Abstract
We describe the case of a 66-year-old woman treated with tyrosine kinase inhibitor Lenvatinib for thyroid carcinoma who had persistent proteinuria above 2 g/24 h despite maximal dose of angiotensin-converting enzyme inhibitor. We initiated a treatment with SGLT2 inhibitor Dapagliflozin. Three months after Dapagliflozin initiation, her proteinuria decreased to 1 g/24 h, and after 6 months of follow-up was 0.6 g/24 h. To our knowledge, this is the first case of successful proteinuria reduction with SGLT2i in a patient treated with Lenvatinib. Specific renal effects of SGLT2i seem promising and their effects on tyrosine kinase inhibitor renal adverse effects need to be validated in clinical trials involving cancer patients.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Proteinuria and hypertension are recognized class effects of antiangiogenic therapy, first identified with the vascular endothelial growth factor (VEGF) antibody bevacizumab, but common to most tyrosine kinase inhibitors (TKIs) targeting VEGF receptors. Lenvatinib is a multikinase inhibitor of VEGF receptors 1–3, fibroblast growth factor receptors 1–4, RET, KIT and platelet-derived growth factor receptor-α which is approved for the treatment of radioiodine-refractory differentiated thyroid cancer [1]. Proteinuria and hypertension occur in 31% and 68%, respectively, of patients receiving Lenvatinib [2].
Strategies for managing renal adverse effects are always challenging with TKIs, and evidence-based medicine guidelines are lacking. As proteinuria may be frequently associated with hypertension in patients treated with TKIs, the use of angiotensin-converting enzyme inhibitors (ACEi) as a treatment option can be considered to lower proteinuria [3]. It is known that SGLT2 inhibition decreases albuminuria and reduces the risk of kidney disease progression [4]. SGLT2 inhibitors (SGLT2i) could have an impact on renal adverse effects related to TKIs but their effect has not yet been studied in this condition.
We describe the first case of successful proteinuria reduction with SGLT2i in a patient treated with Lenvatinib and we review the pathophysiological effects of SGLT2i in this condition.
Case report
We describe the case of a 66-year-old woman who was diagnosed in 2019 with radioactive iodine-refractory poorly differentiated locally advanced thyroid carcinoma with pulmonary metastases and treated with cervical radiation therapy after incomplete thyroid surgery. Her past medical history was significant for hypertension and multinodular goiter. Patient’s characteristics are summarized in Table 1. She was started on lenvatinib and a partial tumor response was achieved after 2 months with long term disease control. When starting TKI therapy, she had no hypertension and no proteinuria. A few months after Lenvatinib initiation, she developed proteinuria and was treated with perindopril. During the entire follow-up period, renal function remained normal. Estimated glomerular filtration rate and persistent albuminuria category according to KDIGO guidelines are shown in Table 1 [5]. Despite ACEi treatment, with blood pressure < 140/90 mmHg, persistent proteinuria above 2 g/24 h was noted on Lenvatinib treatment maintained at a daily dosage of 18 mg. As the patient had asthenia and diarrhea, Lenvatinib was temporarily discontinued which led to a reduction of proteinuria < 1 g/24 h. One month after Lenvatinib reintroduction, proteinuria was > 3 g/24 h and she was referred to nephrology.
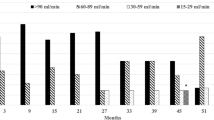
Considering the beneficial effect of this treatment in this patient, we decided to maintain Lenvatinib and initiated treatment with Dapagliflozin 10 mg per day. Three months after Dapagliflozin initiation, her proteinuria dropped to 1 g/24 h despite continuation of Lenvatinib, and after 6 and 9 months of follow-up, proteinuria was 0.6 and 0.7 g/24 h, respectively (Fig. 1). Dapagliflozin treatment tolerance was excellent. The latest 18F-FDG PET-CT scan demonstrated ongoing disease stabilization for more than 3 years.
Discussion
Tyrosine kinase inhibitors inhibit angiogenesis which is caused by various growth factors such as VEGF, epidermal growth factor and platelet-derived growth factor. By inhibiting VEGF, TKIs can alter the integrity of the glomerular slit diaphragm which can cause proteinuria, a commonly described class effect of TKIs. One hypothesis is that that VEGF produced by podocytes travels across the glomerular filtration barrier and reaches the endothelial surfaces where it interacts with several receptors [3]. By inhibiting VEGF, TKIs also reduce nephrin production which can lead to proteinuria [3]. As TKIs have improved progression-free survival for several cancers, it is crucial to very carefully assess the risks and benefits of continuing these agents in situations of proteinuria and/or hypertension.
Lenvatinib is an agent that shows strong tumor suppression, targeting multiple receptors including VEGF receptor 1 to − 3 [6]. The effects of Lenvatinib on renal function are raising new concerns, especially for patients on long-term treatment. Lenvatinib can induce a decline in estimated glomerular filtration rate, particularly with treatment duration > 2 years, and proteinuria is a risk factor for this decline [6].
Considering the favorable endothelial effects of ACEi/angiotensin receptor blockers, they are an interesting first-line agent [3], especially to avoid TKI discontinuation. SGLT2i are being used more and more often and their effect could be interesting in proteinuria related to TKI toxicity. Indeed, the mechanisms of proteinuria due to TKIs appear to be vascular endothelial damage and podocytopathy in glomeruli and it is proven that Dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy [7]. In a recent study, Vart et al. [8] reported that treatment with ACEi/ARBs and SGLT2 inhibitors in patients with albuminuric chronic kidney disease without diabetes but with proteinuria may increase the number of years free from kidney failure and mortality.
The specific effects of SGLT2 inhibitors regarding TKI renal adverse effects have not been proven but several studies have described promising results. Madonna et al. [9] demonstrated that SGLT2 inhibitors empagliflozin and dapagliflozin attenuated the vascular-toxic effect exerted by Ponatinib (a third generation TKI) by reverting endothelial cell senescence and dysfunction in an in-vitro model. In a murine model, Empagliflozin is able to improve Sunitinib-induced cardiac dysfunction via regulating cardiomyocyte autophagy mediated by the AMPK-mTOR signaling pathway [10]. In ob/ob type 2 diabetic mice with albuminuria, Empagliflozin restored the subverted microvascular endothelial ultrastructure [11]. Recently, in a randomized controlled trial in albuminuric type 2 diabetes patients, Tian et al. showed that SGLT2 inhibitors attenuated nephrin loss in the urine; the anti-albuminuric effect of SGLT2i could be attributed to mitigating podocyte apoptosis and attenuating renal fibrosis [12]. This pathophysiology could explain the positive effect on proteinuria induced by TKIs. However, it is important to note that in a randomized controlled trial, 6-week treatment with dapagliflozin did not affect proteinuria in patients with chronic kidney disease without diabetes [13]. This anti-proteinuria effect needs to be evaluated in longer-term kidney outcome trials.
The development of therapeutic strategies for the management of Lenvatinib-associated adverse effects are crucial to improve quality of life, minimize the need for dose reduction, treatment discontinuation and improve patient outcomes [14]. SGLT2i could be a new therapeutic option in situations requiring long-term therapy for recurrent or advanced cancer with few systemic treatment options. As several clinical trials have shown, use of SGLT2i is usually well tolerated and safe and showed an acceptable safety profile in patients with chronic kidney disease [4].
To our knowledge, this is the first reported case of proteinuria reduction with SGLT2i in a patient treated with Lenvatinib. Specific renal effects of SGLT2i seem promising and their effect on TKI renal adverse effects need to be validated in larger studies involving cancer patients.
References
Cabanillas ME, Hu MI, Durand JB, Busaidy NL (2011) Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res 2011:1–9
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372(7):621–630
Kandula P, Agarwal R (2011) Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 80(12):1271–1277
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF et al (2020) Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383(15):1436–1446
Stevens PE (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11):825
Masaki C, Sugino K, Kobayashi S, Hosoi Y, Ono R, Yamazaki H et al (2021) Impact of lenvatinib on renal function: long-term analysis of differentiated thyroid cancer patients. BMC Cancer 21(1):894
Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C et al (2018) SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 3(15):e98720
Vart P, Vaduganathan M, Jongs N, Remuzzi G, Wheeler DC, Hou FF et al (2022) Estimated lifetime benefit of combined RAAS and SGLT2 inhibitor therapy in patients with albuminuric CKD without diabetes. CJASN 17(12):1754–1762
Madonna R, Barachini S, Moscato S, Ippolito C, Mattii L, Lenzi C et al (2022) Sodium-glucose cotransporter type 2 inhibitors prevent ponatinib-induced endothelial senescence and disfunction: a potential rescue strategy. Vascul Pharmacol 142:106949
Ren C, Sun K, Zhang Y, Hu Y, Hu B, Zhao J et al (2021) Sodium-glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK–mTOR signaling pathway-mediated autophagy. Front Pharmacol 29(12):664181
Locatelli M, Zoja C, Conti S, Cerullo D, Corna D, Rottoli D et al (2022) Empagliflozin protects glomerular endothelial cell architecture in experimental diabetes through the VEGF-A/caveolin-1/PV-1 signaling pathway. J Pathol 256(4):468–479
Tian Y, Chen X, Liang X, Wu X, Yao C (2022) SGLT2 inhibitors attenuate nephrin loss and enhance TGF-β1 secretion in type 2 diabetes patients with albuminuria: a randomized clinical trial. Sci Rep 12(1):15695
Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ et al (2020) Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8(7):582–593
Cabanillas ME, Takahashi S (2019) Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin Oncol 46(1):57–64
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to disclose. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Ethical approval was sought but not deemed necessary by the hospital research committee.
Human and animal rights
There are no human and animal right issues to declare.
Informed consent
The authors declare that they have obtained consent from the patient discussed in the report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fages, V., Jannin, A., Maanaoui, M. et al. Proteinuria reduction with SGLT2 inhibitors in a patient treated with tyrosine kinase inhibitor lenvatinib. J Nephrol 37, 187–189 (2024). https://doi.org/10.1007/s40620-023-01701-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01701-0