10.1016/j.biortech.2013.02.039
Liu Y, Chen LG, Wang TJ, Zhang Q, Wang CG, Yan JY, Ma LL (2015) One-pot catalytic conversion of raw lignocellulosic biomass into gasoline alkanes and chemicals over LiTaMoO6 and Ru/C in aqueous phosphoric acid. Acs Sustain Chem Eng 3(8):1745–1755. https://doi.org/10.1021/acssuschemeng.5b00256
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115(21):11559–11624. https://doi.org/10.1021/acs.chemrev.5b00155
Lange H, Decina S, Crestini C (2013) Oxidative upgrade of lignin—recent routes reviewed. Eur Polym J 49(6):1151–1173. https://doi.org/10.1016/j.eurpolymj.2013.03.002
Fache M, Boutevin B, Caillol S (2016) Vanillin production from lignin and its use as a renewable chemical. Acs Sustain Chem Eng 4(1):35–46. https://doi.org/10.1021/acssuschemeng.5b01344
Rautiainen S, Chen J, Vehkamäki M, Repo T (2016) Oxidation of vanillin with supported gold nanoparticles. Top Catal 59(13–14):1138–1142. https://doi.org/10.1007/s11244-016-0633-8
Patil DG, Magdum PA, Nandibewoor ST (2015) Mechanistic investigations of uncatalyzed and ruthenium(III) catalyzed oxidation of vanillin by periodate in aqueous alkaline medium. J Solution Chem 44(6):1205–1223. https://doi.org/10.1007/s10953-015-0341-1
Fache M, Boutevin B, Caillol S (2015) Vanillin, a key-intermediate of biobased polymers. Eur Polym J 68(SI):488–502. https://doi.org/10.1016/j.eurpolymj.2015.03.050
Bomgardner MM (2014) Following many routes to naturally derived vanillin. Chem Eng News 92(6):14–15. https://doi.org/10.1021/cen-09232-bus2
Tarabanko VE, Koropatchinskaya NV, Kudryashev AV, Kuznetsov BN (1995) Influence of lignin origin on the efficiency of the catalytic oxidation of lignin into vanillin and syringaldehyde. Russ Chem Bull 44:367–371. https://doi.org/10.1007/bf00702154
Tomlinson GH, Hibbert H (1936) Studies on lignin and related compounds. XXV. Mechanism of vanillin formation from spruce lignin sulfonic acids in relation to lignin structure. J Am Chem Soc 58:348–353. https://doi.org/10.1021/ja01293a047
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Behling R, Valange S, Chatel G (2016) Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green Chem 18(7):1839–1854. https://doi.org/10.1039/c5gc03061g
Deng WP, Zhang HX, Wu XJ, Li RS, Zhang QH, Wang Y (2015) Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem 17(11):5009–5018. https://doi.org/10.1039/C5GC01473E
Rodrigues Pinto PC, Borges da Silva EA, Rodrigues AE (2011) Insights into oxidative conversion of lignin to high-added-value phenolic aldehyde. Ind Eng Chem Res 50:741–748. https://doi.org/10.1021/ie102132a
Wu GX, Heitz M, Chornet E (1994) Improved alkaline oxidation process for the production of aldehydes (vanillin and syringaldehyde) from steam-explosion hardwood lignin. Ind Eng Chern Res 33:718–723. https://doi.org/10.1021/ie00027a034
Fargues C, Mathias Á, Silva J, Rodrígues A (1996) Kinetics of vanillin oxidation. Chem Eng Technol 19(2):127–136. https://doi.org/10.1002/ceat.270190206
Sultanov VS, Wallis AFA (1991) Reactivities of guaiacyl and syringyl lignin model phenols towards oxidation with oxygen-alkali. J Wood Chem Technol 11(3):291–305. https://doi.org/10.1080/02773819108050276
Augugliaro V, Camera-Roda G, Loddo V, Palmisano G, Palmisano L, Parrino F, Puma MA (2012) Synthesis of vanillin in water by TiO2 photocatalysis. Appl Catal B 111–112:555–561. https://doi.org/10.1016/j.apcatb.2011.11.007
Bjørsvik H-R (1999) Fine chemicals from lignosulfonates. 1. Synthesis of vanillin by oxidation of lignosulfonates. Org Process Res Dev 3:330–340. https://doi.org/10.1021/op9900028
Tarabanko VE, Petukhov DV, Selyutin GE (2004) New mechanism for the catalytic oxidation of lignin to vanillin. Kinet Catal 45(4):569–577. https://doi.org/10.1023/B:KICA.0000038087.95130.a5
Tarabanko VE, Fomova NA, Kuznetsov BN, Ivanchenko NM, Kudryashev AV (1995) On the mechanism of vanillin formation in the catalytic oxidation of lignin with oxygen. React Kinet Catal Lett 55:161–170. https://doi.org/10.1007/BF02075847
Mathias AL, Rodrigues AB (1995) Production of vanillin by oxidation of pine kraft lignins with oxygen. Holzforschung 49(3):273–278. https://doi.org/10.1515/hfsg.1995.49.3.273
Borges da Silva EA, Zabkova M, Araújo JD, Cateto CA, Barreiro MF, Belgacem MN, Rodrigues AE (2009) An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem Eng Res Des 87(9):1276–1292. https://doi.org/10.1016/j.cherd.2009.05.008
Araújo JDP, Grande CA, Rodrigues AE (2010) Vanillin production from lignin oxidation in a batch reactor. Chem Eng Res Des 88(8):1024–1032. https://doi.org/10.1016/j.cherd.2010.01.021
Klinke HB, Ahring BK, Schmidt S, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82(1):15–26. https://doi.org/10.1016/S0960-8524(01)00152-3
Fargues C, Mathias Á, Rodrigues A (1996) Kinetics of vanillin production from kraft lignin oxidation. Ind Eng Chem Res 35(1):28–36. https://doi.org/10.1021/ie950267k
Shilpy M, Ehsan MA, Ali TH, Abd Hamid SB, Ali ME (2015) Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound. Rsc Adv 5(97):79644–79653. https://doi.org/10.1039/c5ra14227j
Lahive CW, Deuss PJ, Lancefield CS, Sun Z, Cordes DB, Young CM, Tran F, Slawin AM, de Vries JG, Kamer PC, Westwood NJ, Barta K (2016) Advanced model compounds for understanding acid-catalyzed lignin depolymerization: identification of renewable aromatics and a lignin-derived solvent. J Am Chem Soc 138(28):8900–8911. https://doi.org/10.1021/jacs.6b04144
Jiang ZC, Zhang H, He T, Lv XY, Yi J, Li JM, Hu CW (2016) Understanding the cleavage of inter- and intramolecular linkages in corncob residue for utilization of lignin to produce monophenols. Green Chem 18(14):4109–4115. https://doi.org/10.1039/c6gc00798h
Yang HT, Xie YM, Zheng X, Pu YQ, Huang F, Meng XZ, Wu WB, Ragauskas A, Yao L (2016) Comparative study of lignin characteristics from wheat straw obtained by soda-AQ and kraft pretreatment and effect on the following enzymatic hydrolysis process. Bioresour Technol 207:361–369. https://doi.org/10.1016/j.biortech.2016.01.123
Froass PM, Ragauskas AJ, J-e Jiang (1996) Chemical structure of residual lignin from kraft pulp. J Wood Chem Technol 16(4):347–365. https://doi.org/10.1080/02773819608545820
Acknowledgements
This work was supported by NSFC (National Natural Science Foundation of China) project (nos. 51476175, 51606205), the National Natural Science Foundation of China (no. 51536009) and Chinese Academy of Sciences “one hundred talented plan” (no. y507y51001).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Lignin Chemistry”; edited by Luis Serrano, Rafael Luque, Bert Sels.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, Y., Liu, J., Liao, Y. et al. Degradation of Vanillin During Lignin Valorization Under Alkaline Oxidation. Top Curr Chem (Z) 376, 29 (2018). https://doi.org/10.1007/s41061-018-0208-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-018-0208-1