Abstract
Geochemical baseline (geogenic+anthropogenic) and background (geogenic) levels of heavy metals in the ground waters from basaltic aquifers of Deccan Volcanic Province of India depicted intra-annual variability on spatial scale. Classified into ‘Group I’ (Fe > Mn > Zn > Pb) and ‘Group II’ (Mo > Ni > Cr > Cu), metals depicted contrasting enrichment and depletion patterns seasonally, attributable to geologic controls and water table fluctuations. While, fortification in Mo–Ni in post-monsoon (high water table) is on account of combined lithogenic+anthropogenic contributions, pre-monsoon augmentation in Fe–Mn (low water table) is entirely lithogenic. Positive values of Normalised Difference Dispersal Index, replicates the dominant role of soil/vadose zone as a chief supplier of metals to the groundwater during post-monsoon, while negative figures recommend host rock as a primary source of accretion of metals in pre-monsoon. Higher Ni/Cr ratios for wells in alluvial aquifer (fertile agriculture plain) than the basaltic and dyke aquifers, further suggest enhanced input of the elements (Mo, Ni > Cu) from soil and agriculture land use in post-monsoon season. Values of Ni/Cr ratio above unity for majority of the wells in post-monsoon and nearly 50 % wells in pre-monsoon suggest privileged weathering of olivine, followed by pyroxene > plagioclase feldspar (Ni/Cr <1) as a major cause of heavy metal load to the groundwater. Pearson correlation coefficients authenticate these inferences by means of elemental associations. The study unveils multi-source derivation of heavy metals related to seasonal fluctuation in the water table conditions leading to range of heavy metals in the groundwater from the study area. The target hazard quotient (THQ) values of heavy metals closer to unity and above unity highlight the possible health risk hazard associated with the consumption of metal contaminated groundwater. The study thus highlights the importance of baseline geochemical mapping to assess the state of near surface environment as heavy metals are closely linked to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amongst several pollutant groups, the heavy metals are kept under surveillance for their mobility and availability from the point of view of biogeochemical and eco-toxicological significance (Tume et al. 2008; Sharma and Subramanium 2010; PuYang et al. 2015; Gupta et al. 2015). Heavy metals present in the form of free cations occur in miniature quantities dissolved in the water in concentrations less than 1 mg per liter (U.S.G.S. 1993). They are the most frequently analyzed among numerous pollutants occurring in water and sediments, as well as in the majority of organisms (Michalec et al. 2014). The heavy metals (Fe, Mn, Ni, Co, Zn, Cu, and Pb) have also been used as indicators to ascertain the degree of contamination and/or pollution (Engin et al. 2015). Accrual of some of these metals in aquatic environment has direct consequences on human health and ecosystem (Adomako et al. 2008; Gupta et al. 2015). Further, concerns about food safety have been raised because of heavy metal contamination of soils (Zhang et al. 2015). Their significance to human health and relation to plant growth have therefore prompted many workers to initiate studies on understanding their relative contributions from different sources and mechanisms of metal transport (Pawar and Nikumbh 1999; Alam et al. 2003; Rajmohan and Elango 2005; Kelepertzis 2014; Arinze et al. 2015; Shirke and Pawar 2015). Health risks to humans via dietary intake of fish were then assessed based on the target hazard quotient (THQ) and hazard index (PuYang et al. 2015) in China. Similarly, Gupta et al. (2015) have conducted studies along Gomati River in India by using THQ to determine quality of dietary intake of fish and water. Fernandez and Nayak (2015) have attempted to study the speciation of metals and their distribution in tropical estuarine mudflat sediments, to understand the depositional environment, post depositional processes, bioavailability of metals and their toxicity.
In aquatic environment heavy metal pollution can result from direct atmospheric deposition, weathering of rocks and through anthropogenic sources (Han et al. 2006; Challenger 2007; Suyash et al. 2008; Kelepertzis 2014; Tiwari et al. 2015). To discriminate between anthropogenic and natural sources, distinction of background and baseline values of toxic elements from anomalies is achieved through environmental geochemical mapping (Albanese et al. 2007; Galan et al. 2008; Yuan et al. 2014). While, background values point to sources of metals related exclusively to geogenic origin, the geochemical baseline (anthropogenic and geogenic inputs) is used to predict future trends in metals accumulations. The International Geological Correlation Program initiated IGCP Project 360 on Global Geochemical Baselines to assess the natural variations in the concentration of an element in the superficial environment (Galan et al. 2008). In the case of water resources in general and groundwater resources in particular, the geochemical mapping of background values is not attempted so far in many countries and India is no exception to this. However, in absence of any availability of regional level geochemical maps, using small area based isolated studies as a ready reference in identifying lithogenic/anthropogenic derivation of metals in the aquatic environment is imperative. Although for most anthropogenically derived metallic load, the soils are regarded as an ultimate sink, nonetheless the difficulty lies in the complex nature of the soils and their differing capacity for receiving and purifying metal pollutants (Fazeli et al. 1998; Wong et al. 2006; Krishna and Govil 2007; Yuan et al. 2014). Because, the metal retention capacity of the soils is largely dependant on the soil structure, humus content, calcareousness, pH, exchange capacity and water regime (Orlov 1992); lack of such a favorable soil structure for metal retention finally allows them to enter into the aqueous system. Though, heavy metals occur in minute quantities in water as compared to soils, their distribution is influenced by (a) nature of parent material, (b) climate and (c) their relative mobility depending on soil parameters such as mineralogy, texture and classification (Krishna and Govil 2007; Galan et al. 2008; Shirke and Pawar 2015). Metals enter the natural aqueous system through several sources: (a) weathering of rocks and soils directly exposed to surface and ground waters forming the largest natural source (Patino et al. 2003) and (b) the anthropogenic sources viz. urbanization, agriculture and industrialization (Pawar and Nikumbh 1999; Wong et al. 2006; Krishna and Govil 2007; Challenger 2007; Suyash et al. 2008; Suyash and Pawar 2010; Michalec et al. 2014; Tiwari et al. 2015; Arinze et al. 2015). Out of these two main contributing sources, to infer the background (geogenic) contributions (Albanese et al. 2007) from natural sources (rock weathering) requires studies initiated on aqueous system in pristine natural environment fairly unaffected by anthropogenic causes. In view of this, investigations were conducted in the headwater portion of the Panjhara River in the northern part of Deccan Volcanic Province (DVP) with the main aim of studying the (1) intra-annual distribution of geochemical baseline levels of ecotoxic heavy metals in the ground waters on spatio-temporal scale and (2) identifying their lithogenic and anthropogenic sources. As stated before, heavy metals persist in the environment, possess bio-accumulative nature and toxicity they are the serious cause of concern from human health point of view.
The study area
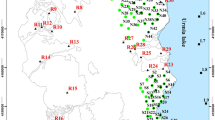
The study area is in the northern part of the Deccan Volcanic Province (DVP) of India (Fig. 1). It is drained by Panjhara River, which is a tributary of E-W flowing Tapi, a major river of Central India that finally empties into and merges with Arabian Sea on the west coast of India. The river originates at an altitude of 760 m amsl in the E-W trending Galna hills of the Western Ghats mountain range. Quite a few tributary streams concoct the adjoining hills of Dhep Dongar in the West and Kalmal and Mandya Dongars in the northwest. Hilly and precipitous landscape with deciduous forest characterizes the source area of the river that merges into rolling hummocky background and gradually unwraps into broad and flat valley floor in the downstream. The headwater part of the basin covering an area of 434 km2 was chosen for the baseline and background geochemical mapping of the eco-toxic heavy metals as it is untouched by industrialization and urbanization except elfin patches of agricultural activities.
The watershed is in the rain shadow zone of Western Ghats region with annual rainfall ranging from 300 to 862 mm (average 512 mm/year) and average precipitation is nearly constant for over one decade. More than 90 % of the rainfall is confined to south-west monsoon season lasting for 4–5 months from June to October. Most of the rivers originate in the high rainfall Western Ghat’s region and flow towards semi-arid east, wherein to augment the needs of water numerous percolation tanks and irrigation dams have been constructed. Amongst them two medium sized irrigation dams (Latipada and Jamkheri) and several percolation tanks have been erected across the Panjhara and its tributaries that have amplified the groundwater as well as irrigation potential of the area. Agricultural land with varied cropping pattern is spread only along watercourses, while mountainous and hilly parts are under degraded forest wrap besides grasslands on hill slopes and rolling plains. Entire population of the area is dependant on surface and groundwater resources for both drinking and agricultural needs. Livestock population of the area also forms an important part of agricultural activity, thus emphasizing the necessity for evaluating the suitability of water for drinking purpose from heavy metals viewpoint.
Geological and petrochemical characteristics
The study area is typified by oldest and moderately weathered basaltic flows of Kalasubai subgroup of DVP (Fig. 2) embracing multi-aquifer system separated by thin tuffaceous red bole layers. On the basis of geochemical data, the Kalasubai subgroup has been divided into five different formations from base to top namely Jawahar, Neral, Igatpuri, Thakurwadi and Bhimashankar each separated by marker Giant Plagioclase Basalt (GPB) flows (Bean et al. 1986). The lava flows from the study area have been divided into Thakurwadi Formation (TF) underlain by Igatpuri Formation (IF) (Bean et al. 1986; Subbarao et al. 1994), separated by Kashele GPB flow. The uppermost part of the IF is exposed along Panjhara riverbed and remaining portion of the watershed is covered by entire TF. Thin section studies of basalts from these formations (samples of dug well sections DW-21, DW-22) show clinopyroxenes (both Ca-rich and Ca-poor), plagioclase feldspar, iron oxides (magnetite and ilmenite) and small amount of olivine (2–2.5 %) divulging typical textures such as fine grained inter-granular to porphyritic and ophitic to sub-ophitic (Subbarao and Hooper 1988). Chemically, flows of IF express MgO content of 4.41–8.82 % and of TF between 3.51 and 11.86 %. While CaO % (8.36–10.85 %), Na2O is <3 % and Fe (total) is as high as 15 %, in both the formations variation in trace elements viz Cr, Cu, Ni and Zn is noticeable (Bean et al. 1986). The Ni and Cr in both the formations are abundant trace elements followed by Zn > Cu > Co (Subbarao and Hooper 1988). Mutually doleritic and gabbroic dykes unveil fine grained inter-granular to coarse-grained hypidiomorphic textures with ophitic and sub- ophitic characters infesting the flows (Sethna et al. 1996).
Hydrogeological set up
Meager incidence of primary openings has imparted low porosity and permeability to basaltic flows in the area. Despite the fact that cavities, vesicles, flow contacts, lava pipes and tunnels can build up principal porosity in the basalt (Pawar and Shaikh 1995) the flows in the study area are relatively less to moderately porous. Conversely, jointing and fracturing by way of interconnectivity have conveyed sufficient secondary porosity and permeability to form suitable groundwater reservoirs at places (Pawar et al. 2008a, b). Further, the cooling features such as columnar joints serve as hydrologic discontinuities, which in turn function as pathways for rainwater that is chief agent of weathering. Resultant porosity is responsible for restraining petite aquifers positioned usually in the fracture systems. From hydrogeological standpoint, Naik and Awasthi (2003) have divided the basaltic flows into two main units (1) lower massive component and (2) upper vesicular part. The massive section clutches trivial primary porosity or permeability and performs as an unyielding zone yet on account of jointing, fracturing and weathering, intermittently moderately porous rock structure is developed. On the other hand, defenseless to weathering, the vesicular entity with sufficient primary porosity helps in shaping good aquifers. Vesicular crust is the main water-bearing horizon in compound flows of TF in the study area (Pawar et al. 2008a). The flows of the two formations are plausibly weathered at the surface down to the depth of about 10–15 m depending upon local slope conditions. Underneath the weathered echelon, rock illustrates unpredictable degree of fracturing and jointing that goes on diminishing with depth. It is not easy to comprehend the geometry of fracture network due to ample variation in their densities, lengths and apertures (Tarits et al. 2006). In the well sections DW-12 and DW-41, it is seen that density of jointing and fracturing goes on waning with depth, as a consequence of this only shallow water table aquifers with limited thickness (not exceeding 15–20 m) subsist in the area. Being fractured rock with very low matrix permeability, the fluid flow in the basalt is often very heterogeneous and confined to few fractures, which is evident from some yielding bore wells placed very close to dry ones.
The well inventory data indicate that shape of all the dug wells is circular and they are fully or partly lined. While, the old wells are lined with stones, the concrete lining is observed in the case of new wells. Diameter of the dug wells varies from 4 to 8.5 m and the depth between 5.5 and 23 m. Wells in alluvial aquifer are deeper (av. depth = 13.28 m) than the basaltic aquifers (av. depth = 8.48 m). Depths of the bore wells vary from 70 m to nearly 100 m. Analysis of water level data indicates maximum fluctuation in alluvial aquifer (av. 8.55 m) and least in the dyke rock (3.5 m). They are indicative of topographic control in general and localized overexploitation in particular. The wells in the vicinity of Lathipada dam show less fluctuation in water table due to recharge from the dam. In general, the thickness of aquifers in dyke is minimum (<12 m), moderate in the case of basalts (5–15 m) and maximum for alluvium (up to 20 m). Pawar et al. (2008a) on the basis of electrical resistivity surveys have identified multiple aquifers in the area occurring at different depths viz. 30, 50 and 70 m, below this hard and compact rock structure without any joints and fractures prevails in the area. The bore wells in the area tap these aquifers.
Methodology
The watershed under review displays relatively less diversity in geological, hydrogeological and environmental conditions except a little modification by agricultural activity. This set up has provided opportunity to identify imprints of lithogenic factors on background and baseline heavy metal chemical composition of groundwater followed by anthropogenic sources.
Sample locations and collection of water samples
In view of the above geological and hydrogeological conditions in the study area sampling wells were selected randomly (Fig. 3) based on type of lithology, aquifers, slope conditions and land use besides their availability and accessibility in the field for groundwater monitoring. Sampling locations covering post- and pre-monsoon seasons, (35 in post-monsoon and 37 in pre-monsoon) were established for groundwater monitoring. In pre-monsoon, the samples represented 29 dug wells (23 in post-monsoon), 7 bore wells (6 in post-monsoon), and 1 surface water sample (6 in post-monsoon) wrapping alluvial, basaltic, and dyke aquifers. Samples were collected as per standard methods (APHA 2005). Due care was taken to avoid contamination of samples during handling. One-liter capacity pre cleaned plastic containers were used for the collection of water samples. Groundwater samples were collected after the well was subjected to pumping for half an hour. In the case of bore wells fitted with hand pumps, samples were collected after hand pumping the bore well for a few minutes. Surface water samples were collected from flowing streams. The pH, electrical conductivity (EC), well depth, static water level, lithologs and aquifer type were recorded in the field. Suction-filtered (through 0.45 µm membrane filter paper) samples were immediately acidified with high purity concentrated nitric acid to pH <2 to avoid Fe-oxide precipitation, to minimize absorption of metal on the container wall, to retard biological action, hydrolysis of chemical compounds and complexes, and reduction of volatile constituents and separately collected for analyses of heavy metals (De 1990). In all 72 samples representing post-monsoon (December, 2004) and pre-monsoon (May, 2005) were collected to find out the intra-annual trends (over 1 year period) in the heavy metal geochemistry of groundwater (Tables 1, 2). All the samples were stored in a refrigerator at 4 °C to prevent any change in volume before laboratory analyses (APHA 2005). The remaining unacidified portion of the sample was subjected for analyses of the major parameters including Ca, Mg, Na, K, SO4, Cl, HCO3 in the laboratory and results obtained were published (Pawar et al. 2008a).
Analyses of water samples and quality control
The refrigerated acidified water samples 100 mL each were subjected for nitric acid digestion (since it is acceptable matrix in flame AAS) on hot plate (150 °C) by adopting the standard procedure (APHA 2005). After complete digestion evidenced by clear solution the samples were filtered through Whatman-42 filter paper. By transferring the filtrate to 100 mL conical flask and spiking it with known concentrations of standard (standard addition method) the volume was made to mark and mixed thoroughly and subjected for heavy and trace element detection on AAS. Using Varian, AA-220 double beam atomic absorption spectrophotometer the heavy metal analysis was performed. The recommended standard working conditions of the instrument varied from 0.01 to 2 ppm (mg/L) for Zn at 231.0 nm wavelength to 0.2–100 ppm in the case of Mo at 313.3 nm wavelength. The lower limit of working range was about ten times the defined detection limit of the metal (Varian 1989). The standard detection limits in ppm (mg/L) are cadmium 0.002, chromium 0.02, copper 0.01, iron 0.02, manganese 0.01, lead 0.05, nickel 0.02, zinc 0.005 and molybdenum 0.1 (APHA 2005). The determinations were done by direct aspiration of sample into the air-acetylene flame for all metals except Mo, where the nitrous oxide-acetylene flame was used. The Merck standards (minimum three) were also used for the calibration of the elements analyzed and the reproducibility of the instrument was 90–95 %. In order to ensure the baseline stability blank was analyzed between the sample and standard reading. Along with every batch of ten samples a black digest containing only acid and standard solution was analyzed. The data obtained was corrected for known concentration of standard added and results were reported in ppb (µg/L).
The chemical data was subjected for statistical analysis such as min. max. average, standard deviation and variance. The Pearson correlation coefficients were calculated using MS-Excel for appraising possible metallic associations and relating them with geochemical processes.
Application of THQ
The consumption of heavy metal containing water is likely to pose risk to human health. The THQ offers an indication of risk level due to pollutant exposure and has been used by many researchers. It was first established by U.S. Environmental Protection Agency (1989) and later on applied for judging the quality of beverages by Hague et al. (2008); seafood by Storelli (2008), Petroczi and Naughton (2009) and for water, sediment and fish by Gupta et al. (2015) and PuYang et al. (2015). The THQ is expressed (U.S. EPA 1989) as follows:
where EFr is exposure frequency (considered as 365 days/year); EDtot is the exposure duration (70 years life expectancy), SFI is the mass of selected dietary ingested (av. water consumption as per Indian Standards (IS 1993) is 5 L/person/day), MCSinorg is the concentration of metal in water (ppb), RfD is the oral reference dose (mg/L based on WHO (1971)/IS (1993) drinking water standards), BWa is the average body weight (55.9 kg for adult) and ATn is the average exposure time for non-carcinogens (365 days/year × EDtot).
Results
Spatio-temporal trends in the heavy metals
The concentrations of heavy metals in the ground waters from Upper Panjhara basin depicted with the help of isoline plots, show remarkable intra-annual variations on spatial scale (Figs. 4, 5) in spite of nearly uniform geological conditions in the area. Although alluvium is present in patches, it has local basaltic provenance and has possibly inherited many metals from weathering of basalt. Based on their intra-annual variability the heavy metals in the Panjhara basin have been grouped into (1) Group I: represented by Fe, Mn, Pb, Zn and Cd and (2) Group II: signifying Mo, Ni, Cu and Cr. The order of abundance of Group I elements in the groundwater is Fe > Mn > Zn > Pb and that of Group II elements is Mo > Ni > Cr > Cu (Tables 1, 2). In general, the concentrations of Group I elements are lower in post-monsoon season when water table is high (av. depth = 3.83 m) and higher in pre-monsoon season. In contrast, the Group II (Mo, Ni, Cu and Cr) depicts higher values in post-monsoon season and lower in pre-monsoon (av. water table depth = 8.23 m). These changes in the temporal behavior of the metals can be attributed to the water table fluctuations related to rainfall recharge inducing changes in redox conditions (Edmunds et al. 2002). Temporal pattern of the elements depicting higher Mo and Ni values in post-monsoon is on account of recharge from monsoon rainfall indicating collective input of elements from lithogenic + anthropogenic factors respectively from higher elevation non agricultural areas (TF flows) and fertile soil zone in the alluvial plains covered by patches of agriculture along water courses. During rainy season due to ‘rinse-out’ effect caused by dissolution and precipitation of soil salts (Smoulders et al. 2004), there is remarkable increase in concentrations of the above elements in the groundwater. The propensity of remaining metallic elements depicting higher concentrations in pre-monsoon is accountable predominantly to rock-water interaction due to higher residence time at moderately deeper depth (IF flows) as rainwater recharge ceases. This is particularly true for low lying discharge areas along the course of main rivers wherein the plots depict higher concentrations.
On the spatial scale Group I (Fe and Mn) isoline pattern reflects the control of groundwater flow on the variations of elements (Fig. 4), with Pb and Zn demonstrating less differences (Tables 2, 3). It is seen from the figure that Fe concentrations increase in the downstream direction with a ‘concentration high’ in low lying area in post-monsoon that expands in pre-monsoon depicting several anomalies spread over entire area. Similarly, Mn also shows small anomalies downstream around dam that disappears in pre-monsoon. However, in the upstream part of dam, expanded Mn anomalies are visible in pre-monsoon. Amongst Group II elements, greater inconsistency in Cu is conspicuous. This necessitates local anthropogenic causative sources of Cu (fungicides and micronutrients) in the groundwater, in addition to the lithogenic inputs (Pawar and Nikumbh 1999). The Ni and Mo (Fig. 4) values though comparable and widely distributed over larger area, enrichment is confined to the alluvial areas with irrigated agriculture. Relatively lower background concentrations of these elements in non–agricultural parts of the basin in upper reaches put forward the view that their input is predominantly from lithological sources such as weathering of olivine, pyroxene and plagioclase feldspar (Subbarao et al. 1994). Elevated values of Group II elements (Ni and Mo) in the central and eastern parts of the area near the confluence of Panjhara and Jamkheri rivers divulge additions possibly from agricultural sources (Pawar et al. 2008a). This high concentration area expands during post-monsoon season indicating likely contribution of both the elements from fertilizer-remains in the soil zone (Pawar and Nikumbh 1999).
Heavy metals drift along groundwater flow lines
Though certain trace and heavy metals illustrate systematic enrichment on temporal scale, their slightly disorganized pattern along groundwater flow lines is apparent (Fig. 6). Several authors have related orderly elemental variations with distance along flow lines (Larocque and Rasmussen 1998; Edmunds et al. 2002; Rajmohan and Elango 2005). As compared to these studies the trends in heavy metal concentrations along three different chosen sections passing through wells 18–17–16–14–12 followed by 10–9–2 and 35–33–32 (Fig. 6) are slightly inconsistent possibly indicating complex groundwater flow patterns, heterogeneity in the rock structure and source composition (Ghosh 1983). This is because, in a fractured rock like basalt with very low matrix permeability, the fluid flow is often very heterogeneous and confined to few fractures, causing random variations in metal elements. Further, the source heterogeneity in DVP is manifested by quartz normative flows, under-saturated basalts and olivine tholeiites. Chemically, quartz normative flows show relatively higher Cu and under-saturated basalts depict elevated Zn, Cr, Ni, Pb and Co, besides olivine tholeiites enriched in Cr, Ni and Co (Ghosh 1983). Therefore, variations in trace elements along groundwater flow in the area can be attributed to uneven distribution and abundance of tholeiites and olivine tholeiites in the area, as silica under saturated varieties are absent. Further, two contrasting patterns can be easily discerned from Fig. 6, (1) post-monsoon season depicting enrichment of groundwater with Mo and Ni and (2) pre-monsoon season showing increase in Fe and Mn. Changes in Cr and Zn are negligible. This pattern suggests that primarily Mo and Ni are contributed by twin sources (1) high altitude TF flows with picritic horizons (Subbarao and Hooper 1988) and (2) flushing of metals to phreatic zone from the soil/vadose stratum by rainwater recharge during post-monsoon season (Pawar and Nikumbh 1999). It is possible that shallow circulation of groundwater due to rising water table (post-monsoon = av. 3.83 m and pre-monsoon = 8.23 m) induced suitable (oxidizing?) conditions that are probably responsible for higher concentrations of Mo and Ni (Kafri et al. 2002). Moreover, Mo has relatively higher geochemical mobility under ambient earth surface environment wherein the predominant state is Mo6+, which forms soluble molybdate anions in water (Hem 1991). However, this process would close in summer distinguished by (1) relatively deeper circulation of water swayed possibly through reducing environment and (2) break in the supply of oxygen by nonattendance of rainwater recharge, thereby diminishing the contribution of these elements to the groundwater (Pawar and Nikumbh 1999). In contrast, these conditions are more suitable for Fe and Mn that amplify during summer probably as a result of higher residence time and moderate rock-water interaction involving pyroxenes and opaque minerals and less abundant olivine in IF flows, thus revealing predominantly geogenic input. The dissolved heavy metals in water are sensitive to pH values (Drever 1982). They are also controlled by their adsorption on manganese and iron oxy-hydroxides (Das and Krishnaswami 2007). It is therefore possible that with increase in pH (av. 8.17) in pre-monsoon season, the surface negative charge of manganese and iron oxy-hydroxides is also amplified, thereby allowing sequestration of metals on Fe-oxy-hydroxides and thus lowering the concentrations of associated Group I heavy metals (Pawar and Nikumbh 1999; Das and Krishnaswami 2007).
Spatial and temporal trends of heavy metals (Fig. 6) along groundwater flow could well explain accretion of these constituents possibly derived from diffuse soil sources and sediment disturbance by runoff (Zhou et al. 2007). High concentrations of these elements in post-monsoon suggest contribution from both geogenic and anthropogenic sources as Mo is potential micronutrient used for enhanced crop productivity (Orlov 1992) and has an ability to get enriched in the soil zone due to its strong affinity for near surface earth environment (Hem 1991). Similarly, besides rock, supplementary Ni and Pb can come from agrochemicals (as impurities) and electroplating activity (Liu et al. 2011). Relatively constant or almost stable concentrations of other elements except Fe and Mn specify their geochemical stability under prevailing environmental conditions (Edmunds et al. 2002). Background Cr concentrations are higher on account of its abundance as a trace constituent in basaltic rocks containing plenteous olivine in the picritic horizons of basalt (Bean et al. 1986; Subbarao et al. 1994). Cr is present generally as oxy-anion (CrO3) and pattern of its occurrence and spatial distribution reflects its natural background concentration in basaltic aquifer under available redox environment (Edmunds et al. 2002). Ni although geochemically related to Mg-olivine (picritic basalt) dominant lithology (Bean et al. 1986), enhances in winter pointing to additional source from soil zone altered by agricultural activity.
Depth integrated deviations in the heavy metals
Though the difference in the depth of bore (av. 50–100 m) and dug wells (5.5–23 m) is remarkable, depth integrated sampling was not possible during the present study. However, to get some idea about depth related variations, the waters collected from different sources indicating different depths such as surface, dug and bore wells have been compared (Fig. 7). It is seen from the figure that there is no noticeable change in the ionic concentration of surface waters, shallow dug and deeper bore wells of Group I elements, demonstrating dominance of single source contribution evidently lithology. Remaining Group II elements show greater disparity in elemental values, which is indicative of their multiple source origin with respect to water types. This is to say that the surface and shallow ground waters tapped by dug wells depicting higher concentrations of the heavy metals at-least in winter season reflect combined participation of dominantly lithological and in part the anthropogenic sources. As against this, the pre-monsoon trends reflect not as much of dissimilarity in the elemental values of dug as well as bore wells suggesting contribution predominantly from geogenic sources.
Discussion
Elucidation of heavy metal eccentricity
Two possible sources of heavy metals in the ground waters from Panjhara basin have been inferred on the basis of their spatio-temporal, depth-wise and groundwater flow related variations. These are (1) lithogenic contributions from weathering of basaltic rocks in the study area and (2) anthropogenic link with the discontinuous patches of agricultural activity as the area is devoid of industrialization and urbanization.
Lithogenic contributions
Geological factors control the distribution of heavy metals, as most of them are direct derivatives of local bedrock composition and hence, it is important to know the geochemical characteristics of rock (Koljonen 1992). The basaltic lithology in the area forms shallow weathered and fractured basaltic aquifers; therefore the sources of heavy metals could be related to their distribution and concentration in the aquifer media (Salonen and Neimi 2007). Because the study area is dominantly under basaltic rock formation, (with minor spread of alluvium and a few doleritic dykes), plausibly metal concentrations are likely to show systematic variations depending upon hydrogeological and geomorphological factors in the study area. However, most of the metals illustrate incoherent behavior that needs explanation based on lithological factors as the anthropogenic influences in the area are least. It is pointed out (Mysen and Kushiro 1976) that the trace and heavy metal content of the basalt depends on a number of factors for instance (1) proportion of crystalline mineral phases, (2) participating phases making liquids and crystals, (3) composition of mantle source, (4) effects of degree of partial and fractional melting, (5) partition coefficients, (6) wall rock alteration and volatile transfer. Further, the quartz normative basaltic flows in the DVP contain relatively higher Cu; the under-saturated variety portrays higher Zn, Cr, Ni, Pb and Co, whilst the olivine tholeiites are more enriched in Cr, Ni and Co than others (Ghosh 1983). The Panjhara basin is typified by both quartz normative as well as olivine tholeiitic basaltic flows of both TF and IF, comprised of plagioclase feldspars, pyroxenes, olivine and opaque minerals, which are potential sources of heavy metals. Atypical patterns of metallic elements in the groundwater therefore could be the result of (1) differential weathering of silicate minerals in the basalt, (2) sulphide-oxidation reactions with sulphide minerals in basalt and (3) reductive dissolution of metal hydroxides (Robertson and Blows 1995). The silicate mineral dissolution reactions of primary minerals resulting from lithological divergence in the area (Pawar et al. 2008a) are typically incongruent type (except congruent dissolution of olivine) and can be summarized as follows:
Rainwater charged by CO2 in the atmosphere, permeates through soil zone and invigorates the basaltic aquifers in the area, which is the source of protons in the above reactions. These rock-water interaction processes unshackle not only the base cations (Ca, Mg, Na, K) but also the associated heavy metals with them such as Zn, Cr, Ni, Pb, Cu and Co besides bicarbonate along with concurrent increase in pH (Rabemanana et al. 2005). In the above reactions, privileged dissolution of certain minerals, their sequence of weathering, modal proportion of different mineral phases and spatial variability in mobilization can lead to differences in the release of metallic ions, thus causing inconsistent patterns (Pawar et al. 2008a).
The basaltic lithology holds nearly 13–15 % Fe–Ti oxide minerals (Sen 1986). The oxidation of ferrous sulphide occurring as accessory mineral (Sen 1986) and reductive dissolution of Fe–Ti oxides (Robertson and Blows 1995) occurring as opaques in basaltic rocks could be responsible for releasing metals such as Fe, Mn and associated metals into the aquifer. Additionally, hydrous manganese and iron oxides occurring as coatings around silicate grains are ubiquitous in soils and alluvial sediments in the study area. The hydrous iron oxides are often amorphous, whereas Mn generally occurs as poorly crystalline birnessite or todorokite (Drever 1982). Since, these hydrous Fe–Mn oxides have extremely high adsorption capacity and affinity for heavy metals, many metals like Cu, Pb, Zn, Cr, Ni etc. that are released along with other cations from several mineral components are largely adsorbed by these oxide phases (Das and Krishnaswami 2007). Due to seasonally variable redox conditions, reductive dissolution of these metal oxides or hydroxides is expected to release the heavy metals in the surface and ground waters as follows:
Thus, ferric iron hydroxide and manganese oxide reduction not only releases Fe and Mn to the groundwater, but also metals co-precipitated or adsorbed onto these metal oxides. This could be the reason for some of the metals (Zn and Pb) to depict complimentary trends to Fe and Mn. For example, at pH values above 7 aqueous complexes of zinc begin to portion to particulate zinc as a result of sorption onto iron oxy-oxide (Challenger 2007). Further, the soils in the area are rich in montmorillonite type clay mineral (Pawar et al. 2008a), which is considered to be very effective in removing the metals from the water. In spite of these natural controls, some of the metals have been found to be occurring in higher concentrations in the groundwater as compared to drinking water standards.
Mineral dissolution
Compositionally, lithological divergence in Panjhara basin is evidently epitomized by varying abundances of heavy elements in two formations TF and IF. The order of abundance of the trace elements in IF is Cr > Ni > Zn > Cu > Co with concentrations in the range of 106–240 > 70–182 > 94–142 > 45–224 > 40–49 ppm respectively. This sequence remains same in TF except for the fact that their values are on much higher side (105–821 > 65–484 > 96–143 > 70–223 > 37–58 ppm) due to higher MgO (8.36 and 10.85 %) content (Subbarao and Hooper 1988; Khadri et al. 1989). Therefore, privileged abundance of heavy metals in the groundwater points to their origin from weathering of mafic phases in the basalt namely olivine and clinopyroxenes (Subbarao and Hooper 1988). Higher concentrations of trace elements recount to greater modal abundance of olivine occasionally attaining 25–30 % proportion (Khadri et al. 1989). Higher values of Ni/Cr (0.79), Ni/Cu (1.28) and Ni/Zn (1.55) for TF than IF (Table 3) supports above observation. Further, the Ni/Cr ratio of pyroxene (0.55–0.59) and plagioclase feldspar (0.67–1.15) is low as compared to olivine (5.62–115). Compared to these values the Ni/Cr ratio for majority of the wells in post-monsoon and >50 % in pre-monsoon are above unity suggesting advantaged weathering of olivine, which could be major supplier of heavy metal load to the waters. This is because the silicate tetrahedra in olivine are linked through chemical bonding of single oxygen with divalent Mg, grading it as nesosilicate, which is weak structure and relatively less stable for chemical attack by water (Hem 1991). On the other hand, pyroxene with double oxygen ions in tetrahedra form long chain inosilicate structure (strong) and plagioclase feldspar amid three oxygen ions in tetrahedra shared with other tetrahedra shape tectosilicate configuration (stronger) appear to be supplementary in role due to relatively higher stability. This inference confirms the findings (based on major element data) of Pawar et al. (2008a) indicating olivine > augite > plagioclase feldspar as the sequence of mineral weathering in the Panjhara basin. It is therefore proposed that heavy metal intra-annual variability in the groundwaters is mainly the result of selective dissolution of mineral constituents in the basalt and their sequence of weathering. Needless to say high proportion of bonding between divalent cations and oxygen in nesosilicate structure of olivine followed by inosilicate configuration of pyroxene outline preferred zones of weaknesses that are easily disrupted by water as compared to silicon-oxygen or aluminum–oxygen bonds in tectosilicate makeup of plagioclase feldspar in the basalt (Hem 1991). Thus, weak structure and relatively less stability for chemical attack by water have possibly favored selective dissolution of olivine followed by pyroxene and plagioclase to release heavy metals in the groundwater, which is reflected in incoherent pattern.
Anthropogenic input
The heavy metals can also be derived from multitude of anthropogenic emissions in the environment (Challenger 2007). Though, mining and smelting, fossil fuel burning, municipal waste incineration, industrialization (Adomako et al. 2008; Suyash et al. 2008; Suyash and Pawar 2010) are important anthropogenic sources, none of these activities are prevalent in the study area. However, the basin is a part of tribal belt of central India; wherein agricultural practices followed by the local population are more traditional, yet some agricultural sources of heavy metals could be envisaged in the area. Use of commercial fertilizers and pesticides, animal waste and waste water discharges has potential to contaminate the water resources (Challenger 2007). Therefore, in order to find out agricultural bustle as an additional source of heavy metals in the groundwater, intra-annual geochemical data were normalized and quantified with the help of Normalised Difference Dispersal Index (NDDI) by using following expression (Suyash and Pawar 2008):
The values of NDDI range between −1 and +1 suggesting absolute dilution and absolute accretion respectively and make the intra-annual comparative evaluation possible. NDDI values for the wells portrayed three distinct patterns (1) Ni–MO displaying positive index values for all the wells (except DW-29), (2) Fe–Mn witnessing negative numbers (except DW-1, DW-29 and DW-31) and (3) the remaining elements (Cu, Zn and Cr) exhibiting mixed pattern (Fig. 8). The positive values of Ni and Mo, and partly Cu imply widespread and uniform, sampling-spot-specific enrichment of these elements related to embellishments from soil/vadose echelon to the phreatic zone during post-monsoon. Negative indices for Fe and Mn point to their consistent and absolute dilution possibly due to near absence or inactive sources in the upper soil/vadose horizon. Hence, groundwater is depleted with respect to them in post-monsoon. The mixed pattern implies depletion and enrichment in parts with respect to Cu, Zn and Cr, limited to explicit well locations possibly due to localized presence or absence of metal source. In nutshell, positive NDDI values replicate the dominance of soil/vadose zone as a chief transmitter of metals in the groundwater during post-monsoon, while negative values recommend host rock as a primary source of accretion of metals in pre-monsoon. Relatively higher values of Ni/Cr ratio for the wells in alluvial (post-monsoon av. = 5.99) and dyke (post-monsoon av. = 7) aquifers surrounded by fertile agriculture plain than the basaltic aquifer (post-monsoon av. 4.04) support this inference (Fig. 9a), suggesting profound input of the elements (Mo, Ni > Cu) from agriculture land use (animal waste related sources) in post-monsoon season. Increase in the values of the Ni/Cr in basaltic/dyke aquifers is spot-specific, thus indicating localized additions of these elements wherever patches of agriculture exist. On the other hand, there is substantial decrease in the Ni/Cr ratios in pre-monsoon (Fig. 9b) pointing to discontinued rainwater recharge dominated by heavy metals from soil/agricultural areas. Negative values of NDDI have been obtained for Fe and Mn and in part for Zn reflecting negligible role of recharge waters. This in turn puts forward the view that there is meager addition of these heavy metals from secondary sources in the soil zone. In view of this, it is plausible that elements that depict positive NDDI are supplied to the groundwater dominantly by soil and sediment as well as anthropogenic sources related to the agriculture. Negative NDDI values reflect involvement of primarily geogenic factors, when recharge through soil zone is absent. Thus, both NDDI and Ni/Cr ratios support the inferences made earlier.
Elemental alliances
In order to authenticate above inferences by means of exploring possible associations amongst the metals, Pearson correlation coefficients were determined (Table 4). Two distinct groups of the chemical components were formed on the basis of their seasonal relationships (1) static coalition and (2) dynamic assemblage. The static group does not illustrate changing inter-ionic links seasonally, whereas the dynamic group demonstrates distinct alteration in relationships seasonally (Pawar et al. 2008a). For instance, pH portrays positive affiliation with Zn, EC with Mo, Cu with Cr > Ni, Mn with Zn > Fe, Ni, and Cd with Mo, followed by pH vs. Ni and Fe vs. Mo negative alliance in pre-monsoon that remains identical even in post-monsoon. Further, heavy metal association indicates that majority of heavy metals are positively correlated with each other in post-monsoon season implying dominant role of soil sources and sediment disturbance by runoff (Zhou et al. 2007) and negatively correlated with each other in summer signifying geochemical diversity in the lithology (Ghosh 1983) and differing response of mineral constituents to weathering of rock affecting heavy metal release (Pawar et al. 2008a). Negative correlation between Fe and Mo in both the seasons shows their mobility as well as stability under contrasting redox conditions (Hem 1991). This is also evidenced by distinct NDDI patterns depicted by these elements. Interestingly, the affirmative link between EC and Mo (r = 0.23) in pre-monsoon becomes stronger in post-monsoon (r = 0.64) reflecting control of soil water recharge on Mo. Moderately strong relationship between Pb and Cu > Zn indicates their common source of origin from sulphide minerals (Sen 1986). Positive correlation of Cu further with Mo > Cr > Ni in pre-monsoon suggests their contribution from quartz normative tholeiitic basaltic flows as a common source (Ghosh 1983). Mn correlates positively with Zn > Fe (similar NDDI pattern) in pre-monsoon indicating Fe–Ti oxides as their possible source and strengthens the relationship with almost all elements such as Cr > Cd > Ni > Zn > Mo > Fe in post-monsoon reflecting their derivation from diffuse soil sources and sediment disturbance by runoff (Zhou et al. 2007). From the positive correlation coefficients it is inferred that heavy metals have been introduced in the water column from a common source (soil/sediment) and lack of significant positive correlation between them suggests that these elements are associated with different aluminosilicate minerals in the basalts (Adomako et al. 2008). In nutshell, the elemental alliances point to their multi-source derivation and seasonal fluctuation in the water table conditions leading to the intra-annual variability in the heavy metal content of groundwater from the study area.
THQ and implications on human health
In the study area metals have been introduced in the aquatic environment primarily from rock weathering, soil and sediment sources followed by localized additions from anthropogenic factors (animal waste) related to agriculture. Since the area is tribal belt and least affected by industrial and urban sources, accumulation of metals in the groundwater is likely to have direct consequences on human health and ecosystem. It is to be noted that the local population is dependant on groundwater as their primary source of drinking water. Knowing the fact that certain heavy metals like Cu and Zn are essential for metabolic activity in the organisms, it is implicit that their lies narrow window between essentiality and toxicity (Adomako et al. 2008). Some of the constituents like cobalt, chromium, cadmium, lead etc. should be virtually absent from the drinking water supply however, in some instances values of metals (Mn, Cr, Cd and Pb) are though higher in the study area are comparable with the studies on Narmada and Tapi river basins (Sharma and Subramanium 2010). Further, pre-monsoon water quality with respect to heavy metals is poor (particularly in basaltic aquifer) as the number of wells exceeding standard limits for drinking water is higher in pre-monsoon than in post-monsoon season. Most of the wells in winter show heavy metal concentrations below desirable limit except in a few cases. Therefore, though it is suggested that metal speciation is more important in assessing the toxicity and mobility (Frieberg et al. 1986), importance of total concentrations of heavy metals in assessing metal pollution can not be downplayed, especially where baseline geochemical map data are non-existent (Adomako et al. 2008).
Several workers have proposed use of THQ for the assessment of the potential human health risks from exposure to chemical contaminants (Storelli 2008; Naughton and Petroczi 2008; Storelli et al. 2010; PuYang et al. 2015; Gupta et 2015). THQ is typically non-cancer risk assessment method based on a ratio between the estimated dose of contaminant and the reference dose below which there will not be any appreciable risk (Storelli 2008). Therefore, THQ was determined for all the metals using WHO and IS recommendations for consumption of drinking water. The values of THQ have been given in the Table 5a, b. It is observed that during pre-monsoon season most of the metals (except Cr and Cd in a few cases) display THQ values less than one. The THQ of metals varies from Pb = 0.01–0.09, Cu = 0.00–0.05, Mn = 0.02–0.18; Cr = 0.01–0.6; Zn = 0.00–0.01; Ni = 0–0; Fe = 0.05–0.23 and Cd = 0.11–4.56. These results are comparable with those obtained by Gupta et al. (2015) for Gomati River in the northern part of India. The results further suggest insignificant non-cancer health hazard from majority of metals except a few wells with respect to values of THQ for Cd. In general, amongst all metals the values of Cd are on higher side and three wells (DW33, DW34 and DW37) exceed the THQ values above unity suggesting potential human health risk hazard if consumption of water is continued. It is possible that these wells are contaminated from chemical fertilizers and organic matter related to agriculture and animal waste (Han 2006). In the post-monsoon season all the wells display THQ values of metals far below unity except a few wells with respect to Cr and Cd. The THQ values for various metals in post-monsoon vary from Pb = 0.01–0.07; Cu = 0.00–0.04; Mn = 0.00–0.16; Cr = 0.06–1.23; Zn = 0.00–0.01; Cd = 0.02–0.8 and Fe = 0.03–0.1. The THQ values of Cr in four wells (DW1, DW5, DW11 and DW21) are close to one or above one reflecting possibility of health risk hazard linked to chromium. In Raipur area of central India, Tiwari et al. (2015) reported much higher values of Cr in contaminated waters and opined that, a frequent ingestion of Cr contaminated water can cause anemia and stomach cancer. Further, the THQ values for Cd (post-monsoon) vary from 0.02 to 0.8 (DW21) indicating reduced health risk hazard as compared to pre-monsoon possibly due to rainwater recharge causing dilution of groundwater. The THQ values reported for sea foods range from <1 (safe) to >10 (of concern) to greater than 100 for red wine (Naughton and Petroczi 2008; Petróczi and Naughton 2009). In addition, Hague et al. (2008) and Naughton and Petroczi (2008) are of the opinion that the THQ is not a measure of risk but it indicates a level of concern. He also stresses that as it is additive function and not multiplicative, the level of concern for THQ of 20 is larger but not tenfold of those at THQ = 2. Nonetheless, in absence of any data on occurrence of heavy metals in groundwater from basaltic aquifers of the area, the results generated have highlighted the concern for human health from metal toxicity point of view. Since, heavy metals are extremely persistent in the environment; they are nonbiodegradable and nonthermodegradable and readily accumulate to toxic levels (Sharma et al. 2007); environmental baselines are needed in order to assess the present status of the surface environment (Galan et al. 2008). In view of the fact that geochemical baselines provide guidelines and quality standards for environmental legislation and decision making, the present study calls for taking necessary steps to establish periodic monitoring network to assess intake of metals due to consumption of poor quality water and the level of health risk associated with it.
Summary and conclusions
Studies were undertaken in the headwater unit of Panjhara basin in the northern part of Deccan Volcanic Province (DVP), India with the main aim of studying the (1) intra-annual distribution of geochemical baseline and background (geogenic) levels of harmful heavy metals in the groundwater on spatial scale and (2) identifying their lithogenic and anthropogenic sources. Geochemical baseline levels of heavy metals in the groundwater depicted variability on spatio-temporal scale. Classification of metals has been done into Group I (Fe > Mn > Zn > Pb) and Group II (Mo > Ni > Cr > Cu), which indicated contrasting enrichment and depletion patterns on the intra-annual scale related to the changing water table conditions attributable to climatic fluctuations. While, fortification in Mo–Ni in post-monsoon is on account of combined lithogenic + anthropogenic contribution, pre-monsoon augmentation in Fe–Mn is entirely lithogenic. Positive values of NDDI, replicate the dominance of soil/vadose zone as a chief supplier of metals in the groundwater during post-monsoon, while negative values recommend host rock as a primary source of accretion of metals in pre-monsoon. This inference is supported by higher Ni/Cr ratio for the wells in alluvial aquifer and dyke surrounded by fertile agriculture plain than the basaltic aquifers, suggesting input of the elements (Mo, Ni > Cu) from agriculture land use in post-monsoon season. Values of Ni/Cr ratio above unity for majority of the wells in post-monsoon and >50 % wells in pre-monsoon suggest privileged weathering of olivine, followed by pyroxene > plagioclase feldspar (Ni/Cr <1) as major contributor of heavy metal load to the groundwater. Pearson correlation coefficients authenticate above inferences by means of elemental associations. Elemental alliances also point to the multi-source derivation of metals and seasonal fluctuation in the water table conditions leading to intra-annual variations in heavy metal content in the groundwater from the study area. As heavy metals persist in the environment and they possess bio-accumulative nature and toxicity, they are the serious cause of concern from human health point of view. Therefore, though it is suggested that metal speciation is more important in assessing the toxicity and mobility, importance of total concentration of heavy metals in assessing metal pollution can not be downplayed, especially where baseline geochemical map data are non-existent. The THQ values exceeding unity reflect possibility of health risk hazard that further emphasizes the need to establish periodic monitoring network for baseline geochemical mapping to avoid health risk hazards.
References
Adomako D, Nyrko BJB, Dampare SB, Serfor-Armah Y, Osae S, Fianko JR, Akoho EHK (2008) Determination of toxic elements in waters and sediments from River Subin in the Ashanti Region of Ghana. Environ Monit Assess 141:165–175. doi:10.1007/s10661-007-9885-x
Alam MGM, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308:83–96
Albanese S, Vivo BD, Lima A, Cicchella D (2007) Geochemical background and baseline values of toxic elements in stream sediments of Campenia region (Italy). J Geochem Explor 93:21–34
APHA, AWWA, WPCF (2005) Standard methods for the examination of water and waste water, 14th edn. American Public Health Association
Arinze IE, Igwe O, Una CO (2015) Analysis of heavy metals contamination in soils and water at automobile junk markets in Obosi and Nnewi, Anambra State, Southeastern Nigeria. Arab J Geosci. doi:10.1007/s12517-015-2001-6
Bean JE, Turner CA, Hooper PR, Subbarao KV, Walsh JN (1986) Stratigraphy, composition and form of Deccan Basalts, Western Ghats, India. Bull Volcano 48:61–83
Challenger E (2007) Heavy metals in the environment—historical trends. Treatise in Geochemistry ISBN (SET) 9 (ISBN: 0-08-044344-3) 67–105
Das A, Krishnaswami S (2007) Elemental geochemistry of river sediments from Deccan Traps, India: implications to sources of elements and their mobility during basalt–water interaction. Chem Geol 242:232–254
De AK (1990) Environmental geochemistry. Wiley, New Delhi 361p
Drever J (1982) The geochemistry of natural waters. Prentice Hall, New York 182
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258:1–24
Engin MS, Uyanik A, Kutbay HG (2015) Accumulation of heavy metals in water, sediments and wetland plants of Kizilirmak Delta (Samsun, Turkey). Int J Phytorem 17(1):66–75. doi:10.1080/15226514.2013.828019
Fazeli MS, Khosravan F, Hossini M, Satyanarayan S, Satish PN (1998) Enrichment of heavy metals in paddy crops irrigated by paper mill effluents near Nanjangud, Mysore district, Karnataka, India. Environ Geol 34(94):287–302
Fernandez M, Nayak GN (2015) S[peciation of metals and their distribution in tropical estuarine mudflat sediments, southwest coast of India. Ecotoxicol Environ Saf 122:68–75
Frieberg L, Elinder CG, Kjellstroem T, Nordberg CF (eds) (1986) Cadmium and health: a toxicological and epidemiological appraisal, effects and response, vol 11. CRC, Boca-Raton
Galan E, Fernandez-Caliani JC, Gonzalez I, Aparicio P, Romero A (2008) Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. J Geochem Explor 98:89–106
Ghosh NC (1983) The trace element studies on Deccan basalts and co-magmatic rocks: a review. Geol Surv India Rec 113(2):71–95
Gupta SK, Chabukdhara M, Singh J, Bux F (2015) Evaluation and Potential Health hazard of selected metals in water, sediments and fish from Gomati River. Hum Ecol Risk Assess 21(1):227–240
Hague T, Petroczi A, Andrews PLR, Barker J, Naughton DP (2008) Determination of metal ion content of beverages and estimation of target hazard quotients: a comparative study. Chem Central J 2:13
Han WY, Zhao FJ, Shi YZ, Ma LF, Ruan JY (2006) Scale and causes of lead contamination in Chinese Sea. Environ Pollut 139:125–132
Hem JD (1991) Study and interpretation of chemical characteristics of natural water. US Geol Survey. Water Supply Paper No. 2254
IS (1993) Indian Standard Specifications for Drinking Water, IS: 10500
Kafri U, Lang B, Halicz L, Yoffe O (2002) Geochemical characterization and pollution phenomenon of aquifer waters in northern Israel. Environ Geol 42:370–386
Kelepertzis E (2014) Accumulation of heavy metals in agricultural soils of Mediterranean: insights from Argolida basin, Peloponnese, Greece. Geoderma 221–222(2014):82–90
Khadri SFR, Subbarao KV, Hooper PR, Walsh JN (1989) Stratigraphy of Thakurwadi Formation, Western Deccan basaltic Province, India. In: Subbarao KV (Ed) Deccan flood basalts. Mem. Geol. Soc. Ind., vol 10, pp 281–304
Koljonen T (1992) Results of the mapping. In: Koljonen T (ed) The geochemical atlas of Finland, part 2: Till. Geological Survey of Finland, Espoo, pp 106–218
Krishna AK, Govil PK (2007) Soil contamination due to heavy metals from an industrial area of Surat, Western India. Environ Monit Assess. doi:10.1007/s10661-006-9224-7
Larocque ACL, Rasmussen PE (1998) An overview of trace metals in the environment, from mobilization to remediation. Environ Geol 33(2/3):85–91
Liu WX, Shen LF, Liu JW, Wang YW, Li SR (2011) Uptake of toxic heavy metals by rice (Oryza sativa L.), cultivated in the agricultural soil near Zhengzhou City, People’s Republic of China. Bull Environ Contam Toxicol 79:209–213
Michalec BK, Lenart-Boroń AM, Cupak AK, Wałęga AS (2014) The evaluation of heavy metal content in water and sediments of small reservoirs in light of various environmental quality regulations. J Environ Sci Health Part A 49:827–832. doi:10.1080/10934529.2014.882645
Mysen BD, Kushiro I (1976) Partioning of iron, nickel and magnesium between metal oxides and silicates in Allende meteorite as a function of CO2. Ann Rep Director Geophys Lab 1975–1976(1700):656–659
Naik PK, Awasthi AK (2003) Groundwater resources assessment of Koyna River basin, India. J Hydrol 11(5):582–594
Naughton DP, Petróczi A (2008) Heavy metal ions in wines: meta-analysis of target hazard quotients reveal health risks. Chem Central J Res 2:22. doi:10.1186/1752-153X-2-22
Orlov DS (1992) Soil chemistry. Oxford and IBH publishing Co. Pvt. Ltd, New Delhi, p 390
Patino LC, Velbel MA, Price JR, Wade JA (2003) Trace element mobility during spheroidal weathering of basalts and andesites in Hawaii and Guatemala. Chem Geol 202:343–364
Pawar NJ, Nikumbh JD (1999) Trace element geochemistry of groundwaters from Behedi basin, Nasik district, Maharashtra. J Geol Soc India 54:501–514
Pawar NJ, Shaikh IJ (1995) Nitrate pollution of groundwaters from shallow basaltic aquifers. Deccan Trap Hydrogeologic Province, India. Environ Geol 25:197–204
Pawar NJ, Pawar JB, Suyash K, Supekar Ashwini (2008a) Geochemical eccentricity of groundwater allied to weathering of basalts from the Deccan Volcanic province, India: insinuation on CO2 consumption. Aquat Geochem 14:41–71
Pawar NJ, Pawar JB, Supekar A, Karmalkar NR, Suyash K, Erram V (2008b) Indian Dykes Editors: Rajesh K. Srivastava, Ch. Sivaji and N. V. Chalapathi Rao. Narosa Publishing House Pvt. Ltd., New Delhi
Petroczi A, Naughton DP (2009) Mercury, cadmium and lead contamination in seafood: a comparative study to evaluate the usefulness of target hazard quotients. Food Chem Toxicol 47(2):298–302
PuYang X, Gao C, Han L (2015) Risk assessment of heavy metals in water and two fish species from Golf Course Ponds in Beijing, China. Bull Environ Contam Toxicol 94:437–443
Rabemanana V, Violette S, de Marsily G, Robain H, Deffontaines B, Andrieux P, Bensimon M, Parraux A (2005) Origin of high variability of water mineral content in the bedrock aquifers of Southern Madagaskar. J Hydrol 310:143–156
Rajmohan N, Elango L (2005) Distribution of iron, manganese, zinc and attrazine in groundwater in parts of palar and cheyyar rivers basins, south India. Environ Monit Assess 107:115–131
Robertson WD, Blows DW (1995) Major ion and trace metal geochemistry of an acidic septic system plume in silt. Groundwater 33(2):275–283
Salonen VP, Neimi KK (2007) Influence of parent sediments on the concentration of heavy metals in urban and suburban soils in Turku, Finland. Appl Geochem 22:906–918
Sen G (1986) Mineralogy and petrogenesis of the Deccan Trap Lava Flows around Mahabaleshwar, India. J Petrol 27(3):627–663
Sethna SF, Ateeq K, Javeri P (1996) Petrology of basic intrusives in the Deccan Volcanic Province South of Tapti Valley and their comparison with those along the west coast. Gondwana Geol Mag Special 2:225–232
Sharma SK, Subramanium V (2010) Source and distribution of trace metals and nutrients in Narmada and Tapti river basins, India. Environ Earth Sci 61(7):1337–1352
Sharma RK, Agrawal M, Marshall F (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66(2007):258–266
Shirke KD, Pawar NJ (2015) Enrichment of arsenic in the Quaternary sediments from Ankaleshwar industrial area, Gujarat, India: an anthropogenic influence. Environ Monit Assess 187:593. doi:10.1007/s10661-015-4815-9
Smoulders APJ, Hudson-Edwards KA, Vander Velde G, Roelofs JGM (2004) Controls on water chemistry of the Pilcomayo river (Bolivia, South-america). Appl Geochem 19:1745–1758
Storelli MM (2008) Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46(8):2782–2788
Storelli MM, Barone G, Cuttone G, Giungato D, Garofalo R (2010) Occurrence of toxic metals (Hg, Cd and Pb) in fresh and canned tuna: public health implications. Food Chem Toxicol 48(11):3167–3170
Subbarao KV, Hooper PR (1988) Reconnaissance map of the Deccan basalt group in the Western Ghats, India. In: Subbarao KV (ed) Deccan flood basalts. Mem. Geol. Soc. India No. 10
Subbarao KV, Chandrashekharam D, Navaneethakrishanan P, Hooper PR (1994) Stratigraphy and structure of parts of the Central Deccan Basalt Province: eruptive models. Volcanism, pp 321–332
Suyash K, Pawar NJ (2008) Quantifying spatio-temporal variations in heavy metal enormity of groundwaters from Ankaleshwar area: GIS based Normalised Difference Dispersal Index mapping. Curr Sci 94(7):905–990
Suyash K, Pawar NJ (2010) Site-specific accentuation of heavy metals in groundwaters from Ankaleshwar industrial estate. Environ Earth Sci, India. doi:10.1007/s12665-010-0879-6
Suyash K, Shirke KD, Pawar NJ (2008) GIS based color composites and overlays to delineate heavy metal contamination zones in the shallow alluvial aquifers, Ankaleshwar Industrial Estate, south Gujarat, India. Environ Geol 54:117–129
Tarits C, Aquilina L, Ayraud V, Pauwel H, Davy P, Touchard F, Bour O (2006) Oxido-reduction sequence related to flux variations of groundwater from fractured basement aquifer (Ploemeur area, France). Appl Geochem 21:29–47
Tiwari MK, Bajpai S, Dewangan UK, Tamrakar RK (2015) Assessment of heavy metal concentrations in surface water sources in an industrial region of central India. Karbala Int J Modern Sci
Tume P, Bech J, Tume L, Bech J, Reverter F, Longan L, Cendoya P (2008) Concentrations and distributions of Ba, Cr, Sr, V, Al and Fe in Torrelles soil profiles (Catalonia, Spain). J Geochem Explor 96:94–105
U.S. Environmental Protection Agency (1989) Guidance manual for assessing human health risks from chemically contaminated, fish and shellfish. U.S. Environmental Protection Agency, Washington, D.C. EPA-503/8-89-002
U.S.G.S. (1993). National water summary—1990–1991: Stream water quality; U.S. Geol. Sur. Water Supply Paper, 2400, p 590
Varian (1989) Analytical methods, flame atomic absorption spectrometry, publication no. 85-100009-00, Mulgrave, p 146
Wong CSC, Li X, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142:1–16
World Health Organisation (1971) International Standards for drinking water, 3rd edn. WHO, Geneva, p 70
Yuan GH, Sun TH, Han P, Li J, Lang XX (2014) Source identification and ecological risk assessment of heavy metals in topsoil using environmental geochemical mapping: typical urban renewal area in Beijing, China. J Geochem Explor 136:40–47
Zhang K, Dearing JA, Dawson TP, Dong X, Yang X, Zhang W (2015) Poverty alleviation strategies in eastern China lead to critical ecological dynamics. Sci Total Environ 506–507:164–181
Zhou JM, Dang Z, Cai MF, Liu CQ (2007) Soil heavy metal pollution around the Dabaoshan Mine, Guangdong Province, China. Pedosphere 17(5):588–594
Acknowledgments
The authors gratefully acknowledge the facilities provided by Department of Geology, Savitribai Phule Pune University, Pune. Thanks are also due to DST-FIST funding for equipment and UGC, Government of India for granting study leave to JBP. Assistance in the field by colleagues Dr. N.R. Karmalkar, Suyash Kumar and Dr. Vinit Erram is thankfully acknowledged. The authors sincerely thank the anonymous reviewers for making many meaningful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pawar, N.J., Pawar, J.B. Intra-annual variability in the heavy metal geochemistry of ground waters from the Deccan basaltic aquifers of India. Environ Earth Sci 75, 654 (2016). https://doi.org/10.1007/s12665-016-5450-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5450-7