Abstract
The utilization of iron coke hot briquette (ICHB) prepared by carbonizing iron ore–coal composite agglomerate made from hot-pressing the mixture of iron ore and blended coal has been considered to be an effective countermeasure to improve blast furnace ironmaking reaction efficiency and to reduce carbon emissions. The strength of ICHB after gasification reaction is overestimated by the Chinese National Standard (GB/T 4000/2008, equivalent to the Nippon Steel Corporation method) and should be evaluated by different methods. In this study, the post-reaction strength of ICHB with the addition of different ratios of iron ore under various conditions was experimentally investigated to illuminate the degradation mechanism of ICHB reacted with CO2. The results showed that, with increasing the iron ore addition ratio from 0% to 20%, the reactivity of ICHB reacted with CO2 at 1100°C for 2 h is remarkably increased, due to the catalytic effect of metallic iron in ICHB, while the post-reaction strength is distinctly decreased. Furthermore, stopping at the weight loss ratio of 20%, the strengths of ICHB after reaction at 1100°C under a CO2 atmosphere and a CO2/CO=1/1 atmosphere are clearly reduced, from 89.74% to 75.93% and from 85.24% to 73.65%, respectively. Meanwhile, the post-reaction strength of ICHB under CO2 is greater than that obtained under CO2/CO=1/1 atmosphere, since there is more time for the reaction gas to diffuse from the exterior to the interior of the ICHB under the latter condition. Additionally, the post-reaction strength of ICHB decreases with increasing weight loss ratio regardless of the reaction gas composition; however, it can be maintained at a high level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, global warming has become increasingly severe and has given rise to considerable concern. The steel industry, through the consumption of substantial amounts of fossil fuel, should be responsible for reducing CO2 emissions and reducing energy consumption, since it contributes to 7% of global CO2 emissions.1 However, CO2 discharged from steel works accounts for more than 15% of the total CO2 emissions in China.2 Blast furnace (BF) ironmaking is a widely used process in the steel industry for converting iron ore to liquid iron, and 80% CO2 per ton of liquid steel is released from BFs.3,4 Consequently, reducing CO2 emissions from BFs is highly significant for energy conservation and emission reduction practices of the steel industry.
BF operation with a low reducing agent rate is considered to be an effective countermeasure to realize low carbon emissions, and could be achieved by improving BF reaction efficiency. Natio et al.5,6 noted that lowering the temperature of the thermal reserve zone (TRZ) by using highly reactive coke can enhance the BF reaction efficiency. Coke is considered to be one of the most significant raw materials for BF ironmaking in terms of its effect on hot metal quality and BF operation.7 Normally, coke in BFs plays four roles: as a source of reduction agent, a source of energy and heat, supporting the stock column, and a carburizer for hot metal.8 The solution loss reaction is considered to be a dominant factor affecting coke degradation in BFs, which mainly occurs in the TRZ and the raceway. Currently, the most widely used indexes of coke for BF operation are the CRI (coke reaction index) and CSR (coke strength after reaction) developed by the Nippon Steel Corporation (NSC), as long as a coke with normal reactivity is used.9 However, it was reported10 that the CRI tested by the NSC method, especially for highly reactive coke, can be overestimated, since the CO2/(CO2 + CO) of ascending gas in actual BFs is approximately 0–0.5. Furthermore, the post-reaction strength of coke taken out from the shaft of the Hyundai Steel Company’s No. 1 BF is substantially higher than that from NSC tests. Some previous studies9,11,12 reported that the post-reaction strength of coke, especially that of highly reactive coke, should be evaluated by the constant weight loss percentage because the solution loss in actual BFs is approximately 20–30 wt.%. Additionally, it is well known that CSR is the strength index after a constant period of the gasification reaction, and thereby the highly reactive coke with higher CRI inevitably shows lower CSR. As a result of its low CSR, the generation of coke breeze can be promoted by using highly reactive coke in an actual BF, and this is one of the barriers to the promotion of the practical application of highly reactive coke. In this sense, the post-reaction strength of highly reactive coke is highly important and should be rationally evaluated. Nomura et al.13 investigated the post-reaction strength of catalyst-added highly reactive coke prepared by two catalyst addition methods,14 with simulated coke oven conditions under reaction stopping at a weight loss ratio of 20 wt.%. It was found that the post-reaction strength of the highly reactive coke produced by the post-addition method is equal to or greater than that of conventional coke. The type of catalyst and the catalyst-adding method could strongly affect the coke strength. However, the effects of catalyst addition ratio on the post-reaction strength of the highly reactive coke have not been intensively studied. Additionally, there are few reports on the post-reaction strength of carbon iron composite made by carbonizing iron ore–coal briquettes in a shaft furnace15,16,17,–18 under different conditions.
Depending on the Chinese raw material conditions, the preparation process of iron coke hot briquette (ICHB) has been proposed and optimized.19 In this paper, the post-reaction strength of ICHB with the addition of different ratios of iron ore was first investigated according to the NSC method. Simultaneously, the strength of ICHB after reaction was evaluated by another two methods. Finally, the ICHB strength after stopping the reaction at different weight loss ratios was estimated. Moreover, the degradation mechanism of ICHB reacted with CO2 was analyzed by modern measurement technologies. This paper could provide technological support and a theoretical basis for the practical application of ICHB.
Experimental
Raw Materials
Iron ore concentrate and blended coal from a steel works in China were used in the tests. The chemical composition of the iron ore (mass fraction %) was TFe 64.28%, FeO 7.86%, SiO2 2.85%, CaO 0.07%, Al2O3 0.06%, and MgO 0.35%. The proximate analysis of the blended coal (mass fraction %) was fixed carbon 67.95%, ash 8.95%, volatile matter 21.69%, and moisture 1.41%. The ash composition of the blended coal (mass fraction %) was CaO 6.65%, SiO2 45.18%, MgO 1.89%, Al2O3 26.99%, and TFe 6.05%.
The raw materials were dried at 105°C for 5 h in a draught drying cabinet. Then, they were crushed and adequately blended with 0–20 wt.% iron ore. Then, the mixed samples were heated to 300°C and immediately pressed to form the coal–iron ore agglomerate (hot briquette) by using a self-regulating hot press system with a pressure of 50 MPa. Finally, the hot briquette was carbonized at 1000°C for 4 h in a carbonization furnace with a heating rate of 3°C/min in the heating stage from room temperature to 1000°C, and the carbonized product was cooled in a N2 atmosphere with a cooling rate of 15°C/min to acquire ICHB containing carbon and metallic iron. The external sizes of the hot briquette and the ICHB sample were approximately 21 × 19 × 16 mm and 18 × 16 × 14 mm, respectively. The masses of the hot briquette and the ICHB were 5.3 g and 4.0 g, respectively. The chemical composition of ICHB with the addition of different ratios of iron ore are given in Table I.
Method and Procedure
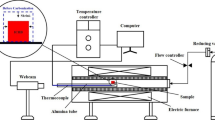
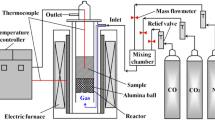
The gasification reaction test of ICHB was carried out in a macro-thermobalance reactor, as shown in Fig. S1a (see supplementary material). Approximately 200-g samples were reacted with CO2 at 1100°C for 2 h, and the changes in weight with time were automatically recorded by computer through measurement of the total weight of the samples and the reactor. The weight loss ratio of the samples before and after the reaction was defined as the reactivity index, according to the Chinese National Standard (GB/T 4000-2008, equivalent to the NSC method).20,21 The post-reaction strength of the reacted samples [PSR (CO2, 1100°C, 2 h)] was evaluated by \( {\text{I}}_{10}^{600} \), which is the percentage of the sample weight retained on a sieve with 10-mm apertures to the weight of the reacted samples after 600 revolutions (20 rpm × 30 min) in an I-type drum tester (Φ 130 × L 700 mm), as shown in Fig. S1b (see supplementary material).
Additionally, ICHB samples were reacted with CO2 at 1100°C until the weight loss reached 20% in the above macro-thermobalance reactor. The post-reaction strength was evaluated by \( {\text{I}}_{10}^{600} \) and specified as PSR (WL = 20%, CO2, 1100°C) in the following part. Furthermore, the post-reaction strength of ICHB was also evaluated by \( {\text{I}}_{10}^{600} \) after the reaction with 20% weight loss ratio at 1100°C under an atmosphere of CO2/CO=1/1, closely resembling that in the TRZ of BF,13 and was abbreviated as PSR (WL = 20%, CO2/CO=1/1, 1100°C) hereafter. Meanwhile, the post-reaction strength of ICHB with different weight loss ratios at 1100°C was investigated under two kinds of reaction conditions (CO2, CO2/CO=1/1), which were called PSR (CO2, 1100°C) and PSR (CO2/CO=1/1, 1100°C) in the following part.
After the reaction, the internal microstructure of ICHB was evaluated by scanning electron microscopy (SEM) with energy dispersive x-ray spectrometrr (EDS) (Zeiss, Ultra Plus). The surface of a half-cut ICHB was inlaid by thermosetting resin in a metallographic specimen inlaying machine, and was ground on different grades of abrasive paper. After grinding, the sample was polished and the polished samples were mounted on a conductive support and coated with a thin layer of gold–palladium alloy using a sputter coater.
Results and Discussion
Post-reaction Strength of ICHB Under CO2 Atmosphere with a Constant Reaction Time
Figure 1 gives the reactivity and post-reaction strength of ICHB under a CO2 atmosphere for 2 h at 1100°C and their relationship. It is clear from Fig. 1a that the reactivity of ICHB is gradually enhanced from 46.74% to 69.54%, while the PSR (CO2, 1100°C, 2 h) of ICHB is dramatically decreased from 74.08% to 36.81% with increasing the addition ratio of iron ore from 0% to 20%. Under the same experimental conditions, the reactivity and the PSR (CO2, 1100°C, 2 h) of conventional coke are approximately 31.39% and 57.08%, respectively.19 Metallic iron and iron oxide in ICHB increase with the addition ratio of the iron ore. In the gasification of ICHB, metallic iron and iron oxide have catalytic effects on the gasification reaction of carbon,22,23,–24 suggesting that the gasification reaction of ICHB is strengthened with the addition of iron ore. It was reported13 that highly reactive coke inevitably shows a lower post-reaction strength. Therefore, the PSR (CO2, 1100°C, 2 h) of ICHB gradually reduces. Furthermore, the line in Fig. 1b was calculated by the method of least squares.13 It was found that the PSR (CO2, 1100°C, 2 h) of ICHB decreases linearly with the increase of the reactivity.
Post-reaction Strength of ICHB Under CO2 Atmosphere with a Constant Weight Loss
Figure S2 (see supplementary material) shows the weight loss rates of the gasification reaction (CO2, 1100°C) stopped at the weight loss of 20% for conventional coke and ICHB with the addition of different ratios of iron ore. It was observed that the weight loss rates of conventional coke and ICHB are approximately straight lines. Moreover, the weight loss rates of ICHB are higher than those of coke. Furthermore, the weight loss rate of ICHB can be enhanced by increasing the addition ratio of iron ore. The main reason is that ICHB has a higher reactivity because of the catalytic effects of metallic iron and iron oxide in ICHB compared with those in coke.
The PSR (WL = 20%, CO2, 1100°C) values of conventional coke and ICHB are shown in Fig. 2. It can be seen that the PSR (WL = 20%, CO2, 1100°C) of ICHB decreases from 89.74% to 75.93% by increasing the addition ratio of iron ore from% 0 to 20%. For comparison, the post-reaction strength of normal coke stopped at the weight loss ratio of 20% is approximately 73.41%, which is lower than that of ICHB. The gasification reaction of highly reactive coke proceeds from the external region to the internal region due to its high reactivity and low porosity, while the reaction process of conventional coke occurs simultaneously in the outer and inner parts because of its low reactivity and high porosity.25,26,–27 The microstructure of coke and ICHB after the gasification reaction tested by SEM/EDS analyses is shown in Fig. 3. From Fig. 3a and b, it is distinguishable that, after the reaction, the microstructure of coke in the external region is similar to that in the internal region, which indicates that coke gasification follows the homogeneous reaction model. However, the external region of ICHB with the addition of 20% iron ore after the reaction presents an apparent stratification phenomenon (as seen in Fig. 3c), namely, an eroded layer, a transition layer and an uncorroded layer; simultaneously, the internal region of ICHB shows no change (as seen in Fig. 3d). Meanwhile, the carbon content of region 1 in the eroded layer (62.02%) is clearly lower than that of region 2 in the uncorroded layer (83.87%). Therefore, the gasification reaction of ICHB conforms to the unreacted-core reaction model. In other words, the gasification reaction proceeds preferentially at the surface in the case of the highly reactive ICHB. The highly reactive ICHB is more resistant to breakage after reaction compared with conventional coke for the same weight loss ratio.
The relationship between the PSR (WL = 20%, CO2, 1100°C) and the PSR (CO2, 1100°C, 2 h) is described in Fig. 4. Clearly, the PSR (WL = 20%, CO2, 1100°C) is greater than the PSR (CO2, 1100°C, 2 h), regardless of whether conventional coke or ICHB is compared. This is reasonable since the reactivity values (weight loss percentage in the NSC tests10) of conventional coke and ICHB used in this study are more than 20%, ranging from 30% to 70%. Furthermore, a linear relationship between the PSR (WL = 20%, CO2, 1100°C) and the PSR (CO2, 1100°C, 2 h) of ICHB is observed, and the correlation coefficient is approximately 0.94. Additionally, the difference in the PSR (WL = 20%, CO2, 1100°C) of ICHB is approximately 13.81%, which is smaller than the difference in the PSR (CO2, 1100°C, 2 h), 37.27%.
Post-reaction Strength of ICHB Under CO2/CO=1/1 Atmosphere with a Constant Weight Loss
Figure S3 (see supplementary material) presents the weight loss rates of conventional coke and ICHB gasification reaction stopped at the weight loss of 20% at 1100°C under the feed gas atmosphere of CO2/CO=1/1. It can be seen that the weight loss rate of coke is inferior to that of ICHB. Meanwhile, the weight loss rate of ICHB is accelerated with increasing the addition ratio of iron ore. Simultaneously, the gasification process of coke and ICHB can be divided into two stages. The weight loss rates of coke and ICHB are relatively higher in the first stage and slow down in the second stage. The main reason could be that the concentration of carbon monoxide in the gas phase is gradually increased, since it is the reaction resultant of carbon reacted with CO2.
The PSR (WL = 20%, CO2/CO=1/1, 1100°C) of conventional coke and ICHB and the relationship between the PSR (WL = 20%, CO2/CO=1/1, 1100°C) and the PSR (WL = 20%, CO2, 1100°C) for coke and ICHB are described in Fig. 5. It can be seen from Fig. 5a that, with increasing the addition ratio of iron ore, the PSR (WL = 20%, CO2/CO=1/1, 1100°C) of ICHB is visibly decreased from 85.24% to 73.65%, but the values are still superior to that of conventional coke (72.51%). Under the same experimental conditions, the reaction amounts of ICHB clearly exceed that of coke after the reaction was stopped at a fixed weight loss ratio because of the high reactivity of ICHB.
Figure 5b gives the relationship between the PSR (WL = 20%, CO2/CO=1/1, 1100°C) and the PSR (WL = 20%, CO2, 1100°C) for coke and ICHB. With adding different ratios of iron ore, the PSR (WL = 20%, CO2/CO=1/1, 1100°C) of ICHB always falls behind the PSR (WL = 20%, CO2, 1100°C) of ICHB. However, the post-reaction strengths of coke are nearly equal under the two conditions. Additionally, the linear relationship between the PSR (WL = 20%, CO2/CO=1/1, 1100°C) and the PSR (WL = 20%, CO2, 1100°C) of ICHB can be observed. The above phenomena can be explained by the microstructures of coke and ICHB after the reaction, as shown in Fig. 6. It is clear from Fig. 6a and b that, after the reaction under CO2/CO=1/1 atmosphere, coke has more and larger pores compared with that of coke reacted in CO2 atmosphere, and the loose structure of coke is observed in the former condition. Furthermore, the exterior of ICHB with adding 15% iron ore is seriously eroded in the CO2/CO=1/1 atmosphere, while it is partly eroded in the CO2 atmosphere, as seen in Fig. 6c and d. Meanwhile, macropores are generated in the interior of ICHB after reaction in the atmosphere of 50% CO2 together with 50% CO, and fine pores are formed in the interior of ICHB under CO2 atmosphere, as seen in Fig. 6e and f. Additionally, it is clear from Figs. S2 and S3 that the reaction times for coke and ICHB under the CO2/CO=1/1 atmosphere are longer than those in the atmosphere of CO2 when the weight loss ratio is approximately 20%, although the former condition has a lower partial pressure of CO2. Under these circumstances, the internal structures of coke and ICHB can be easily destroyed under the CO2/CO=1/1 atmosphere since the gas has more time to diffuse into the inner parts. Therefore, the PSR (WL = 20%, CO2/CO=1/1, 1100°C) has lower values.
Microstructures of coke and ICHB with the addition of 15% iron ore after reacting at 1100°C and stopping at the weight of 20% under different conditions: (a) exterior of coke under CO2 atmosphere, (b) exterior of coke under CO2/CO=1/1 atmosphere, (c) exterior of ICHB under CO2 atmosphere, (d) exterior of ICHB under CO2/CO=1/1 atmosphere, (e) interior of ICHB under CO2 atmosphere, and (f) interior of ICHB under CO2/CO=1/1 atmosphere
Post-reaction Strength of ICHB at Different Weight Loss Ratios
Figure 7 shows the strength of ICHB after the gasification reaction at 1100°C was stopped at different weight loss ratios. Clearly, with increasing weight loss ratio, the post-reaction strength of ICHB decreases regardless of the type of reaction gas used in the experiment. Simultaneously, the post-reaction strength of ICHB in the atmosphere of CO2/CO=1/1 is inferior to that of ICHB in the CO2 atmosphere, when the weight loss ratio is 10–40 wt.%. Additionally, the post-reaction strengths of ICHB under the above two conditions show a good linear relationship, with a correlation coefficient of about 0.995. Under these two conditions, the post-reaction strength of ICHB shows relatively high values compared with those of the NSC tests. Therefore, there is strong potential for the practical application of ICHB since it has sufficient post-reaction strength in terms of the actual operating conditions in BFs.
Conclusion
The metallurgical strength of ICHB and the related mechanism were experimentally investigated under different conditions. The following conclusions can be drawn:
-
1.
With increasing the addition ratio of iron ore from 0% to 20%, the reactivity of ICHB clearly increases from 46.74% to 69.54%, while the strength of ICHB after the gasification reaction (CO2, 1100°C, 2 h) decreases from 74.08% to 36.81%, according to the NSC method.
-
2.
With the addition ratio of iron ore increasing from 0% to 20%, the post-reaction strength of ICHB (WL = 20%, CO2, 1100°C) is remarkably decreased from 89.74% to 75.93%. Meanwhile, the strength of ICHB after the reaction was stopped at the weight loss of 20% at 1100°C in atmosphere of CO2/CO=1/1 distinctly decreases from 85.24% to 73.65%. The values in the former conditions are greater than those in the latter conditions.
-
3.
With increasing weight loss ratio, the post-reaction strength of ICHB decreases regardless of the reaction atmosphere. Additionally, the strength of ICHB after the reaction under the CO2/CO=1/1 condition is lower than that of ICHB in the CO2 atmosphere. At the same time, the resultant strengths present a linear relationship, and the correlation index is approximately 0.995.
References
X. Xing, H. Rogers, G.Q. Zhang, K. Hockings, P. Zulli, A. Deev, J. Mathieson, and O. Ostrovski, Fuel Process. Technol. 157, 42 (2017).
W.Q. Xu, W.J. Cao, T.Y. Zhu, Y.J. Li, and B. Wan, Steel Res. Int. 86, 1063 (2015).
Z.L. Zhang, J.L. Meng, L. Guo, and Z.C. Guo, Metall. Mater. Trans. B 47B, 467 (2016).
C. Zhou, G.W. Tang, J.C. Wang, D. Fu, T. Okosun, A. Silaen, and B. Wu, JOM 68, 1353 (2016).
M. Naito, A. Okamoto, K. Yamaguchi, T. Yamaguchi, and Y. Inoue, Tetsu-to-Hagané 87, 357 (2001).
M. Naito, A. Okamoto, K. Yamaguchi, T. Yamaguchi, and Y. Inoue, Nippon Steel Tech. Rep. 94, 103 (2006).
F.Y. Meng, S. Gupta, D. French, P. Koshy, C. Sorrell, and Y.S. Shen, Powder Technol. 320, 249 (2017).
M. Lundgren, R. Khanna, L.S. Ökvist, V. Sahajwalla, and B. Björkman, Metall. Mater. Trans. B 45, 603 (2014).
R. Guo, L. Sun, and Q. Wang, Coke Chem. 55, 300 (2012).
J.Y. An, J.B. Seo, J.H. Choi, J.H. Lee, and H. Kim, ISIJ Int. 56, 226 (2016).
K. Yamaguchi and T. Uno, J. Jpn. Inst. Energy 78, 760 (1999).
P. Barnaba, Coke Mak. Int. 92, 114 (1985).
S. Nomura, M. Naito, and K. Yamaguchi, ISIJ Int. 47, 831 (2007).
S. Nomura, H. Ayukawa, H. Kitaguchi, T. Tahara, S. Matsuzaki, M. Naito, S. Koizumi, Y. Ogata, T. Nakayama, and T. Abe, ISIJ Int. 45, 316 (2005).
T. Yamamoto, T. Sato, H. Fujimoto, T. Anyashiki, M. Sato, and K. Takeda, Tetsu-to-Hagané 96, 683 (2010).
T. Anyashiki, K. Fukada, and H. Fujimoto, JFE Tech. Rep. 13, 1 (2009).
M. Sato, T. Yamamoto, and M. Sakurai, JFE Tech. Rep. 19, 103 (2014).
B.D. Flores, A. Guerrero, I.V. Flores, A.G. Borrego, M.A. Díez, E. Osório, and A.C.F. Vilela, Fuel Process. Technol. 155, 238 (2017).
H. Wang, M. Chu, W. Zhao, R. Wang, Z. Liu, and J. Tang, Ironmak. Steelmak. 43, 571 (2016).
Chinese National Standard (GB/T 4000–2008): Coke-Determination of reactivity and strength after reaction.
M. Lundgren, L.S. Ökvist, and B. Björkman, Steel Res. Int. 80, 396 (2009).
H.T. Wang, W. Zhao, M.S. Chu, Z.G. Liu, J. Tang, and Z.W. Ying, Powder Technol. 318, 318 (2018).
Y. Yamamoto, Y. Kashiwaya, S. Miura, M. Nishimura, K. Katou, S. Nomura, M. Kubota, K. Kunimoto, and M. Naito, Tetsu-to-Hagané 96, 297 (2010).
A.P. Dhupe, A.N. Gokarn, and L.K. Doraiswamy, Fuel 70, 839 (1991).
S. Watakabe, K. Takeda, and K. Igawa, Tetsu-to-Hagané 88, 8 (2002).
T. Nishi, H. Haraguchi, and Y. Miura, Tetsu-to-Hagané 70, 43 (1984).
T. Nishi, H. Haraguchi, and T. Okuhara, Tetsu-to-Hagané 73, 1869 (1987).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (50804008), the China Postdoctoral Science Foundation (2016M601321), and the Fundamental Research Funds of the Central Universities of China (N162503003).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Chu, M., Wang, Z. et al. Research on the Post-reaction Strength of Iron Coke Hot Briquette Under Different Conditions. JOM 70, 1929–1936 (2018). https://doi.org/10.1007/s11837-018-3036-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-3036-4