Abstract
With the increasing demand for low-carbon metallurgy, large-scale hydrogen-rich smelting has become an effective way to realize low-carbon ironmaking. The injection of hydrogen-rich fuel into blast furnace changed the reduced atmosphere, affecting the reaction behavior and structure of the burden. Production practice has proved that the use of nut coke is an important measure for alleviating the increasingly tense relationship between coke supply and realizing low reducing agent ironmaking. In this paper, the effect of mixed charging of nut coke and sinter on reduction and softening-melting properties in the hydrogen-rich smelting process of blast furnace is studied. The results showed that the close contact between nut coke and sinter promoted carbon dissolution and water–gas reaction to generate more CO and H2, thus increasing the reduction potential in the gas mixture. With the increase of nut coke and hydrogen-rich ratio, the softening–melting properties of the burden were improved. This change narrowed and lowered down the position of cohesive zone, triggering to an enlargement of indirect reduction zone, which was conducive to strengthening blast-furnace smelting and saving coke. The permeability resistance of the burden bed was reduced, which was beneficial to the good airflow distribution and the gas utilization rate. There was a slag layer between nut coke and metal iron phase in the softening–melting process, and the slag could quickly fuse with the ash distributed on the nut coke surface to dissolve it, thus promoting the occurrence of carburizing reaction and improving the dripping behavior of the burden.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the aggravation of global warming, low-carbon production by reducing CO2 emissions has become the focus of social attention [1, 2]. The energy structure of the iron and steel industry is dominated by coal, which leads to an increase in CO2 emissions with the increase in iron and steel production. The traditional blast-furnace ironmaking technology has been mature, and both energy utilization and pollution emissions are close to its limit. Only by developing breakthrough blast-furnace ironmaking technology can we further save energy and reduce emissions [3]. Hydrogen is an excellent reducing agent and clean energy. Replacing carbon with hydrogen on a large scale is an effective way to reduce CO2 emission for blast-furnace ironmaking and finally realize the low-carbon ironmaking and green sustainable development [4, 5]. However, the reduction of coke resulted in a thinner coke split, increasing the coke load and worsening the gas permeability. To ensure the stable operation of hydrogen-enriched blast furnace with low coke ratio, the coke properties had to be improved to meet the higher requirements such as higher strength, relative larger, and more even particle size [6, 7]. Therefore, the small particle size coke will be produced more in the coke screening process, if they could not be used highly valuable and sufficiently, the cost of coke would be improved inevitably.
Mixing appropriate amount of nut coke into the sinter and loading into the blast furnace on the premise of stabilizing the quality of hot metal has been widely used. Many investigators studied the effect of nut coke on the blast-furnace smelting. Mousa’s [8] research results show that mixing nut coke in the sinter bed inhibits the reduction retardation phenomenon and improves the sinter reducibility. Kashihara’s [9, 10] research results show that simultaneous use of coke mixing and hydrogen addition accelerated the reduction rate through the carbon gasification rate, and it also decreased pressure drop. Grvel's [11] research results show that the nut coke addition shortens the softening, melting, and dripping temperature ranges, which shows improved properties of the cohesive zone. Chang [12] investigated the effects of CO2 and H2O on the dissolution loss of coke at different temperatures. The results show that the average reaction rate of coke with H2O is 1.3–6.5 times higher than that with CO2, and the water vapor diffuses more easily into the coke. The previous studies mainly focused on the influence of nut coke mixed charging on the smelting process of traditional blast furnaces; however, facing the increasing desire for using hydrogen-rich blast-furnace smelting to reduce CO2 emission, there still was a lack of systematic analysis on the influence of nut coke charging under the hydrogen-enriched smelting conditions. Imagine a further increase in the proportion of hydrogen rich in blast furnace. The injection of hydrogen-rich fuel into the blast furnace changed the reduction atmosphere, affecting the reaction behavior and structure of the burden [13, 14]. Therefore, it is of great significance to investigate the influence of nut coke mixed charging on the smelting process of the hydrogen-rich blast furnace.
The effects of nut coke and hydrogen-rich ratio on the reduction and softening–melting behavior of burden were studied by program reduction simulation experiment and self-designed high-temperature softening–melting experimental device. In addition, quenching experiments were carried out at different temperatures to explore the microstructure characteristics of the interface reaction between coke and slag iron at high temperatures, and to analyze the change mechanism of softening–melting behavior under the mixed charging of nut coke and sinter. This paper is expected to provide a theoretical basis for the application of nut coke in the smelting process of the hydrogen-rich blast furnace.
Experimental Materials and Methods
Experimental Materials
The sinter ore and coke used in this experiment were taken from a commercial steel company. The sinter used in the experiment was 10.0–12.5 mm in diameter. The coke with a diameter of 10.0–12.5 mm acted as regular coke, and the particle size of nut coke was 6.3–8.0 mm. The chemical analysis of sinter and coke is shown in Tables 1 and 2.
Isothermal Reduction Experiment
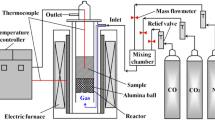
The simulation experimental device of coke and sinter mixed charging program reduction is shown in Fig. 1. The gas was introduced from the bottom gas inlet. At the beginning of the experiment, the burden was heated from room temperature to 950 °C at a rate of 8 °C/min in N2 atmosphere and then switched to reducing gas and kept at that temperature for 60 min. After that, the burden was cooled to room temperature in N2 atmosphere. During the experiment, the volume percentages of H2, CO, and CO2 in tail gas were recorded in real time by the gas infrared analyzer. The reduction degree of sinter and the mass loss rate of nut coke were calculated by weight change, and the calculation formulas are shown in formula (1) and formula (2). Based on the national standard of iron ores—determination of reducibility (GB/T13241-91) [15, 16]—the isothermal reduction testing was carried out and the reduction degree of sinter was calculated. The mass of sinter and coke (regular coke+nut coke) remained unchanged in each experiment, which were 50 g and 15 g, respectively, and the thickness of regular coke burden decreased with the increase of the coke ratio. During the experiment, the reducing gas was kept at 50%N2-30%CO-20%H2, and the influence of the nut coke ratio (0%, 20%, 40%) on the reduction was explored. The ratio of nut coke was kept at 40%, and the effect of gas composition (70%N2-30%CO, 60%N2-30%CO-10%H2, 50%N2-30%CO-20%H2) on the reduction was explored.
where RI(%) = Reducibility index, %; m0 = Initial mass of the sinter, g; m1 = Mass of the sinter before starting the reduction, g; mt = Mass of the sinter at the end of reduction, g; A = Content of FeO in the sinter before the test, (%); B = Content of total iron content before the test, (%).
where x(%) = Mass loss rate of nut coke, %; m0 = Initial mass of the nut coke, g; mt = Mass of the nut coke at the end of reduction, g
Softening–Melting Experiment
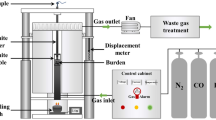
The softening–melting properties of the burden were tested by using the self-designed high-temperature softening–melting experimental device, as shown in Fig. 2. Based on the national standard of iron ores-method for determination of iron reduction softening dripping performance under load (GB/T34211-2017), the experiment was carried out by adjusting the proportion of coke and the composition of reducing gas. The furnace burden was loaded in a graphite crucible (φ94 × 210), and three layers of burden were placed in the crucible. When being free of nut coke, 40 g and 80 g regular coke were loaded acting as upper and lower burden layers, respectively, and 500 g sinter lied between the two coke layers. When the nut coke was loaded, it was mixed into the sinter layer, correspondingly the amount of regular coke was reduced according to the proportion of nut coke. The burden was heated from room temperature to 900 °C in N2 atmosphere and then switched to reducing gas meanwhile continued improving the temperature. When the droplet mass reached the maximum value, the experiment ceased, and the burden was cooled to room temperature in N2 atmosphere. During the process of the experiment, the softening–melting dropping parameters of iron-bearing burden were automatically recorded and generated by a computer. The softening–melting dropping parameters of the iron-bearing burden are shown in Table 3. During the experiment, in order to make the results more accurate, the experimental method commonly used by many researchers was adopted, that is, the method of enlarging the experimental conditions in equal proportion. The reducing gas was kept at 60%N2-30%CO-10%H2, and the influence of the nut coke ratio (0%, 20%, 40%) on the softening–melting properties was explored. The ratio of nut coke was kept at 20%, and the effect of gas composition (70%N2-30%CO, 60%N2-30%CO-10%H2, 50%N2-30%CO-20%H2) on the softening–melting properties was explored.
Quenching Experiments
To explore the microstructure characteristics of the interface reaction between coke and slag iron in the high-temperature zone, and analyze the change mechanism of the softening–melting behavior of the burden, quenching experiments (20%nut coke, 60%N2-30%CO-10H2) under different temperature (T40 = 1291 °C, Ts = 1443 °C) conditions were carried out. After reaching the desired temperature, the burden was cooled to room temperature in N2 (5L/min, gas quenching) atmosphere. After cooling, the sample crucible was cut vertically, and the experimental sample was embedded with epoxy resin for further analysis.
Results and Discussion
Effect of Nut Coke and Hydrogen-Rich Ratio on Sinter Reduction and Coke Reaction Behavior
The reduction degree of sinter and weight loss of nut coke in different reduction conditions are shown in Fig. 3. The experimental results showed that using the same reducing gas with 50%N2-30%CO-20%H2, when improving the nut coke from 0 to 40%, the reducibility index of the sinter increased from 20 to 54%, and the mass loss rate of nut coke increased from 0 to 8%. When keeping the nut coke ratio at 40%, the reducibility index of the sinter increased from 29 to 54% and the mass loss rate of nut coke increased from 3 to 8% with the hydrogen ratio increasing from 0 to 20%.
The reducibility index of sinter and the mass loss rate of nut coke were promoted with the increase of the coke ratio. The possible reactions in the reduction process are shown in formulas (3–12). The reduction of iron oxide by H2 and CO followed the principle of step-by-step transformation, iron oxides were reduced to produce CO2 and H2O, and nut coke and sinter were mixed in close contact. Therefore, the CO2 and H2O produced by reduction could immediately gasify the nut coke to regenerate CO and H2. The generated reduction gas reduced the nearest sinter in the burden, and the reduction speed was accelerated, consequently the reduction degree was improved. The indirect reduction rate in the upper part of the blast furnace was accelerated, and the reduction degree increased when the sinter arrived to the lower part of the blast furnace, which was a very effective factor to reduce the coke consumption of blast furnace. In the high-temperature zone, the solid-state reaction between carbon and FeO was carried out through physical contact, and the nut coke was in close contact with the sinter, and the contact surface increased, which was beneficial to the improvement of the solid-phase reaction rate. However, when the surface of iron oxide had been reduced, it was difficult for solid carbon to permeate and diffuse to the center of sinter for reduction, so the reaction rate between carbon and FeO was limited. At this time, the solid-phase reaction was restricted by water–gas reaction (formula 10) and water–gas replacement reaction (formula 11). The water–gas replacement reaction was reversible, and it was affected by temperature. When the temperature was higher than 1000 °C, the reaction proceeded in positive direction to form CO and H2O, under the condition of high temperature, H2 acted as an intermediary, promoting the direct reduction of carbon [4]. In addition, according to the electron cyclic donor–acceptor catalyzing theory, metallic iron could increase the number of active sites on the coke surface, thus improving the gasification reactivity of coke [19]. During the reduction process, H2 and CO diffused to the sinter surface through the gas boundary layer, and the iron oxides on the sinter surface were first reduced to metallic iron, and the reduced iron was used as a catalyst to promote the gasification of coke. To sum up, the reduction potential was improved by the carbon dissolution reaction and water–gas reaction promoted by nut coke. These continuous reactions were the key factors for the improvement of the reduction degree of the sinter.
The effect of nut coke ratio on tail gas composition is shown in Fig. 4a. The experimental results showed that mixing the nut coke into sinter burden increased the proportion of CO and H2 in reducing gas, which verified our inference above. In addition, compared to the absence of nut coke, the proportion of CO2 in the early stage of the reduction process with nut coke used was lower but increased in the later stage, which was different from the research results of Mousa in pure CO atmosphere. Mousa's [8] research results showed that the CO2 produced in the process of reduction with nut coke was always lower than that of no nut coke employment. We speculated that this was mainly because the hydrogen-rich reduction process of iron oxide was not only affected by coke solution loss and water–gas reaction but also restricted by water–gas displacement reaction (formula 11). When the temperature was lower than 1000 °C, the reaction proceeded toward the positive direction to form CO2 + H2 [20]. The reduction potential was improved by adding nut coke, and the content of H2O generated by the reduction in the burden bed increased. Part of the generated H2O reacted with CO, thus resulting in the increase of CO2 in the gas mixture.
The reducibility index of sinter and the mass loss rate of nut coke increased with a rise in hydrogen ratio. The increase of the proportion of hydrogen and the occurrence of water–gas reaction promoted the reduction potential of the mixture, thus promoting the reduction of sinter. The effect of hydrogen ratio on tail gas composition is shown in Fig. 4b. The experimental results showed that the proportion of CO in reducing gas increased significantly in the process of hydrogen-rich reduction. In addition, the content of CO2 increased significantly in the initial stage of hydrogen-rich reduction. The reason for this was that H2 had stronger diffusion and reduction ability than CO, and the rate of H2O produced by H2 reduction was higher than that of CO2 by CO reduction [21]. It could be inferred that the reaction rate of H2O and coke in the mixture burden bed at the initial stage of the reaction was higher than that of CO2 and coke. At the beginning of reduction, the water–gas replacement reaction led to the rapid increase of CO2 in the tail gas.
To sum up, the small specific surface area of nut coke had high reactivity, the sinter was in close contact with nut coke, and the reduced H2O and CO2 reacted quickly with nut coke to form CO and H2 to promote the reduction reaction. In addition, the coke consumption prevented the loss of conventional coke in the lump zone and maintains a good quality when it reached the lower part of the blast furnace, which was beneficial to improving the gas flow distribution, and reduce the coke ratio and stabilizing the operation of the blast furnace. But it should also be noted that the reaction (6–10) was endothermal, thus decreasing the temperature in the upper part of the blast furnace [22]. However, the water–gas replacement reaction was exothermal, and the decrease of temperature in the furnace promoted the positive formation to produce CO2 and H2, which led to the increase in the utilization rate of CO gas on the top of the furnace and the decrease of the utilization rate of hydrogen.
The microstructure changes of sinter and coke under different nut coke and hydrogen-rich ratio were analyzed by SEM–EDS (Scanning Electron Microscope–Energy-Dispersive Spectrometer), and the results are shown in Fig. 5.
In the absence of coke, the structure of the sinter was dense (a1), and the dense structure was unfavorable to the diffusion of reducing gas, thus reducing the reduction rate. For the mixed burden with nut coke and sinter, the surface structure of the sinter was relatively porous (a2), so the reducibility of the sinter was improved, which was consistent with the research results of Mousa [23]. The cracks and pores of sinter using pure CO reduction were less (a3), and they mainly concentrated on the surface, correspondingly the internal structure was dense, while the overall cracks and pores of the reduction sinter increased with the addition of H2, which contributed to the strong penetration and diffusion ability of H2. Along with the reduction, H2 diffused into the sinter through the cracks and pores, thus accelerating the internal reduction of iron oxides in the sinter. The rapid internal reduction reaction of the sinter produced a large amount of iron, forming a large number of cracks due to the shrinkage of internal volume. In addition, the water–gas reaction between H2O and the residual carbon in the sinter increased the internal pressure, leading to an increase in porosity [4].
Under the same experimental conditions, the microstructure of regular coke and nut coke is shown as (b1) and (b2). The regular coke exhibited more pores at the edge and fewer inside of the coke, while the overall pores of nut coke were increased and the solution loss was obvious, indicating that the coke consumption prevented the loss of regular coke in the lump zone. The effect of hydrogen rich on the microstructure change of nut coke is shown as (b2) and (b3). Compared with pure CO, the pore size of coke was significantly larger and the solution loss was obvious due to the increase of H2O content in a hydrogen-rich atmosphere.
Effect of Nut Coke and Hydrogen-Rich Ratio on Softening and Melt-Dropping Properties
Effect of Nut Coke and Hydrogen-Rich Ratio on Softening–Melting Properties
The softening–melting properties of the burden under different reduction conditions are shown in Fig. 6. The experimental results showed that when the reducing gas was 60%N2-30%CO-10%H2, with the nut coke content increasing from 0 to 40%, the T10 increased from 1140 to 1178 °C and the T40 increased from 1269 to 1345 °C. Compared to the rapid growth of T40, the change of T10 was slight, correspondingly the △Ta increased from 129 to167 °C. The Ts increased from 1432 to 1503 °C, and the Td decreased from 1508 to 1505 °C, finally the △Tm decreased from 76 to 2 °C.
The results of chapter 3.1 showed that the increase of nut coke ratio promoted the indirect reduction of iron ore and the FeO content increased when the burden reached the cohesive zone. With the increase in temperature, a large amount of FeO was reduced to form a thicker shell of metallic iron and the shell had strong deformation resistance. The reduction of FeO led to the decrease of low melting point compounds and an increase in porosity; moreover, the skeleton action of nut coke in the burden bed led to the slow shrinkage of the burden column. Therefore, the T10 and T40 increased and the △Ta became narrow. The mixed charging of nut coke and sinter strengthened the reduction of FeO, improving the melting point of the slag, thus the TS increased. The close contact between nut coke and sinter created a good condition for the carburization, which decreased the melting point of iron, and therefore, the dripping temperature dropped slightly. Because of the increases of Ts and the decreases of Td, the range of △Tm decreased, when the ratio of nut coke was 40%, the △Tm approached to be zero, meaning that the air permeability of the burden column was significantly improved. In addition, the heat transfer condition between the gas flow and the burden bed was improved by the mixed charging of nut coke and sinter and improved the heat transfer efficiency, thus the cohesive zone was narrowed and moved down.
When the nut coke ratio was 20%, with the hydrogen-rich ratio increasing from 0 to 20%, the T10 increased from 1149 to 1173 °C, and the T40 increased from 1254 to 1335 °C, correspondingly the △Ta increased from105 to162 °C. The Ts increased from 1332 to 1456 °C, and the Td dropped from 1516 to 1490 °C, and finally, the △Tm narrowed from 184 to 34 °C.
The increase of the proportion of hydrogen promoted the reduction potential of the mixture, and the sinter was rapidly reduced to form a thick metallic iron shell. Furthermore, the reduction of FeO led to the decrease of low melting point compounds. Therefore, the T10 and T40 increased and the △Ta became narrow. With the increase in the hydrogen-rich ratio, the FeO content decreased and the melting point of the slag phase was improved as a result the Ts increased. The Td decreased with the rise of the hydrogen-rich ratio. However, Qie [24] and Lan's [25] research results showed that the Td increased with the increase of hydrogen-rich ratio without adding nut coke, which was contrary to the experimental results. Our previous research results also showed that the Td increased from 1508 to 1533 °C with an increase in hydrogen-rich ratio from 10 to 20%. The Td of the burden in the high hydrogen-rich atmosphere was reduced by adding nut coke [26]. The Td of molten iron was mainly determined by carbon content. The content of cementite increased with carbon content, compared to the metallic iron, and the melting point of cementite was lower, so the Td decreased with the increase of cementite content. Combined with the study of the influence of nut coke on the reduction process in chapter 3.1, we speculated that three reasons contributed to the results: (1) The addition of nut coke promoted the reduction of sinter, the amount of sponge iron increased, and the newly reduced sponge iron as a catalyst to promote the CO decomposition and form carbon black. Carburizing reaction between sponge iron and precipitated carbon increases the carbon content of hot metal; (2) The iron shell formed by sinter reduction at high temperature was in close contact with the nut coke in the burden bed, and the carburizing rate of the iron shell was increased compared with being free of nut coke, which reduces the dripping temperature; (3) The FeO content in the slag determined the carbon content in iron, and the decarburization reaction of slag to hot metal is shown in formula (13). The addition of nut coke promoted the reduction of sinter, and the decrease of FeO content retarded the decarburization reaction of slag to hot metal [27], leading to the increase of carbon content in pig iron. To sum up, although the addition of nut coke made the regular coke bed thinner and the carbon accumulated when molten iron flows through the regular coke bed at high temperature, more carbon was accumulated from sponge iron carburizing and direct contact.
To sum up, with the increase of nut coke and hydrogen-rich ratio, the softening–melting properties of the burden were improved, and the cohesive zone was narrowed and moved down. The narrowing of the cohesive zone could effectively improve the air permeability of the burden column, and the downward movement of the cohesive zone expands the indirect reduction zone, which is beneficial to strengthen blast-furnace smelting and save coke.
The cohesive zone of the blast furnace with the largest differential pressure loss accounts for more than 50% of the total resistance loss of the burden column. As the furnace burden begins to soften and the volume shrinks, the porosity decreases continuously, which leads to a sharp increase in the resistance loss when the gas passes through [28]. The effects of nut coke and hydrogen-rich ratio on the S and △Pm of the softening–melting process are shown in Fig. 7.
When the reducing gas was 60%N2-30%CO-10%H2, with the nut coke content increasing from 0 to 40%, the △Pm declined from 2.4 to 1.2 kPa, and the S value decreased from 95.3 to 1.0 kPa °C. Nut coke acted as the role skeleton, improving the permeability of the cohesive zone of the original sinter. In addition, combined with the research results of chapter 3.1, it showed that the dissolution loss of nut coke increased the porosity, which provided excessive micropores for the gas flow to diffuse to the sinter surface. At the same time, the addition of nut coke could reduce the carbon dissolution of regular coke, which was beneficial to improving the skeleton function of regular coke. Besides, the cohesive zone was narrowed and characterized with a thin and non-dense shell, permitting the gas permeated easily.
When the nut coke ratio was 20%, with the hydrogen-rich ratio increasing from 0 to 20%, the △Pm declined from 5.3 to 2.0 kPa, and the S value significantly decreased from 517.5 to 42.9 kPa· °C. The reduction was strengthened through improving the hydrogen-rich ratio, and a large amount of FeO in the burden was reduced to metallic iron, forming a smaller amount of liquid oxides and more porous metallic iron particles, and the cohesive zone changed in the shape to form a thin shell with non-dense and good permeability. In addition, hydrogen rich reduced the dripping temperature and narrowed the melting temperature zone. When the temperature raised to a certain level, a large number of metallic iron melted and dropped, thus providing a lot of space and reducing the resistance of the updraft. At the same time, because the smaller atomic radius of hydrogen had strong permeability, the resistance of the burden column decreased, and thus, the improvement of hydrogen content could promote the gas permeability of the burden bed.
Effect of Nut Coke and Hydrogen-Rich Ratio on the Melt-Dropping Property
The drop of molten iron and the separation of slag and iron during blast-furnace smelting are closely related to the productivity of the blast furnace. The increase of nut coke and hydrogen-rich ratio leads to an enhanced reduction atmosphere in the furnace, changing the reaction behavior and structure of the burden. This may affect the separation and dripping behavior of iron slag in the softening–melting process. Therefore, it is of great significance to explore the influence of the nut coke and hydrogen-rich ratio on melt-dropping behavior.
The macroscopic morphology of droplets under different nut coke and hydrogen-rich ratio is shown in Fig. 8. When the hydrogen-rich ratio was 20% and the nut coke ratio was 20%, the slag and iron were separated more thoroughly, which was attributed to the acceleration of reduction when improving the nut coke and hydrogen-rich ratio. At the same time, the carburizing reaction in the reduction process was promoted, and the dripping temperature was reduced. Therefore, when the temperature arrived at the dripping temperature, a large number of iron dropped, and a good separation of slag and iron was realized. The drop of a large number of iron resulted in the release of space in the furnace burden, which was consistent with the above law of pressure difference.
In the process of the softening–melting experiment, starting from the first drop of molten iron, the weight changes of drops at different times were automatically recorded by a computer. The slag on the surface of the drop was separated to get iron and slag, and the carbon content in iron and the chemical composition of slag were detected. The weight of drop and the carbon content in iron under different nut coke and hydrogen-rich ratio are shown in Fig. 9. The chemical composition of the slag is shown in Table 4.
When the reducing gas was 60%N2-30%CO-10%H2, with the nut coke content increasing from 0 to 40%, the dripping quality increased at first and then decreased, and the carbon content in iron increased gradually. With the increase of nut coke content, the shrinkage of regular coke beds was beneficial to the increase of dripping quality. However, improving the nut coke ratio further, the enhanced wettability between slag and coke and the deterioration of slag fluidity made the hold-up of slag increase [29]. Therefore, there was a certain limit to the proportion of nut coke. With the increase of sinter reduction degree and carburization amount in metallic iron, the erosion of coke bed by dripping zone was alleviated, and the protection to the regular coke will be more obvious, which is beneficial to improving the air permeability of burden column. The fusion of the slag and the ash distributed on the nut coke surface increased the content of SiO2 and Al2O3 in the slag, and the addition of nut coke promoted the reduction, thus reducing the content of FeO in the slag.
When the nut coke ratio was 20%, with the hydrogen-rich ratio increasing from 0 to 20%, the dripping quality decreased, and the carbon content in iron increased gradually. With the decrease of FeO content in slag, the melting point and viscosity of slag increased, resulting in the decrease in dripping rate. In addition, Pan’s [30] research results showed that an over-high reduction degree would cause difficulty in dripping due to more iron remaining in the slag. As a result, the dripping quality was reduced. The carbon content in iron increased along with the hydrogen-rich ratio, which was consistent with the dripping temperature analysis above.
Characteristics of Coke-Slag-Iron Interface in Softening–Melting Process
The macroscopic morphology and interface microstructure of the burden column when the quenching temperature was at 1291 °C and 1443 °C are shown in Figs. 10 and 11.
The macrostructure of burden bed quenched at 1291 °C is shown in Fig. 10a. It could be seen that nut coke played a skeleton role in maintaining the burden bed structure, hindering the bonding between sinter ores, ensuring the formation of voids and gas channels, and promoting the diffusion of reducing gas. Because the bonding strength between sinter and coke was low, the sinter and nut coke were separated during the sampling process, so the sinter and nut coke were embedded, respectively, to observe the interface structure between them. The micro-morphology and element distribution of the sinter are shown in Fig. 10b. The surface part of the sinter was rapidly reduced, and the produced metallic iron on the interface was interconnected to form an iron layer. Entering inside of the sinter, the amount of metallic iron decreased gradually. The micro-morphology and element distribution of nut coke are shown in Fig. 10c. The ash began to accumulate on the surface of nut coke, and the wettability of solid oxide to molten iron was poor. If the nut coke surface was covered with solid ash, it was difficult for the molten iron to pass through, which was disadvantageous for carburizing. Therefore, the carburization of metallic iron was inhibited by the ash layer formed on the interface of nut coke. The key to improving the carburization speed of iron was to remove the solid ash at the interface.
The macrostructure of burden bed quenched at 1443 °C is shown in Fig. 11a. It could be seen that the sinter ores free of nut coke gradually collapsed and stuck together, resulting in a significant decrease in column porosity and hindrance of the gas passage. Where the nut coke existed, the nut coke was used as the skeleton of the burden column, which limited the aggregation of particle clusters, and ensured the existence of voids and channels, thus improving the air permeability of the cohesive zone. Nut coke was in close contact with the sinter, and the reducing gas could diffuse to the sinter through the pores between nut cokes, which promoted the reduction and improved the utilization rate of gas. In addition, Mousa's [23] research showed that the surface structure of the sinter was loose by adding nut coke, which was beneficial to the gas diffusion, thus promoting the reduction. The micro-morphology of the sinter is shown in Fig. 11b. The reduction degree of the sinter was high, the amount of metallic iron increased significantly and connected in a dendritic, and the slag phase was partially wrapped by iron and partly gathered on the surface of the sinter. The micro-morphology and element distribution of the contact interface of coke-iron-slag are shown in Fig. 11c. Iron existed mainly in the form of metallic iron. There was a slag layer between metallic iron and coke, which was evenly distributed and narrow, and a small amount of iron was in direct contact with coke. A small amount of molten slag and iron entered the pores between nut cokes, which might be the result of the improvement of the fluidity of the slag phase infiltrating into nut coke. Compared to that of being quenched at 1292 °C, the reaction interface between coke and slag iron was closely intertwined, and the slag could be rapidly fused with the ash distributed on the coke surface. Shin’s [31] research results show that the wettability of metal iron and carbon was better, and the wettability with molten slag was poor. Therefore, with the preceding the reaction, the slag formed on the surface of metallic iron covered the ash layer on the surface of coke and dissolved it, thus promoting the direct contact between iron and carbon, and then the reaction cycled to promote the carburization to iron. In addition, the graphitization degree of coke increased with the increase of temperature, which promoted the carburizing reaction [25]. All these conditions could lead to carburization and rapid melting in a relatively small temperature range. Therefore, carburizing of metallic iron reduced the dropping temperature.
Conclusions
-
(1)
The reduction degree of sinter and the mass loss of nut coke increased with improving the nut coke content. The reduction of iron oxides was restricted by coke solution loss and water–gas reaction. The gasification reaction produces CO and H2, which increases the reduction potential and accelerates the reduction rate of the sinter. In addition, the reduction process was also restricted by the water–gas replacement reaction, and the gasification heat absorption was beneficial to the positive reaction to produce CO2+H2.
-
(2)
With the increase of nut coke and hydrogen-rich ratio, the softening temperature zone increased, the melting temperature zone decreased, the average differential pressure of the stock column decreased, and the air permeability increased. The cohesive zone was narrowed and moved down. This change was beneficial to strengthen blast-furnace smelting and save coke.
-
(3)
During the softening–melting, the ash was concentrated on the surface of coke, and the poor wettability of solid oxides to hot metal prohibited the direct contact between iron and carbon. As the temperature was improved, the reaction interface of coke and slag iron was closely intertwined, and the slag formed on the surface of metallic iron would cover the ash layer on the surface of coke and dissolved it, which made the carbon exposing to the iron due to removal of ash layer, thus the carburization was promoted.
References
Ma KH, Deng JY, Wang G, Zhou Q, Xu J (2021) Utilization and impacts of hydrogen in the ironmaking processes: a review from lab-scale basics to industrial practices. Int J Hydrogen Energy 46:26646–26664. https://doi.org/10.1016/j.ijhydene.2021.05.095
Zhang B, Guo JZ, Zhang HW, Mao JJ, Li QJ, Wang DY, Hong X (2012) Experimental investigation on optimal carbon/hydrogen ratio for developing iron bath reactor with H2-C mixture reduction. Steel Res Int 83:175–180. https://doi.org/10.1002/srin.201100201
Wang DD, Xu J, Ma KH, Xu Y, Dang J, Kou MY, Lv XW (2017) Innovative evaluation of CO-H2 interaction during gaseous wustite reduction controlled by external gas diffusion. Int J Hydrogen Energy 42:14047–14057. https://doi.org/10.1016/j.ijhydene.2017.04.065
Chen YB, Zuo HB (2021) Review of hydrogen-rich ironmaking technology in blast furnace. Ironmak Steelmak 48:749–768. https://doi.org/10.1080/03019233.2021.1909992
Bilík J, Pustejovska P, Brozova S, Jursova S (2013) Efficiency of hydrogen utilization in reduction processes in ferrous metallurgy. Sci Iran 20:337–342. https://doi.org/10.1016/j.scient.2012.12.028
Chu MS, Guo XZ, Shen FM, Yagi JI (2007) Numerical simulation of innovative ironmaking technologies applied in blast furnace process. J Northeastern Univ 28:829–834
Can Y, Jens W, Thomas T (2017) Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J Clean Prod 154:488–501. https://doi.org/10.1016/j.jclepro.2017.03.162
Elsayed AM, Alexander B, Dieter S (2011) Effect of nut coke-sinter mixture on the blast furnace performance. ISIJ Int 51:350–358. https://doi.org/10.2355/isijinternational.51.350
Kashihara Y, Sawa Y, Sato M (2012) Effect of hydrogen addition on reduction behavior of ore layer mixed with coke. ISIJ Int 52:1979–1985. https://doi.org/10.2355/isijinternational.52.1979
Kashihara Y, Iwai Y, Sato T (2015) Effect of unconsumed mixed small coke on permeability in lower part of blast furnace. ISIJ Int 55:1237–1244. https://doi.org/10.2355/isijinternational.55.1237
Gavel DJ, Adema A, Van Der STELJ (2019) Effect of nut coke addition on physicochemical behaviour of pellet bed in ironmaking blast furnace. ISIJ Int 59:778–786. https://doi.org/10.2355/isijinternational.ISIJINT-2018-580
Chang ZY, Wang P, Zhang JL, Jiao KX, Zhang YQ, Liu ZJ (2018) Effect of CO2 and H2O on gasification dissolution and deep reaction of coke. Int J Min Met Mater 25:1402–1411. https://doi.org/10.1007/s12613-018-1694-4
Nishioka K, Ujisawa Y, Tonomura S (2019) Sustainable aspects of CO2 ultimate reduction in the steelmaking process (COURSE50 Project), Part 1: hydrogen reduction in the blast furnace. J Sustain Metall 2:200–208. https://doi.org/10.1007/s40831-016-0061-9
Tang J, Chu MS, Li F, Feng C, Liu ZG, Zhou YS (2020) Development and progress on hydrogen metallurgy. Int J Min Met Mater 27:713–723. https://doi.org/10.1007/s12613-020-2021-4
Liu YL, Wang JS, Zhang HJ, Liu JL, She XF, Xue QG (2015) Reduction behaviour of ferrous burden under simulated oxygen blast furnace conditions. Ironmak Steelmak 42:358–365. https://doi.org/10.1179/1743281214Y.0000000238
Bai MH, Long H, Ren SB, Liu D, Zhao CF (2018) Reduction behavior and kinetics of iron ore pellets under H2–N2 atmosphere. ISIJ Int 58:1034–1041. https://doi.org/10.2355/isijinternational.ISIJINT-2017-739
An XW, Wang JS, Lan RZ, Han YH, Xue QG (2013) Softening and melting behavior of mixed burden for oxygen blast furnace. J Iron Steel Res Int 20:11–16. https://doi.org/10.1016/S1006-706X(13)60090-4
Wang HT, Zhao W, Chu MS, Wang R, Liu ZG (2017) Effect and function mechanism of sinter basicity on softening-melting behaviors of mixed burden made from chromium-bearing vanadium-titanium magnetite. J Cent South Univ 24:39–47. https://doi.org/10.1007/s11771-017-3406-z
Wang HT, Chu MS, Wang ZH, Zhao W, Liu ZG, Tang J (2018) Research on the post-reaction strength of iron coke hot briquette under different conditions. JOM 70:1929–1936. https://doi.org/10.1007/s11837-018-3036-4
Bernasowski M (2014) Theoretical study of the hydrogen influence on iron oxides reduction at the blast furnace process. Steel Res Int 85:670–678. https://doi.org/10.1002/srin.201300141
Lu F, Wen LY, Zhao Y, Zhong H, Xu J, Zhang SF (2019) The competitive adsorption behavior of CO and H2 molecules on FeO surface in the reduction process. Int J Hydrogen Energy 44:6427–6436. https://doi.org/10.1016/j.ijhydene.2019.01.173
Natsui S, Shibasaki R, Kon T, Ueda S (2013) Effect of high reactivity coke for mixed charge in ore layer on reaction behavior of each particle in blast furnace. ISIJ Int 53:1770–1778. https://doi.org/10.2355/isijinternational.53.1770
Mousa EA, Senk D, Babich A (2010) Influence of nut coke on iron ore sinter reducibility under simulated blast furnace conditions. Ironmak Steelmak 37:219–228. https://doi.org/10.1179/030192309X12506804200906
Qie Y, Lyu Q, Liu XJ, Li JP, Lan CC, Zhang SH, Yan CJ (2018) Effect of hydrogen addition on softening and melting reduction behaviors of ferrous burden in gas-injection blast furnace. Metall Mater Trans B 49:2622–2632. https://doi.org/10.1007/s11663-018-1299-3
Lan CC, Zhang SH, Liu XJ, Lyu Q (2020) Change and mechanism analysis of the softening-melting behavior of the iron-bearing burden in a hydrogen-rich blast furnace. Int J Hydrogen Energ 45:14255–14265. https://doi.org/10.1016/j.ijhydene.2020.03.143
Pan YZ, Zuo HB, Wang JS, Xue QG, Wang G (2020) Review on improving gas permeability of blast furnace. J Iron Steel Res Int 27:121–131. https://doi.org/10.1007/s42243-019-00321-y
Du CB, Liu ZJ, Zhang JL, Wang YZ, Niu LL (2018) Effect of hydrogen content on reduction and softening-melting properties of iron bearing charges. China Metallurgy 28:19–23. https://doi.org/10.13228/j.boyuan.issn1006-9356.20180158
Ueda S, Kon T, Miki T, Kim SJ, Nogami H (2016) Effects of Al2O3 and MgO on softening, melting, and permeation properties of CaO-FeO-SiO2 on a coke bed. Metall Mater Trans B 47:2371–2377. https://doi.org/10.1007/s11663-016-0683-0
Liu Y, Xue Q, Guo W (2016) The dynamic dissolution of coke with slag in melting and dropping zone: 7th International Symposium on High-Temperature Metallurgical Processing. Springer
Pan Y, Zuo HB, Wang BX, Wang JS, Wang G, Liu YY (2018) Effect of reduction degree on cohesive zone and permeability of mixed burden. Ironmak Steelmak 47:322–327. https://doi.org/10.1080/03019233.2018.1493762
Shin M, Oh JS, Lee J (2015) Carburization, melting and dripping of iron through coke bed. ISIJ Int 55:2056–2063. https://doi.org/10.2355/isijinternational.ISIJINT-2015-115
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (U1960205) and China Minmetals Corporation (2020ZXA01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Veena Sahajwalla.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Yu, Y., Gao, Y. et al. Effect of Mixed Charging of Nut Coke and Sinter on Hydrogen-Rich Smelting Process of Blast Furnace. J. Sustain. Metall. 9, 280–293 (2023). https://doi.org/10.1007/s40831-022-00645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00645-2