Abstract
Background
Mesangial proliferative glomerulonephritis is a common glomerular disorder that may lead to end-stage renal disease. Epidermal growth factor (EGF) plays an important role in the regulation of cell growth, proliferation, and differentiation and in the pathology of various renal diseases. Erlotinib is a novel, oral, highly selective tyrosine kinase inhibitor of the EGF receptor. It is clinically used to treat non-small cell lung and pancreatic cancers. Here, we investigated the effect of erlotinib on the progression of mesangioproliferative glomerulonephritis in an experimental model.
Methods
Mesangial glomerulonephritis was induced with anti-rat Thy-1.1 antibody in male Wistar rats weighing 150–160 g. Rats were treated with erlotinib (10 mg/kg/day p.o.) or vehicle only (polyethylene glycol). Native Wistar rat kidneys were used as histological controls. Serum creatinine levels were measured at day 7. Kidneys were harvested 7 days after antibody administration for histology.
Results
Native controls showed no histological signs of glomerular pathology. In the vehicle group, intense glomerular inflammation developed after 7 days and prominent mesangial cell proliferation and glomerular matrix accumulation was seen. Erlotinib was well tolerated and there were no adverse effects during the follow-up period. Erlotinib significantly prevented progression of the glomerular inflammatory response and glomerular mesangial cell proliferation as well as matrix accumulation when compared with the vehicle group. Erlotinib also preserved renal function.
Conclusion
These results indicate that erlotinib prevents the early events of experimental mesangial proliferative glomerulonephritis. Therefore, inhibition of the EGF receptor with erlotinib could prevent the progression of glomerulonephritis also in clinical nephrology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glomerular diseases and particularly glomerulonephritis are major problems in clinical nephrology [1]. Glomerulonephritis may lead to end-stage renal disease (ESRD) and declining renal function, resulting in dialysis or renal transplantation. Although there have been many studies on glomerulonephritis in recent years, there is no cure for this condition and the current medication only slows down the progress of the disease. The pathogenesis of glomerulonephritis varies. It is likely that genetic factors, immunological abnormalities, and exposure to antigens play a critical role in the initial steps of the disease [2, 3]. Glomerulonephritic changes lead to increased permeability of the glomerular capillary wall and, thus, massive proteinuria, blood hypoalbuminemia, generalized edema, a drop in plasma volume, and diminished glomerular filtration. At this stage, the disease is incurable and leads evidently to ESRD and dialysis.
Epidermal growth factor (EGF) mediates cell proliferation and differentiation through multiple pathways. On the cell surface, it binds to EGF-receptor (EGFR), which belongs to the ErbB family of receptor tyrosine kinases [4]. EGFR can also be activated by various other ligands, like transforming growth factor beta (TGF-β), angiotensin II (ANG II), endothelin, aldosterone, and thrombin through transactivation [5]. Therefore, EGFR plays a role in mediating the effect of ANG II on renal cell proliferation and accumulation of fibrosis [6, 7].
In the human kidney, EGF is mainly expressed in the tubular area [8], whereas EGFR is widely distributed in the glomeruli, proximal tubuli, and the medullae [9]. EGF has an important role in controlling renal hemodynamics. It decreases renal blood flow and the glomerular filtration rate [10]. Prolonged activation of EGFR may affect renal function by epithelial-mesenchymal transition, leading to fibrosis and renal cell carcinoma [11–14]. In addition, EGFR mediates the effect of heparin-binding epidermal growth factor-like growth factor (HB-EGF), which is active in clinical glomerulonephritis [15].
Erlotinib is a tyrosine kinase inhibitor, which is clinically used to treat non-small cell lung and pancreatic carcinomas [16, 17]. Erlotinib is a highly specific EGFR inhibitor that prevents the proliferation and differentiation of cancer cells. It has been widely studied in other pathological conditions and was shown to prevent experimental rapidly progressive glomerulonephritis (RPGN) in mice [18] and partially inhibit cisplatin-induced nephrotoxicity in a rat-model [19]. We previously demonstrated that erlotinib prevented chronic allograft injury in an experimental kidney transplantation model [20]. This effect appears to be mediated, in part, by decreasing the proliferation and differentiation of monocyte-macrophages [21].
Here, we investigated the effect of EGFR inhibition by erlotinib on the development of kidney damage in an antibody-mediated glomerulonephritis model. Our hypothesis was that EGF has a major role in the development of the pathological changes seen in glomerulonephritis.
Materials and methods
Animals
Specific, pathogen-free, male Wistar rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 150–160 g were used. They received regular rat food and tap water ad libitum, and were maintained on a 12-h light/dark cycle. The animals received human care in compliance with the Guide for the Care and Use of Laboratory Animal Resources published by the National Institutes of Health and Office of Animal Care and Use (National Academy Press 2011, National Research Council, Washington, DC, USA). There were five animals in each study group.
Model of anti-Thy 1.1-induced glomerulonephritis
Glomerulonephritis was induced by intravenously injecting the monoclonal anti-Thy 1.1 antibody (as74-p, 3 mg/kg, Antibody Solutions, Sunnyvale, CA, USA). This antibody causes mesangial cell lysis in the kidney within 24 h by binding to a Thy 1-like antigen on the surface of renal mesangial cells, leading to mesangial cell proliferation, accumulation of the mesangial matrix, and, therefore, massive mesangial injury causing proteinuria [22]. This antibody-induced glomerulonephritis animal model mimics mesangioproliferative glomerular diseases, such as IgA nephropathy in humans. Animals were sacrificed at day 7 for histology. The creatinine levels were determined from the serum samples taken before sacrifice.
Treatment
After induction of glomerulonephritis, the animals were treated either with erlotinib (10 mg/kg/day) or vehicle only (polyethylene glycol, Sigma-Aldrich, St. Louis, MO, USA). Erlotinib (kindly provided by Roche, Basel, Switzerland) and vehicle were administered orally once a day.
Histopathology
The kidneys were bisected horizontally and fixed in 4 % paraformaldehyde. The specimens were cut in 4 µm thick sections and stained with Mayer’s hematoxylin–eosin, Masson’s trichrome, and diastase-periodic acid-Schiff to investigate histopathological changes.
Quantification of histopathology
Histopathological analysis was done by blind review. The intensity of glomerular damage was scored by analysis of glomerular matrix expansion of approximately 50 full-sized, randomly selected glomeruli from each specimen, and the lesion was graded from 0 to 4, according to the percentage of glomerular involvement as follows: 0, 0–4 %; 1, 5–24 %; 2, 25–49 %; 3, 50–74 %; 4, 75 % or more [23]. The mean number of inflammatory cells in the glomerulus was calculated from ten randomly chosen visual fields per sample (magnification ×20). To define the glomerular tuft area, all glomeruli seen in three randomly chosen visual fields (magnification ×20) were digitally analyzed using Axioplan 4.2 analysis software (Carl Zeiss, Oberkochen, Germany).
Immunohistochemistry
For immunohistochemistry, the samples were incubated in 4 % paraformaldehyde for 4 h and then fixed for paraffin blocks. Four-µm thick sections were cut in series on glass slides. Before staining, the samples were deparaffinized. For epitope retrieval, the glass slides were heated in a microwave oven for 20 min in sodium citrate buffer (pH 6.0) and then allowed to cool at room temperature. To demonstrate the expression and localization of EGF and EGFR, the samples were immunostained using the Vectastain Elite ABC Kit (Vector Laboratories Inc., Burlingame, CA, USA),according to the manufacturer’s instructions. The final staining reaction was revealed by NovaRed (Vector Laboratories). After the staining, the slides were counterstained with Mayer’s hemalum, incubated in an alcohol series and xylene, and permanently mounted. Polyclonal goat immunoglobulin (Ig)G antibodies to EGF (1 µg/ml; sc-1343; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and EGF-R (2 µg/ml; sc-03, Santa Cruz Biotechnology) were used to investigate growth factor expression.
To demonstrate the infiltration of activated macrophages and T-cells, a 3-layer indirect immunoperoxidase technique was used. Monoclonal mouse antibodies for ED3 (5 µg/ml, Serotec Ltd, Oxford, UK), rat CD4, and rat CD8 (5 µg/ml, BD PharMingen, San Diego, CA, USA) were used to define acute cell infiltration. Peroxidase-conjugated rabbit anti-mouse IgG (DAKO A/S, Copenhagen, Denmark) and peroxidase-conjugated goat anti-rabbit IgG (Caltag Laboratories, Burlington, CA, USA) were used sequentially. The final staining reaction was revealed by AEC (3-amino-9-ethylcarbazole, Vector Laboratories). A specimen stained without primary antibody was used as a negative control in all immunohistochemical staining.
Quantification of immunohistochemistry
Immunohistochemical analysis was done by blind review. The intensity of the staining of the samples was scored from 0 to 3 as follows: 0, no visible staining; 1, cells with faint staining; 2, moderate intensity with multifocal staining; and 3, intense diffuse staining.
Statistical analysis
Statistical analyses were performed using the SPSS program (SPSS version 10; SPSS, Chicago, IL, USA). The significance between the groups was determined by parametric analysis of variance and the Least Significant Difference test, ANOVA, or by non-parametric analysis by Mann–Whitney test.
Results
Clinical course and creatinine levels
Erlotinib was well tolerated and no side effects were observed. No animals were lost during the follow-up. Serum creatinine levels were significantly lower in the erlotinib-group compared with the vehicle group (p = 0.008, Fig. 1), indicating better renal function in the erlotinib-treated animals.
Histology
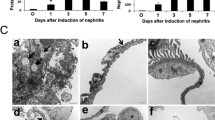
In native rat kidneys, there were no signs of glomerular pathology. However, moderate to intense glomerular changes were seen in the vehicle group 7 days after anti-Thy injection. Erlotinib ameliorated these histological changes and nearly normal histology was seen (Fig. 2a–d). Erlotinib treatment significantly decreased the glomerular cell count compared with the vehicle-treated control group (p = 0.001, Fig. 2a). In addition, the glomerular tuft area was significantly smaller in the erlotinib group than in the control group (p = 0.01, Fig. 2b), indicating decreased mesangial matrix expansion and tissue edema. Erlotinib treatment also decreased the glomerular matrix expansion score (p = 0.008, Fig. 2c) when compared with vehicle.
Glomerular infiltration: a erlotinib prevented glomerular cell accumulation when compared with the vehicle group and the cell count was similar to the level seen in native rat kidney-controls (p = 0.001). Erlotinib also prevented glomerular tissue edema (p = 0.01) (b) and glomerular mesangial matrix expansion (c) (p = 0.008). d Hematoxylin–eosin staining showing an increased glomerular tuft area and glomerular cell count in the vehicle group (magnification ×63 and ×20)
Glomerular lymphocyte and macrophage infiltration
Glomerular cell infiltration was further analyzed by immunohistochemistry. In the control group, there was intense infiltration of glomerular CD4 and CD8 positive lymphocytes and ED3 positive macrophages (Fig. 3a–d). Erlotinib significantly decreased the infiltration of CD4 positive lymphocytes (p = 0.001, Fig. 3a, d), CD8 positive lymphocytes (p = 0.001, Fig. 3b, d), and ED3 positive activated macrophages (p = 0.001, Fig. 3c, d).
Growth factor expression
In the vehicle group, there was moderate to intense expression of glomerular EGF and EGFR (Fig. 4a–c). Erlotinib significantly decreased EGF (p = 0.016, Fig. 4a, c) and EGFR (p = 0.016, Fig. 4b, c) expression.
Discussion
Glomerulonephritis is a common kidney disease that leads to declining renal function and ESRD with a need for dialysis or renal transplantation. The mechanisms behind the glomerular inflammation remain unknown and currently there is no curative treatment for this pathological process. Growth factors may have a role in the initial pathology of glomerulonephritis and in the development of the subsequent ESRD. The role of EGF in the development of glomerulonephritis is not well established. Here, we show that glomerular expression of EGF and EGFR is elevated in the early phase of experimental glomerulonephritis. In addition, the inhibition of EGF-function with erlotinib significantly decreases glomerular changes and glomerular swelling while preserving renal function. These findings indicate that EGF has a role in the early events of glomerular inflammation as well as in the development of glomerulonephritis.
The infiltration of CD4 and CD8 positive T-cells in glomeruli is one sign of inflammation in glomerulonephritis. According to our results, the inhibition of EGF function by erlotinib decreases the lymphocyte infiltration and inflammation changes in the glomeruli. In addition, it significantly decreases the glomerular infiltration of ED3 + activated macrophages, supporting our previous finding that erlotinib decreases the proliferation and differentiation of monocyte-macrophages in vitro [21].
Erlotinib is clinically used in the treatment of lung and pancreatic cancers and it is well tolerated [16, 17]. The future use of orally administered tyrosine kinase inhibitors will likely expand outside of the field of oncology. We already demonstrated that erlotinib had a beneficial effect in an experimental kidney transplantation [20]. Here, we show that erlotinib decreased expression of the EGF ligand and receptor and maintained better renal function in the early phase of experimental glomerulonephritis. Based on these findings, we hypothesize that EGF-activation is one pathological mechanism mediating the development of glomerulonephritis. Furthermore, these results suggest that EGF-inhibition with erlotinib could prevent the development of glomerulonephritis.
Taken together, our results indicate that EGF expression is increased during the early development of experimental glomerulonephritis. In addition, we demonstrated that EGFR inhibition with erlotinib prevents the development of glomerulonephritis and maintains better renal function. Therefore, erlotinib could be a new intervention for glomerulonephritis in clinical nephrology.
References
Levy M, Berger J (1988) Worldwide perspective of IgA nephropathy. Am J Kidney Dis 12(5):340–347
Emancipator SN, Lamm ME (1989) IgA nephropathy: overproduction or decreased clearance of immune complexes? Lab Invest 61(4):365–367
Couser WG (1993) Mediation of immune glomerular injury. Clin Investig 71(10):808–811
Holbro T, Hynes NE (2004) ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 44:195–217
Wetzker R, Bohmer FD (2003) Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4(8):651–657
Watanabe T, Barker TA, Berk BC (2005) Angiotensin II and the endothelium: diverse signals and effects. Hypertension 45(2):163–169
Mezzano SA, Ruiz-Ortega M, Egido J (2001) Angiotensin II and renal fibrosis. Hypertension 38(3 Pt 2):635–638
Kasselberg AG, Orth DN, Gray ME, Stahlman MT (1985) Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem 33(4):315–322
Harris RC (1991) Potential physiologic roles for epidermal growth factor in the kidney. Am J Kidney Dis 17(6):627–630
Harris RC, Hoover RL, Jacobson HR, Badr KF (1988) Evidence for glomerular actions of epidermal growth factor in the rat. J Clin Invest 82(3):1028–1039
Norman J, Badie-Dezfooly B, Nord EP, Kurtz I, Schlosser J, Chaudhari A et al (1987) EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am J Physiol 253(2 Pt 2):F299–F309
Okada H, Danoff TM, Kalluri R, Neilson EG (1997) Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol 273(4 Pt 2):F563–F574
Creely JJ, DiMari SJ, Howe AM, Hyde CP, Haralson MA (1990) Effects of epidermal growth factor on collagen synthesis by an epithelioid cell line derived from normal rat kidney. Am J Pathol 136(6):1247–1257
Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P et al (2000) Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106(2):225–234
Flamant M, Bollee G, Henique C, Tharaux PL (2012) Epidermal growth factor: a new therapeutic target in glomerular disease. Nephrol Dial Transplant 27(4):1297–1304
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E et al (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11(6):521–529
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M et al (2011) Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17(10):1242–1250
Wada Y, Iyoda M, Matsumoto K, Shindo-Hirai Y, Kuno Y, Yamamoto Y et al (2014) Epidermal growth factor receptor inhibition with erlotinib partially prevents cisplatin-induced nephrotoxicity in rats. PLoS One 9(11):e111728
Rintala JM, Savikko J, Palin N, Rintala SE, Koskinen PK, von Willebrand E (2014) Epidermal growth factor inhibition, a novel pathway to prevent chronic allograft injury. Transplantation 98(8):821–827
Savikko J, Rintala JM, Rintala S, Koskinen P (2015) Epidermal growth factor receptor inhibition by erlotinib prevents vascular smooth muscle cell and monocyte-macrophage function in vitro. Transpl Immunol 32:175–178
Ikezumi Y, Kawachi H, Toyabe S, Uchiyama M, Shimizu F (2000) An anti-CD5 monoclonal antibody ameliorates proteinuria and glomerular lesions in rat mesangioproliferative glomerulonephritis. Kidney Int 58(1):100–114
Tomita M, Sogabe H, Nakazato S, Nakatsuji S, Noto T, Hamada K et al (2005) Monoclonal antibody 1-22-3-induced glomerulonephritis in uninephrectomized rats as a model of progressive renal failure. Nephrol Dial Transplant 20(11):2358–2367
Acknowledgments
This work was supported in part by Finska Läkaresällskapet, the Finnish Medical Foundation, Helsinki University Hospital Research Funds, the Research and Science Foundation of Farmos, Research Funds of Päijät-Häme Central Hospital, and the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Rintala, J.M., Savikko, J., Rintala, S.E. et al. Epidermal growth factor receptor inhibition with erlotinib ameliorates anti-Thy 1.1-induced experimental glomerulonephritis. J Nephrol 29, 359–365 (2016). https://doi.org/10.1007/s40620-015-0233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0233-x