Abstract
In recent years, superparamagnetic iron oxide nanoparticles have attracted a great attention due to their various biomedical applications, such as magnetic resonance imaging, targeted drug delivery, and hyperthermia. In this article, γ-Fe2O3 magnetic nanoparticles (Maghemite) were prepared in oleic acid media by co-precipitation method. The oleic acid, a monounsaturated fatty acid was used as the capping and stabilizing agent during the synthesis of the magnetic nanoparticles. Characterization of obtained nanoparticles were performed using powder X-ray diffraction (PXRD), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectra (FTIR), and vibrating sample magnetometer (VSM). The crystallite size of γ-Fe2O3 nanoparticles was achieved in the range between 16.2 and 26.8 nm. The FESEM demonstrated the regular spheres of γ-Fe2O3 nanoparticles. The obtained nanoparticles were coated with oleic acid indicating by FTIR analysis. The resulted oleic acid-coated nanoparticles were shown superparamagnetic properties (~52 emu/g). This suggested method is simple and rapid to fabricate superparamagnetic nanoparticles which make them appropriate candidates for theranostic application in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic metal nanoparticles have been found attractive in biomedical sciences in recent years due to their non-toxicity, biocompatibility, chemical inactivity, biodegradability, and suitable magnetic properties (Kuznetsov et al. 2001; Ding et al. 2015; Hu et al. 2006; Hayashi et al. 2013). In fact, there are several types of iron oxide nanoparticles but only magnetite (Fe3O4) or its oxidized form maghemite (γ-Fe2O3) are the most frequent kind of nanoparticles employed in medical diagnostics and/or drug delivery (Häfeli et al. 2013; Bee et al. 1995; Gupta and Gupta 2005; Figuerola et al. 2010). The reasons are their superior magnetic moments, sufficient chemical stability in physiological conditions, low particle dimension, large surface area, and prominently their easy and economical synthesis.
There are currently various physical and chemical methods developed for fabricating iron oxide nanoparticles. For instance, sonochemical synthesis, sol–gel reactions, thermal decomposition, electrochemical, ultrasonic assisted, microwave hydrothermal, γ-ray radiation and chemical solution (Wu et al. 2009; Pascal et al. 1999; Shafi et al. 2002; Sreeja and Joy 2007; Wu and Wang 2013; Akbar et al. 2004). In this study, we have employed oleic acid capping agent as a stabilizer on the surface of γ-Fe2O3 nanoparticles to lessen their agglomeration (Jadhav et al. 2013) by a simple co-precipitation method. In addition, we have investigated the magnetic properties of the obtained iron oxide nanoparticles.
2 Experimental Section
2.1 Materials and Reagents
Iron (III) chloride hexahydrate (≥98%) and iron (II) chloride tetrahydrate (≥99%) were purchased from Sigma-Aldrich, Germany; oleic acid (90%) was obtained from Sigma-Aldrich chemical co.) St. Louis, MO, USA); ammonium hydroxide solution (NH4OH, 28–30%) was purchased from Merck, Germany. Acetone (extra pure) was purchased from Dr. Mojallali chemical laboratories, Iran. All the materials and reagents were used without further purification.
2.2 Methods
2.2.1 Synthesis of Iron Oxide Nanoparticles Stabilized with Oleic Acid
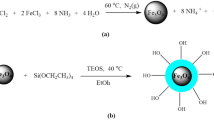
We were synthesized oleic acid-coated magnetite nanoparticles by a simple chemical co-precipitation technique described as following. FeCl2·4H2O (12.0 g) and FeCl3·6H2O (24.3 g) were dissolved in deoxygenated water (50 mL) in a 250-mL three-neck flask under an argon atmosphere at ambient temperature for 30 min. Then, the flask was placed into 80 °C water bath, and 28% ammonium hydroxide (35 mL) was added dropwise with vigorous stirring. Oleic acid (15 mL) was added drop by drop during 10 min and continued heating at 80 °C for 30 min. Stirring constantly lasted to evaporate the remaining ammonia. Finally, the black precipitate was separated by magnetic decantation. After cooling to room temperature, then washed the precipitate three times with deionized water and acetone solution through centrifugation at 10,000 rpm for 15 min. Afterward, the nanoparticles were washed one more time with 50 °C deionized water to remove excess oleic acid. The iron oxide nanoparticles were lyophilized for 2 days at −60 °C and 7 mmHg vacuum (LYPHLOCK 12 LABCONCO, Kansas City, MO).
2.2.2 Characterization of Oleic Acid Stabilized γ-Fe2O3
The prepared superparamagnetic iron oxide nanoparticles (SPIONs) were characterized using powder X-ray diffraction (PW 3040/60, X Pert PRO; Netherland). The size and morphological properties of sample were characterized by field emission scanning electron microscopy (FESEM) (Mira 3-XMU). The FTIR spectra of the oleic acid-coated magnetite nanoparticles were recorded on a FTIR spectrometer (Thermo Nicolet, AVATAR 370, USA) in the range of 400–4000 cm−1 using KBr pellet. Magnetic properties of the sample was measured by a vibrating sample magnetometer (VSM, AGFM/VSM 3886 Kashan, Iran) at ambient temperature in a magnetic field strength of 1.0 T.
3 Results and Discussions
3.1 Powder X-Ray Diffraction (PXRD)
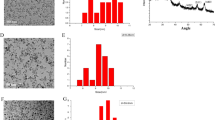
Figure 1 illustrates the XRD pattern obtained from γ-Fe2O3 nanoparticles capped with oleic acid prepared by co-precipitation method. All diffraction peaks of the observed sample are consistent with the standard structure of maghemite (JCPDS card No. 25-1402). Neither diffraction peaks nor impurities are detected, such as ferric nitrate [Fe(NO3)3], goethite [FeO(OH)], and magnetite (Fe3O4) or amorphous phase. The crystallite size (D) of maghemite nanoparticles determined using X-ray diffraction line broadening based on Scherrer’s equation, i.e., (D = Kλ/βcosθ) where D is the particle size, K is a constant (~0.94) related to the particle shape and crystalline plane, λ is the X-ray wavelength (0.15406 nm), β is the full width at half-maximum of diffraction peak, and θ is the X-ray diffraction angle (Nidhin et al. 2008). The crystallite size of the sample coated with oleic acid was estimated to be ~9 nm.
3.2 Field Emission Scanning Electron Microscopy (FESEM)
Figure 2 shows the FESEM images for the oleic acid stabilized γ-Fe2O3 nanoparticles at different magnifications, which confirms that these nanoparticles are semi-spherical in shape and uniformly dispersed. The FESEM measurements of prepared nanoparticles capped with oleic acid were shown to be between 16.2 and 26.8 nm.
3.3 Fourier Transform Infrared Spectra (FTIR)
Figure 3 illustrates the FTIR spectrum of oleic acid stabilized γ-Fe2O3 nanoparticles. The FTIR spectrum confirmed the adsorption of oleic acid on the surface of the γ-Fe2O3 nanoparticles. The bands at around 1420, 1520, and 1620 cm−1 originate from metal oleate (Nyquist and Kagel 2012). In this spectrum, the bands below 3000 cm−1 are the characteristic peaks of aliphatic alkyl groups of oleic acid. The broad bands around 3350 cm−1 relate to the presence of hydrogen-bounded OH groups (Nyquist and Kagel 2012). Moreover, two bands at 2922.07 and 2851.23 cm−1 are attributed to the symmetric CH2 and the asymmetric CH2 stretch, respectively (Zhang et al. 2006; Wang et al. 1998). The analysis indicates the strong peak at 580 cm−1 corresponding to the formation of iron oxide phase.
3.4 Vibrating Sample Magnetometry (VSM)
Magnetic measurements were performed based on vibrating sample magnetometry (VSM) instrument at room temperature (300 K) up to 90,00 Oersted (Oe). Figure 4 shows superparamagnetic behavior of γ-Fe2O3 nanoparticles with saturation magnetization (Ms) value of about 52 emu/g. It means that the obtained γ-Fe2O3 nanoparticles is suitable for biomedical diagnostics and therapy, such as magnetic resonance imaging (MRI) contrast agents, hyperthermia treatment, biomagnetic separation, and magnetic drug targeting and delivery. Compared to the Ms value of bulk γ-Fe2O3 (~74 emu/g) (Berkowitz et al. 1968), the decrease of Ms may be due to difference in the crystallinity of samples (Hong et al. 2006).
4 Conclusion
Superparamagnetic γ-Fe2O3 nanoparticles have been synthesized by an organic material for theranostic applications. Oleic acid-coated γ-Fe2O3 nanoparticles were prepared by co-precipitation method with the size ranging from 16.2 to 26.8 nm. The obtained nanoparticles were semi-spherical in shape and uniform in size. It was confirmed that the oleic acid molecules were adsorbed on the surface of nanoparticles. Besides, oleic acid-coated γ-Fe2O3 nanoparticles revealed superparamagnetic behavior (~52 emu/g). This approach provides a facile, novel, and feasible method for preparing stable magnetic γ-Fe2O3 nanoparticles.
References
Akbar S, Hasanain S, Azmat N, Nadeem M (2004) Synthesis of Fe2O3 nanoparticles by new sol-gel method and their structural and magnetic characterizations. arXiv preprint cond-mat/0408480
Bee A, Massart R, Neveu S (1995) Synthesis of very fine maghemite particles. J Magn Magn Mater 149(1):6–9
Berkowitz A, Schuele W, Flanders P (1968) Influence of crystallite size on the magnetic properties of acicular γ-Fe2O3 particles. J Appl Phys 39(2):1261–1263
Ding Y, Shen SZ, Sun H, Sun K, Liu F, Qi Y et al (2015) Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater Sci Eng C 48:487–498
Figuerola A, Di Corato R, Manna L, Pellegrino T (2010) From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res 62(2):126–143
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Häfeli U, Schütt W, Teller J, Zborowski M (2013) Scientific and clinical applications of magnetic carriers. Springer, Berlin
Hayashi K, Nakamura M, Sakamoto W, Yogo T, Miki H, Ozaki S et al (2013) Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 3(6):366–376
Hong R, Pan T, Li H (2006) Microwave synthesis of magnetic Fe3O4 nanoparticles used as a precursor of nanocomposites and ferrofluids. J Magn Magn Mater 303(1):60–68
Hu F, Wei L, Zhou Z, Ran Y, Li Z, Gao M (2006) Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv Mater 18(19):2553–2556
Jadhav NV, Prasad AI, Kumar A, Mishra R, Dhara S, Babu K et al (2013) Synthesis of oleic acid functionalized Fe3O4 magnetic nanoparticles and studying their interaction with tumor cells for potential hyperthermia applications. Colloids Surf B 108:158–168
Kuznetsov AA, Filippov VI, Alyautdin RN, Torshina N, Kuznetsov OA (2001) Application of magnetic liposomes for magnetically guided transport of muscle relaxants and anti-cancer photodynamic drugs. J Magn Magn Mater 225(1):95–100
Nidhin M, Indumathy R, Sreeram K, Nair BU (2008) Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull Mater Sci 31(1):93–96
Nyquist RA, Kagel RO (2012) Handbook of infrared and Raman spectra of inorganic compounds and organic salts: infrared spectra of inorganic compounds. Academic Press, Cambridge
Pascal C, Pascal J, Favier F, Elidrissi Moubtassim M, Payen C (1999) Electrochemical synthesis for the control of γ-Fe2O3 nanoparticle size. Morphology, microstructure, and magnetic behavior. Chem Mater 11(1):141–147
Shafi KV, Ulman A, Dyal A, Yan X, Yang N-L, Estournes C et al (2002) Magnetic enhancement of γ-Fe2O3 nanoparticles by sonochemical coating. Chem Mater 14(4):1778–1787
Sreeja V, Joy P (2007) Microwave–hydrothermal synthesis of γ-Fe2O3 nanoparticles and their magnetic properties. Mater Res Bull 42(8):1570–1576
Wang W, Efrima S, Regev O (1998) Directing oleate stabilized nanosized silver colloids into organic phases. Langmuir 14(3):602–610
Wu Z-G, Wang Y (2013) One-pot synthesis of magnetic γ-Fe2O3 nanoparticles in ethanol-water mixed solvent. Materials Science-Poland. 31(4):577–580
Wu W, He Q, Jiang C (2009) Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. ChemInform 40(24):i
Zhang L, He R, Gu H-C (2006) Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci 253(5):2611–2617
Acknowledgements
The authors thank the financial support of Mashhad University of Medical Sciences (Grant No. 910808) based on the PhD thesis (No. A-517) of Mrs. Aida Gholoobi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gholoobi, A., Abnous, K., Ramezani, M. et al. Synthesis of γ-Fe2O3 Nanoparticles Capped with Oleic Acid and their Magnetic Characterization. Iran J Sci Technol Trans Sci 42, 1889–1893 (2018). https://doi.org/10.1007/s40995-017-0147-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-017-0147-7