Abstract
In ironmaking, maintaining gas permeability in blast furnace with low coke rate operation is essential to reduce carbon emissions. The high pressure loss in the cohesive zone decreases the gas permeability and affects the productivity of blast furnace. In order to increase the gas permeability in the cohesive zone, the thickness of the cohesive layer should be decreased. For this purpose, increasing softening temperature and decreasing dripping temperature of the iron ore are desired. In this study, softening, melting, and permeation of SiO2-FeO-CaO-Al2O3-MgO on a coke bed were investigated. The oxide sample in a tablet form was heated under CO/CO2 atmosphere, and the shape of the tablet was observed. The softening and melting temperatures of the SiO2-FeO-CaO system changed with the addition of Al2O3 and MgO. Oxide tablets with and without Al2O3 softened below and above the solidus temperature, respectively. The melting temperatures varied with the ratio of CO/CO2 in the gas. The permeation temperature was independent of the melting temperature, but dependent on the wettability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of new processes for mitigating CO2 emissions in ironmaking has been widely studied.[1,2] Coke rate can be reduced from the levels of conventional operation by replacing coke with a CO and H2-based gas mixture. Coke used in the blast furnace acts as structural material for maintaining gas permeability, and thus, low coke rate operation causes an increase in the pressure loss. With the development of low coke rate operation technologies, it is increasingly important to control the flow of solids, liquids, and gases in the blast furnace.[3–5] In the cohesive zone, the thickness of the coke slit decreases with the decreasing coke rate. The iron ore melts partially and fills the vacancy in the packed bed, decreasing the permeability. Even for the conventional operation, the pressure loss is strongly dependent on the softening layer in the cohesive zone.[6,7] Therefore, operation at low coke rate is dependent on increased permeability in the cohesive zone.
The ore layer consists of a solid–liquid mixture in the cohesive zone. The layer thickness can be decreased by delaying the softening and facilitating the dripping of the iron ore. In a previous study,[8] softening, melting, and permeation properties of CaO-FeO-SiO2 on a coke bed were investigated. The following results were obtained: (1) The measured softening and melting temperatures were higher than the calculated solidus and liquidus temperatures, respectively. (2) Deformation of the oxide tablet was affected by the liquid phase ratio. (3) Permeation temperature of the oxide melt was independent of the melting temperature. (4) The permeation of the melt was dependent on the wettability on coke.
The permeability of sinter layer in the blast furnace, including the effects of Al2O3 and MgO in the ore, has been extensively studied. It has been reported that the addition of MgO to the sinter ore increases the softening temperature in the cohesive zone.[9] During the liquid phase formation, addition of Al2O3 or MgO can decrease the melting temperature of the ore.[10] Liquid holdup in the packed bed is increased with the increasing Al2O3 concentration.[11] The sinter ore used in ironmaking contains Al2O3 and MgO. An adequate method for decreasing the thickness of cohesive zone can be developed after clarifying the effects of these compounds on the softening and melting properties of the sinter ore.
In this study, we investigated the softening, melting, and permeation of an oxide tablet of SiO2-FeO-CaO, containing Al2O3 and/or MgO, on a coke bed. The changes in the shape of the tablet were observed during heating. The effects of Al2O3 and MgO addition, reduction ratio, and CaO/SiO2 ratio of the oxide were studied. Differences between the softening and solidus temperatures, and the melting and liquidus temperatures were determined by comparing the experimental findings with the calculation results based on a thermodynamic database.[12]
Experimental Procedure
Softening, melting, and permeation properties of SiO2-FeO-CaO-Al2O3-MgO oxide tablet were observed. As reported previously,[8] a small particle of coke was used for the blast furnace burden, and the oxide tablet was placed on the coke bed in an aluminum crucible.
Measurement
Reagent grade Fe, Fe2O3, CaCO3, SiO2, Al2O3, and MgO were used to prepare the samples. CaO was prepared by decomposing CaCO3 at a temperature of 1673 K (1400 °C) for 14 h. Fe and Fe2O3 were mixed at a stoichiometric ratio, and the mixture was fully melted and quenched to form FeO. The oxides were mixed at the compositions shown in Table I.
The samples s5-8 were prepared by melting the mixed reagents at 1723 K (1450 °C), which was followed by quenching on a water cooled copper plate in a stream of He gas and then by grounding in an agate mortar. Two grams of oxide mixture was pressed in a die to produce a tablet with a diameter of 10 mm. The sample s1 was used as the standard material.[8] The samples s2-4, s5-8, s9-10, and s11-12 were used to investigate the effects of additives, i .e., Al2O3 and MgO, pre-melting, FeO concentration and the basicity (CaO/SiO2), respectively.
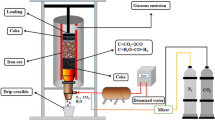
Coke of 1-2 mm diameter was filled into a shallow alumina crucible (φ 23 mm in diameter), and the oxide tablet was placed on the coke bed. The coke was prepared from screening small particles of coke. The concentrations of fixed carbon, volatile matter, ash, and water in the coke were 87.7, 0.3, 12.1, and 0.3 mass pct, respectively. As shown in Figure 1, a horizontal electric resistance furnace with a mullite tube (φ 35 mm ID) was employed for the observation of the tablet. The sample was placed in the center of the tube, and the changes in the appearance of the sample were observed using a camera placed close to the end of the reaction tube. The temperature of the furnace was controlled by a proportional-integral-derivative (PID) controller connected to a thermocouple located near the sample. N2 gas was introduced at a flow rate of 100 ml/min, and the temperature was increased to 1173 K (900 °C) at a heating rate of 10 K/min. The temperature was subsequently increased to 1723 K (1450 °C) at a rate of 5 K/min under a CO/CO2 gas mixture flow and maintained at 1723 K (1450 °C) for 1800 seconds. The mixing ratio of CO/CO2 was controlled at 4/6, 5/5, and 6/4 with the use of mass flow controllers. The softening, melting, and permeation temperatures were measured by observing the changes in the shape of the tablet.
Observation of Samples
In this study, the softening, melting, and permeation processes were observed. The method employed in the determination of process temperature is explained elsewhere.[8] Deformation of the shape of the tablet indicated the formation of a liquid phase during the heating. The temperature of softening was defined as the temperature at which the deformation starts. The melting temperature was defined as the temperature at which the softened sample transformed into a smooth droplet. The permeation temperature was defined as the temperature at which the sample in liquid phase gradually permeated into the coke bed after softening, or the temperature at which the droplet suddenly permeated into the coke bed after remaining on the coke bed for a certain period of time. If a temperature of 1723 K (1450 °C) was reached and the droplets did not permeate into the coke bed, the permeation temperature was recorded as 1723 K (1450 °C) regardless of the time taken for the permeation.
Experimental Results
Effects of Al2O3 and MgO on Softening and Melting
The CaO/SiO2 ratio and FeO concentration of the sample s1[8] were 1.2/1 and 30 mass pct, respectively. The softening, melting, and permeation temperatures of the CaO-FeO-SiO2 mixture containing Al2O3 and/or MgO heated under a gas flow with CO/CO2 = 5/5 are shown in Figure 2. Circle, triangle, square, and inverted triangle represent the samples s1, s2, s3, and s4, respectively.
The softening temperature of samples s2 and s4, which contain Al2O3, are lower than that of sample s1, indicating that Al2O3 decreases the softening temperature. The softening temperature of sample s3 is close to that of sample s1, and higher than that of sample s4, indicating that MgO reduces the influence of Al2O3 on decreasing the softening temperature. Similarly, the addition of Al2O3 and MgO, respectively, decreases and increases the melting temperature.
The softening, melting, and permeation temperatures of pre-melted samples s5-8 are shown in Figure 3. The compositions of the samples s5, s6, s7, and s8 are the same as those of s1, s2, s3, and s4, respectively. Comparing Figure 3 with Figure 2, it can be seen that the softening and melting temperatures overall decrease with pre-melting for all the samples, except for samples s4 and s8, for which the softening temperature increased by pre-melting. The decrease in melting temperature caused by pre-melting is greater than that in softening temperature.
It can be seen that when the structure of sample is uniform, the difference between the softening and melting temperatures is small. Permeation temperature, on the other hand, does not change with pre-melting, and the droplets of samples s5-8 maintained their shape on the coke bed up to 1723 K (1450 °C). There was no difference in time taken for permeation.
The liquid ratios of CaO-SiO2-FeO oxide systems containing Al2O3 and/or MgO were simulated by a thermodynamic database[12] with varying temperatures. For each temperature, the oxygen partial pressure (\( {\text{P}}_{{\text{O}_{2} }} \)) was determined under a gas flow with CO/CO2 = 1/1. The results are shown in Figure 4. The lines indicate liquid ratio of s1-4 calculated. The solid and open circles denote the softening and melting temperatures measured in this study, respectively. The softening of the tablet started at a different temperature than the calculated solidus temperature. For samples s1, s3, s5, and s7, the softening temperature was higher than the calculated solidus temperature. The softening of pre-melted samples s5 and s7 was observed at a temperature lower and closer to the solidus temperature than the softening temperature of the mixing samples s1 and s3.
For the samples s2, s4, s6, and s8, the softening temperature was lower than the calculated solidus temperatures. According to the phase diagram of the FeO-Al2O3-SiO2 system,[13] the lowest eutectic temperature of the system is 1356 K (1083 °C). By contacting SiO2-Al2O3-FeO in the mixed sample, the liquid phase was formed, and the tablet was softened at a temperature lower than the solidus temperature. Similarly, the liquid phase in non-equilibrium state might be formed at a temperature lower than the solidus temperature by contacting CaO-FeO-SiO2-Al2O3 and CaO-FeO-SiO2-Al2O3-MgO.
The results showed that samples containing Al2O3 formed an initial liquid in non-equilibrium condition. Compared to mixed samples, the softening temperatures of the pre-melted samples were close to their respective solidus temperature, regardless of their composition. For uniform samples, the softening temperatures were closer to the solidus temperatures.
When the liquid phase ratio of the mixed sample at equilibrium was 80 pct or more, melting was observed. The melting temperature was lower for the pre-melted samples, when the liquid phase ratio at equilibrium was between 40 and 90 pct. During melting, the oxide tablet became a droplet and was in a solid–liquid mixed state.
The droplets remained on the coke bed for up to 10 minutes. Then, the liquid permeated into the bed. There was no relation between the temperatures of melting and permeation, and pre-melting did not show any effect on permeation.
Effects of FeO Concentration on Softening and Melting
FeO concentration in the sinter ore decreases with the progress of reduction in the blast furnace. In this study, the influence of FeO concentration on the softening and melting behaviors was determined changing the initial concentration of FeO in the sample with fixed CaO/SiO2 = 1.2/1. The experimental results are shown in Figure 5. Solid and open circles denote CaO-FeO-SiO2 and CaO-FeO-SiO2-Al2O3-MgO samples, respectively.
The softening temperatures of samples s1 and s4 increased slightly with a decrease in FeO concentration from 30 to 10 pct. Comparing the samples s1 and s9, it was shown that the melting temperature was increased by 40 K (40 °C) with a decrease in FeO concentration. The melting temperatures of samples s4 and s10 were close. For all the samples, permeation was observed after the formation of droplet on coke bed at 1723 K (1450 °C). FeO concentration did not affect the time required for permeation.
The experimentally measured softening and melting temperatures and the calculated liquid phase ratio are shown in Figure 6. Solid and open circles represent the softening and melting temperatures, respectively. The lines indicate calculated liquid ratios of s1, s4, s9, and s10. The data points fell close to the calculated liquid ratio line. Since a liquid olivine phase was formed as an initial liquid from CaO-FeO-SiO2 samples s1 and s9, the temperatures of initial liquid phase formation and softening were approximately 1573 K (1300 °C). The solid phase equilibrated with the liquid phase was 2CaO·SiO2. Since the solid ratio with 2CaO·SiO2 composition increased with the decreasing FeO concentration, liquid formation was delayed.
The calculated solidus temperatures of samples s4 and s10 were 1513 K (1240 °C), which is the eutectic of melilite, FeO, and 2CaO·SiO2, and 1533 K (1260 °C), which is eutectic of olivine, melilite, and 3CaO·MgO·2SiO2, respectively. Solidus temperatures of samples s4 and s10, which contain both Al2O3 and MgO, were higher than those of samples s1 and s9. The softening temperatures of samples s1 and s9 were higher than those of s4 and s10, and also higher than the calculated solidus temperatures of samples s1 and s9, respectively. The softening temperatures of samples s4 and s10, which contain only Al2O3, were lower than their respective solidus temperatures.
The liquid phase ratios of samples s4 and s10 were significantly different from each other: the calculated liquid ratios of s4 and s10 were 100 and 46 pct at 1573 K (1300 °C), respectively. However, the measured melting temperatures of s4 and s10 were very close to each other. In a previous study,[8] it was reported that FeO concentration has no effect on the melting temperature but causes a change in liquid ratio. The shapes of s4 and s10 at 1523 K (1250 °C) are shown in Figure 7. The softening and melting temperatures of both samples were approximately 1473 K and 1578 K (1200 °C and 1305 °C), respectively. 1523 K (1250 °C) is higher than the solidus temperature of s4 and lower than the solidus temperature of s10. Deformation in sample s4 is clearly shown in Figure 7. The sample 10 was shrunk down in size and had slight deformation at the surface, but maintained its shape. Even if there was a deformation in the surface caused by the liquid phase formation below the solidus temperature, only non-equilibrium liquid phase was formed, and the liquid phase fraction was not increased.
Figure 8 shows the softening, melting, and permeation temperatures of the samples s1-s4 (CaO/SiO2 = 1.2/1 and 30 wt pct FeO) at atmospheric pressure with CO/CO2 ratios of 4/6, 5/5, and 6/4. Influence of CO/CO2 ratio on the softening temperature was small, and temperature difference between the samples was not evident.
The melting temperatures increased with the increasing CO ratio. The melting temperatures of samples s1 and s3, which did not contain Al2O3, increased by 100 K (100 °C) with an increase in the CO ratio from 0.4 to 0.6. The softening behavior in the measurements was changed by changing the CO/CO2 ratio, more specifically by changing the \( {\text{P}}_{{\text{O}_{2} }} \). The influence of \( {\text{P}}_{{\text{O}_{2} }} \) on melting was obtained using a thermodynamic database. Influence of \( {\text{P}}_{{\text{O}_{2} }} \) on the eutectic temperature or liquid phase ratio was not clear. The permeation temperature was 1723 K (1450 °C), and the permeation was observed approximately 10 minutes after the formation of droplet. The effect of \( {\text{P}}_{{\text{O}_{2} }} \) on the permeation behavior was not determined.
The \( {\text{P}}_{{\text{O}_{2} }} \) changed depending on the CO/CO2 ratio in the atmosphere. In this study, FeO exhibited a stable phase at temperatures over 1173 K (900 °C). Based on experimental results and calculated phase diagram of the CaO-FeO-SiO2,[13] it was concluded that the effect of FeO concentration in the samples on the solidus and liquidus temperatures was small. However, the rate of liquid phase formation decreased with the decreasing FeO concentration. FeO in samples would be reduced on contacting the coke bed. Thus, the samples were exposed to both reducing and oxidizing atmospheres.
The oxide phase became a mixture of solid and liquid at temperatures above the softening temperature. Since mass transfer is relatively easy through the liquid phase, reduction with coke would be accelerated. Reduction becomes easier with the decreasing \( {\text{P}}_{{\text{O}_{2} }} \), while it progresses faster at high CO/CO2 ratios. FeO reduction after reaching the solid–liquid mixed state decreased the rate of liquid phase formation, decreasing the melting temperature. It cannot be confirmed from the experiments; however, there is a possibility of increasing the dissolution rate with the increasing \( {\text{P}}_{{\text{O}_{2} }} \) in atmosphere.
Effects of CaO/SiO2 Ratio on Softening and Melting
The softening and melting temperatures of the CaO-FeO-SiO2-Al2O3-MgO mixture heated under a gas flow with CO/CO2 = 5/5 are shown in Figure 9. The ratios of CaO/SiO2 were 0.43, 1.2, and 2.3, and the FeO concentration was 30 mass pct. The influence of CaO/SiO2 ratio on the softening temperature was small: the temperature changed from 1433 K to 1473 K (1160 °C to 1200 °C). Softening temperature of the CaO-FeO-SiO2 ternary system changed with basicity.[8] However, the temperature of the system containing Al2O3 and MgO changed little with basicity. The melting temperature increased with the increasing basicity. The permeation into the coke bed was observed after keeping the system at 1723 K (1450 °C) for approximately 10 minutes. There was no relation between basicity and permeation time at 1723 K (1450 °C).
The measured softening and melting temperatures of samples s4, s11, and s12 and the calculated liquid phase ratios are shown in Figure 10. Solid and open circles represent the softening and melting temperatures, respectively. The lines indicate calculated liquid ratio.
The softening temperatures of samples s11 and s12 were higher than their respective solidus temperatures. The softening temperature of sample s4 was lower than its solidus temperature. For all the samples, the melting and liquidus temperatures were close to each other.
Since the weight ratio of CaO/FeO/SiO2 of sample s11 was close to the composition of eutectic CaCO3-FeSiO2-Fe2SiO4, even with Al2O3 and MgO, the difference between softening and melting temperatures was small.
Since the weight ratio of CaO/FeO/SiO2 of sample s12 corresponded to CaO-2CaO·SiO2-FeO composition, CaO-FeO-rich initial liquid was formed at 1403 K (1130 °C). The initial liquid phase of sample s12 would be in equilibrium with CaO and 2CaO·SiO2. Since the melting temperatures of these solid phases are high, s12 became liquid at or above 1623 K (1350 °C).
Control of Iron Ore Composition to Decrease Cohesive Layer Thickness
In order to decrease the thickness of the cohesive zone in the blast furnace, it is needed to increase the softening and decrease the dripping temperatures of the iron ore. Softening caused by liquid formation leads to deformation and blockage of the packed bed. The liquid phase ratio increases with melting. As the ratio increases, the liquid drops into the coke bed. Experimental results have shown that there is a difference between softening and solidus temperatures, and the permeation or fall of the droplet into the coke bed is determined by wettability and is not affected by the melting temperature.
Addition of Al2O3 to the CaO-FeO-SiO2 causes the formation of an initial liquid with non-equilibrium composition by heating and decreases the softening temperature. Addition of MgO slightly increases the softening temperature of CaO-FeO-SiO2. When components are non-uniform, there is a difference between the temperature of softening initiation and the solidus temperature at equilibrium. Since the influence of the initial liquid with non-equilibrium composition on the rate of liquid formation is small, it is not clear whether there is a correlation between softening and the clogging of the packed bed. For a more detailed analysis of softening, the softening step should be considered separately from the deformation of iron ore by formation of the initial liquid, and blockage of void of the ore layer by liquid.
Addition of Al2O3 and MgO, respectively, decreases and increases the melting temperature of CaO-FeO-SiO2. It also increases the difference between the softening and melting temperatures. It has little effect on the wettability of the oxide with the coke, since there is no relationship between the melting and permeation temperatures.
Addition of Al2O3 and MgO, respectively, increases and decreases the temperature difference between softening and dripping of the iron ore, in the temperature range of solid–liquid coexistence in the cohesive zone. When the \( {\text{P}}_{{\text{O}_{2} }} \) is low, the rate of liquid formation is suppressed, and the temperature difference between softening and melting is increased. Influence of basicity on the softening temperature of CaO-FeO-SiO2-Al2O3-MgO is less than that on the softening temperature of CaO-FeO-SiO2. On the other hand, since the melting temperature of CaO-FeO-SiO2-Al2O3-MgO is close to the melting temperature of CaO-FeO-SiO2, higher basicity has the advantage of suppressing the increasing liquid phase formation rate.
When wettability of slag on coke is poor, decreased Al2O3 concentration, MgO addition, and increased basicity can decrease the temperature difference between softening and dripping. In addition, decreasing concentration or enhanced reduction of FeO is effective on reducing the liquid formation rate.[8] When wettability of slag on coke is good, increased concentrations of Al2O3 and FeO, and decreased basicity can decrease the temperature difference between softening and permeation of ore into the coke bed.
Dynamic wettability between coke and slag is dependent on the reaction conditions at the interface, the properties of coke, and composition of slag, which cannot be predicted by the available data and conventional methods on wettability. Further research on dynamic wettability is needed to determine the optimum properties of the iron ore.
Conclusions
The softening, melting, and permeation properties of a tablet of CaO-FeO-SiO2 system containing Al2O3 and/or MgO were investigated. The changes in the shape of the tablet were observed by heating under CO/CO2 gas flow. The following conclusions were drawn:
-
From CaO-FeO-SiO2 oxide containing Al2O3, an initial melt at non-equilibrium composition is produced by heating at a temperature lower than the eutectic temperature.
-
When Al2O3 and MgO are both added to the oxide system, the variation in the softening temperature due to change in basicity is mitigated.
-
The initial liquid with non-equilibrium composition has a small effect on the liquid ratio.
-
The influence of FeO concentration on softening and melting temperatures is small. However, decrease in FeO concentration in the oxide reduces the liquid phase formation rate.
-
The temperature difference between softening and melting is increased with the decreasing \( {\text{P}}_{{\text{O}_{2} }} \). In addition, \( {\text{P}}_{{\text{O}_{2} }} \) might affect the dissolution rate of solid oxide phase.
-
The influence of basicity on the softening temperature of CaO-FeO-SiO2-Al2O3-MgO is smaller than that on the softening temperature of CaO-FeO-SiO2. Melting temperature of the system increases with the increasing basicity.
References
T. Miwa, H. Okuda, M. Osame, S. Watakabe, and K. Saito: EECR Steel, Metec InSteelCon 2011, Dusseldorf, 2011, ECCR-58, DVD.
2. M. Naito, K. Takeda, Y. Matsui: ISIJ Int., 55(2015), 7-35.
3. S. Ueda, T. Miki, T. Murakami, H. Nogami, T. Sato: Tetsu-to-Hagane, 99(2013), 1-11.
4. M. Hayashi et al.: Tetsu-to-Hagane, 100(2014), 211-226.
5. H. Nogami, Y. Ueki, T. Murakami, S. Ueda: Tetsu-to-Hagane, 100(2014), 227-245.
6. K. Ono, Y. Hida, A. Shigemi, and K. Kodama: Tetsu-to-Hagané, 61(1975), 777-786.
7. Y. Yamaoka, H. Hotta, and S. Kajikawa: Tetsu-to-Hagané, 66(1980), 1850-1859.
8. S. Ueda, T. Kon, T. Miki, S.J. Kim, H. Nogami: ISIJ Int., 55(2015), 2098-2104.
9. M. Matsumura, M. Hoshi and T. Kawaguchi: Tetsu-to-Hagane, 92(2006), 865-874.
10. K. Yamaguchi, H. Ueno, T. Kawaguchi, and S. Matsunaga: ISIJ Int., 34(1994), 964-972.
11. M. Hino, T. Nagasaka, A. Katsumata, K. Higuchi, K. Yamaguchi and N. Kon-no: Metal. Mater. Trans. B, 30B(1999), 671-683.
FACTSAGE, Ecole Polytechnique CRCT, Montreal.
ACerS-NIST Phase Equilibrium diagrams PC database v. 4.0, ACerS/NIST 2014.
Acknowledgment
This paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 25, 2016.
Rights and permissions
About this article
Cite this article
Ueda, S., Kon, T., Miki, T. et al. Effects of Al2O3 and MgO on Softening, Melting, and Permeation Properties of CaO-FeO-SiO2 on a Coke Bed. Metall Mater Trans B 47, 2371–2377 (2016). https://doi.org/10.1007/s11663-016-0683-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0683-0