Abstract
Background
Leptin and ghrelin appear to play a role in weight regain after a successful weight loss. The pre-treatment plasma levels of leptin/ghrelin ratio (L/G) could have power to predict this clinically relevant issue in the obesity treatment.
Objective
To evaluate the ability of the L/G as a non-invasive tool for the early discrimination of obese patients who are more likely to regain weight after an energy restriction program (regainers) from those who maintain the lost weight (non-regainers).
Subjects and methods
Fasting leptin and ghrelin levels were evaluated in 88 overweight/obese patients who followed an 8-week hypocaloric diet program and were categorized as regainers (≥10 % weight-lost regain) and non-regainers (<10 % weight-lost regain) 6 months (32 weeks) after finishing the dietary treatment. A receiver operating characteristic (ROC) curve analysis was employed to evaluate the diagnostic value of the L/G ratio and to establish a cut-off point to differentiate regainers from non-regainers.
Results
Regainers showed a statistically higher baseline (week 0) and after treatment (week 8) L/G ratio than non-regainers. The baseline L/G ratio was associated with an increased risk for weight regain (odds ratio 1.051; p = 0.008). Using the area under the ROC curve (AUC), the L/G ratio significantly identified female (AUC = 0.69; p = 0.040) and male regainers (AUC = 0.68; p = 0.030). The maximum combination of sensitivity and specificity was shown at the cut-off point of 26.0 for women and 9.5 for men.
Conclusions
The pre-intervention fasting leptin/ghrelin ratio could be a useful non-invasive approach to personalize obesity therapy and avoid unsuccessful treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Weight regain after hypocaloric diet-induced weight loss has emerged as one of the most significant obstacles in obesity therapy [1, 2]. This fact perpetuates the obesity epidemic that affects more than 30 % of U.S. adults [3] and 23 % of Spanish adults [4]. Diverse therapeutic strategies have been adopted to decrease body adiposity. Nutritional interventions, such as caloric restriction diets, can be an efficient therapeutic approach to promote weight loss in obese patients [5, 6]. Although most therapeutic trials involving drugs [7] or dietary treatments show mean weight losses higher than 5 % of body weight [5], the long-term success of maintaining the weight loss is usually poor [8]. Therefore, the identification of factors associated with weight regain can enhance our understanding regarding energy homeostasis regulation and would have positive implications in the personalization of therapeutic approaches to obesity.
Several biological and psychological factors may contribute to weight regain after a weight loss treatment [9]. There is also evidence that some weight reduction therapies fail due to compensatory metabolic adaptations, which promote rapid and efficient weight regain [1, 8]. Among the mechanisms implicated, orexigenic and anorexigenic hormones appear to have a clinically relevant role [10].
Ghrelin is a circulating orexigenic hormone [11] implicated in both the short-term control of food intake at single meals and long-term body weight regulation [12]. Moreover, this hormone improves the cardiovascular function [13] and it exerts an anti-inflammatory effect that contributes to extend the life span [14]. Leptin is a key anorexigenic hormone that relays information about peripheral adiposity by signaling to the central nervous system to regulate food intake and energy expenditure [15]. There is a complex interaction between leptin and ghrelin, which are reciprocally regulated during the physiological response to food intake, inducing changes in body weight [16].
Nutrigenomic/nutrigenetic and proteomic studies have been performed to identify markers with the potential to predict successful weight maintenance [17–20]. We have previously demonstrated [21] that patients who regained ≥10 % of the lost weight after weight reduction induced by an 8-week-balanced hypocaloric diet (regainers) have higher leptin levels and lower ghrelin levels than those who maintained the lost body weight (non-regainers). Interestingly, this result suggests the existence of two different predetermined populations of patients according to the leptin and ghrelin levels conditioning the outcomes [21]. However, there are no studies that effectively demonstrate clinical usefulness in discriminating between regainers and non-regainers of diet-induced weight loss. In fact, a recent systematic review concluded that little evidence within the reviewed studies support pre- or post-weight loss leptin, ghrelin or insulin sensitivity as predictor of regain, suggesting that these hormones taken alone, are not sufficient predictors of weight regain in free-living humans [22]. Under these evidences, we suggest that the leptin/ghrelin ratio could be a better predictor of weight regain than either leptin or ghrelin alone.
The aims of this study performed in obese patients were the following: (1) to evaluate the ability of the leptin/ghrelin ratio as a non-invasive tool for the early identification of obese patients more likely to regain the lost weight after an energy restriction program and (2) to assess a leptin/ghrelin ratio cut-off that may help to differentiate weight loss regainers from non-regainers.
Methods
Study patients and design
The trial was based on a nutritional intervention monitored by trained dieticians from the Department of Nutrition, Food Science and Physiology of the University of Navarra, as previously described [21]. The protocol used for this study was approved by the Clínica Universidad de Navarra Ethical Committee (Ref. 54/2006) in agreement with the Helsinki Declaration and followed national and EU guidelines.
A group of overweight and obese patients (n = 104) were enrolled in a nutritional weight loss program that occurred over 2 months [21]. At 32 weeks, i.e., 6 months after dieting ended, the patients returned to the clinical research unit for further assessment (Fig. 1). The patients were then categorized as “regainers” or “non-regainers” depending on whether they regained more than 10 % of the lost weight. The circulating leptin/ghrelin ratio (L/G) was evaluated in 48 (29 men/19 women) obese patients that maintained the initial weight reduction (non-regainers) and 40 (20 men/20 women) obese patients that regained the lost weight (regainers). Sixteen patients were excluded due to the lack of completed data for leptin and ghrelin levels.
Schematic representation of the experimental design. Unsuccessful weight loss maintenance was defined as weight loss regain of at least 10 % of the lost weight after the energy restriction treatment. Based on these criteria, 40 patients (20 men/20 women) regained >10 % of the lost weight (regainers), and 48 patients (29 men/19 women) maintained the initial weight reduction (non-regainers). Preliminary results of this work were presented at the 53rd Congress of the Spanish Society of Endocrinology and Nutrition (SEEN; May 2011. Endocrinol Nutr 58:80)
Weight loss was induced with a hypocaloric diet designed to produce a 30 % energy restriction (500–600 kcal/day) of the subject’s total energy expenditure. The nutritional intervention program consisted of an 8-week consumption of a balanced hypocaloric diet containing 55 % of the energy supply as carbohydrates, 15 % as protein and 30 % as fat, which was implemented by a food exchange system. The dietary compliance was assessed through 3-day weighed food records [23]. Upon completion of the dietary intervention (week 8), the patients were only instructed on a healthy lifestyle such as dietary intake and physical activity [21].
Anthropometric measurements were performed according to standardized procedures at baseline (week 0), the endpoint (week 8) and the follow-up period (week 32). Venous blood samples were collected after a 12-h overnight fast at the beginning of the restriction diet (baseline; week 0) and at the end of the diet intervention (endpoint; week 8). The EDTA-plasma and serum of patients were separated from whole blood and immediately frozen at −80 °C until assayed.
Calculation of the leptin/ghrelin ratio
Plasmatic ghrelin levels were measured with a radioimmunoassay (RIA) using a commercially available kit (Linco Research Inc., St Charles, MO, USA). This assay detects both acylated and deacylated ghrelin. The serum concentrations of leptin were assessed using a radioimmunoassay-based method (DPC, Los Angeles, CA, USA). To express the L/G ratio in arbitrary units, it was calculated as leptin in ng/mL multiplied by 103 and divided by ghrelin in pg/mL.
Because the function of these hormones is mediated by a molecule-receptor interaction, the L/G ratio was also studied on a molar basis to further evaluate its implication in weight loss regain at a more physiological level. Thus, the number of leptin and ghrelin molecules was determined through Avogadro’s constant (N A = 6.022 × 1023 mol−1).
Statistical analysis
The sample size of this interventional trial was estimated accounting for the weight loss after treatment (main variable) and calculated according to the equation reported by Mera et al. [24]. Thus, to detect differences, the sample size was established at a minimum of 85 obese/overweight patients who finished the nutritional intervention.
Weight regain was calculated (in percentage) as the difference in body weight between the follow-up period (week 32) and the endpoint (week 8) and related to the endpoint. Successful weight loss maintenance was considered with the criterion of 10 % weight regain, according to a previously published report [21].
The normal distribution of variables was performed using the Kolmogorov–Smirnov and the Shapiro–Wilk tests. Accordingly, the parametric Student’s t or nonparametric Mann–Whitney U test was applied to detect differences between regainers and non-regainers. The Spearman coefficient was used to evaluate the potential association between body weight regain and the leptin/ghrelin ratio (L/G). A logistic regression analysis was applied to assess the potential predictive capacity of the L/G ratio in weight loss regain.
The discriminatory ability of the L/G ratio at baseline (L/GB) and after 8 weeks of nutritional treatment (L/G8W) was estimated between regainers and non-regainers with the area under the receiver operating characteristic (ROC) curve (AUC) [25]. The AUCs of the L/GB and L/G8W differences were assayed according to the method of comparing the areas under ROC curves described by Hanley and McNeil [26].
Using the ROC curve analysis, the L/G cut-off value was defined as the level that yielded the maximum sensitivity and specificity for predicting weight regain propensity. Additionally, the diagnostic efficiency (percent of correctly classified) of the L/G ratio was calculated as the percent of agreement. These results are often interpreted as negligible efficiency (<20 %), minimal (20–40 %), moderate (41–60 %), good (61–80 %) and excellent (>80 %) [27]. The guidelines for the publication of studies of the diagnostic accuracy of medical tests were followed [28, 29].
Statistical analysis was performed with SPSS 15.0 software (SPSS Inc., IL, USA) for Windows XP (Microsoft, WA, USA). Results are presented as mean ± standard deviation and a p ≤ 0.05 was considered statistically significant.
Results
The analysis of the clinical characteristics of the patients categorized according to the 10 % of weight regain classification criterion showed no significant differences in age, body weight, body fat mass or waist circumference between regainer and non-regainer patients at baseline (Table 1). Significant differences were found between both groups for the BMI in women, the body weight decrease induced by the dietary treatment in men, and the weight loss regain in both genders, according to the study design.
As previously described [21], baseline leptin levels were statistically higher in regainer women and baseline ghrelin levels were statistically lower in regainer men compared with the same genders in the non-regainer group (Table 1). When the leptin/ghrelin ratio (L/G) was calculated, both the L/G ratios at baseline (L/GB) and after 8 weeks of hypocaloric treatment (L/G8W) were approximately twofold higher in regainers than non-regainers for both women and men (Fig. 2). On a molar basis, the L/G ratio difference was maintained at approximately twofold higher in regainers than non-regainers (p < 0.05). In fact, for women, the L/GB was 5.44 ± 2.49 for non-regainers vs. 9.03 ± 6.81 for regainers, and for men, the ratio was 3.36 ± 2.22 for non-regainers vs. 5.67 ± 4.44 for regainers. The L/G8W for female non-regainers was 4.03 ± 4.05 vs. 8.03 ± 7.87 for regainers, and in men, the ratio was 1.84 ± 3.00 for non-regainers vs. 2.88 ± 1.97 for regainers.
Box and whiskers plots comparing the circulating leptin/ghrelin ratio (L/G) between the non-regainer and the regainer group at baseline and after 8 weeks of an energy restriction treatment in men and women. The lower and upper end of the box represents the 25th and 75th percentile of the distribution, respectively. The center line inside the boxes indicates the median or 50th percentile, and the whiskers show the interquartile range. The L/G values are shown as leptin (ng/mL × 103)/ghrelin (pg/mL). a.u denotes arbitrary units
Interestingly, a correlation analysis showed that higher weight regain (%) was associated with higher values of L/GB (r = 0.30; p = 0.005) and L/G8W (r = 0.43; p < 0.001) (Fig. 3).
Association between the leptin/ghrelin ratios at baseline (L/GB) and after 8 weeks of energy restriction treatment (L/G8W) with body weight regain 6 months after finishing dietary intervention (32 weeks). The patients who presented higher values of the L/G ratio at baseline and after energy restriction treatment regained the lost body weight in the 6 months of follow-up. a.u denotes arbitrary units
To further investigate the potential predictive ability of the L/G ratio in weight regain, a logistic regression analysis was performed. Thus, after adjusting for age, gender and body weight at baseline, L/GB appeared to be a potential independent predictor of weight regain (odds ratio 1.051 (95 % CI 1.013–1.091); p = 0.008), whereas a non-statistically significant tendency was observed for L/G8W (odds ratio 1.031 (95 % CI 0.998–1.065); p = 0.067).
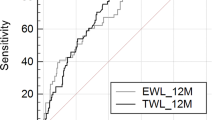
Receiver operating characteristic curves were used to evaluate the diagnostic power of L/G to discriminate regainer from non-regainer patients. The area under the ROC curve (AUC) of L/GB was 0.69 (95 % CI 0.52–0.86; p = 0.040) for women and 0.68 (95 % CI 0.53–0.84; p = 0.030) for men (Fig. 4). According to the combination of maximum sensitivity and specificity calculated with the ROC curve, the optimal cut-off value for L/GB was 26.2 units for women and 9.5 units for men (Fig. 4). Using this optimal L/GB cut-off value, the positive (PPV) and negative predictive values (NPV) were approximately 70 % in women. For men, the optimal L/GB cut-off value was associated with a PPV and NPV of 50 and 90 %, respectively. To further validate the usefulness of the L/G ratio as a novel marker of weight regain predisposition, the patients included in the initial sample of the current study were reclassified. In this analysis, 70 % of women and 60 % of men were correctly classified (efficiency) by the L/GB ratio (Fig. 4).
Receiver operating characteristic (ROC) curves for the circulating leptin/ghrelin ratio before (L/GB) calorie restriction treatment to discriminate between regainer and non-regainer women and men. The arrows indicate the location of the selected cut-off point with the best sensitivity and specificity. The data of sensitivity (S), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and the proportion of patients correctly classified (efficiency) are shown
The circulating L/G8w also showed the capacity to discriminate between regainers and non-regainers in women (AUC = 0.73 (95 % CI 0.58–0.89); p = 0.012) and men (AUC = 0.77 (95 % CI 0.63–0.91); p = 0.002). However, although the AUC for L/G8w was slightly higher than the L/GB, this difference was not statistically significant in women (z = 0.353; p > 0.05) or men (z = 0.792; p > 0.05).
Discussion
This study demonstrates the clinical value of the circulating leptin/ghrelin ratio as a novel biological marker to discriminate between overweight/obese patients with a high predisposition to significantly regain weight after an energy restriction treatment and patients able to maintain the lost weight. Thus, a leptin/ghrelin ratio value before treatment higher than 26.2 units for women and 9.5 units for men was able to detect the majority of patients more likely to regain the lost weight. Thus, the leptin/ghrelin ratio appears to be a useful, non-invasive tool to avoid the efforts of both patients and health professionals in following a hypocaloric diet in those patients with a high probability of regaining weight.
Identifying robust biological markers to guide appropriate therapy and thereby improve clinical outcomes is an important task [29]. Classically, markers have been used as a clinical tool to facilitate the diagnosis, classification, prognostication and therapy of several diseases [30–32]. In obesity therapy, the prevention of weight regain after a nutritional intervention to induce weight loss is the most clinically relevant issue [1]. Observations of regainers and non-regainers in clinical studies focused on weight loss therapies are common. This important weight loss variability among individuals is due to several factors, including metabolic adaptations [33] and genetic predisposition [17, 34]. In this regard, several markers have been suggested as predictors of weight regain, including nutrigenomic/nutrigenetic [17, 18, 20] or proteomic [19] approaches. However, as far as we know, the current study shows for the first time the clinical usefulness of a marker to detect, at an early time, patients who are more likely to regain the diet-induced weight loss.
The most basic requirement for a novel risk marker is that the association between the marker and the outcome of interest be statistically significant when tested as a predictor of future events [29]. In fact, it was previously described that high leptin and low ghrelin levels were associated with weight loss regain [21]. These results are counterintuitive because ghrelin is a potent orexigenic signal and leptin is a satiety signal, suggesting a disruption in the sensitivity to these hormone signals, probably in the central nervous system of those subjects with a higher predisposition to regain body weight [21] which even could be determined by an epigenetic regulation [35]. Interestingly, this association was strengthened in the present study when the L/G ratio was evaluated, which was found to be associated with the percentage of weight regain. Those patients who regained the lost weight showed an L/G ratio approximately twofold higher than the weight maintenance group in both women and men. Reinforcing these results, this difference between regainers and non-regainers was conserved when the L/G ratio was evaluated with leptin and ghrelin on a molar basis.
The inverse, i.e., the G/L ratio was also evaluated, but the differences between regainers and non-regainers were not statistically significant (data not shown), and therefore the G/L was not employed. Since leptin levels are increased with increased body weight, it could be hypothesized that the lack of statistical significance in G/L ratio contrary to the L/G could suggests that the leptin resistance could be the main compound of the weight regain predisposition rather than the compensatory low levels of ghrelin observed in a state of excess body weight.
Based on these results, the diagnostic accuracy of the L/GB ratio for the detection of regain in women and men was demonstrated. Moreover, the leptin/ghrelin ratio after 8 weeks of energy restriction treatment (L/G8w) showed a similar ability to detect failure in weight loss maintenance. Leptin and ghrelin levels were also different between the two weight maintenance groups, but the diagnostic accuracy of these hormones evaluated separately showed no individual power to differentiate between both groups (data not shown).
Since the laboratory test provided good discrimination, the next step was to determine a cut-off point for clinical use [36]. In the current study, the optimal cut-off point, defined as the level with a maximum combination of sensitivity and specificity, appears to have an adequate efficiency to discriminate regainers from non-regainers, classifying more than 60 % of patients correctly [27].
Taken together, the calculation of the L/G ratio at baseline could allow health professionals to identify whether an obese patient should initially be treated with a conventional dietary program or with a personalized treatment, such as a special dietary program with the capacity to improve weight loss maintenance [37]. Moreover, this ratio could even help in making a decision on the advisability of bariatric surgery in obese patients with a detected resistance to maintain the weight loss after an energy restriction treatment.
A limitation of the current study could be the limited number of patients, which may affect the accuracy of the L/G ratio as a clinically relevant biomarker of weight regain predisposition. However, the results of this study add important information because it is common to find a low discriminative ability between case and control patients for a common heterogeneous disease, such as obesity [38], metabolic syndrome [39] or cancer [32]. Moreover, the current study is in the exploratory phase (Phase I), in which a new test is first evaluated in a clinical setting to determine whether the test has any ability to discriminate between disease and non-disease patients [25]. In this type of trial, the sample size estimation is based on an AUC >0.50, and at least ten disease patients and ten controls should be included in the study [25].
In conclusion, these results show for the first time that the determination of the fasting leptin/ghrelin ratio before beginning a weight loss therapy program could be a useful non-invasive approach to personalize obesity therapy. A baseline leptin/ghrelin ratio higher than 9.5 units for men and 26.2 units for women identified most of the patients who regained more weight after a successful initial weight loss. Further studies are needed to validate the leptin/ghrelin ratio as a useful marker of weight regain.
References
Maclean PS, Bergouignan A, Cornier MA, Jackman MR (2011) Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 301:R581–R600
ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K (2011) Preventing weight gain by lifestyle intervention in a general practice setting: three-year results of a randomized controlled trial. Arch Intern Med 171:306–313
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497
Gutierrez-Fisac JL, Guallar-Castillon P, Leon-Munoz LM, Graciani A, Banegas JR, Rodriguez-Artalejo F (2012) Prevalence of general and abdominal obesity in the adult population of Spain, 2008–2010: the ENRICA study. Obes Rev 13:388–392
Abete I, Parra MD, Zulet MA, Martinez JA (2006) Different dietary strategies for weight loss in obesity: role of energy and macronutrient content. Nutr Res Rev 19:5–17
Fernandez JM, Rosado-Alvarez D, Da Silva-Grigoletto ME et al (2012) Moderate-to-high intensity training and hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with metabolic syndrome. Clin Sci (Lond) 123:361–373
Astrup A, Rossner S, Van Gaal L et al (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374:1606–1616
Sumithran P, Prendergast LA, Delbridge E et al (2011) Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 365:1597–1604
Schur EA, Cummings DE, Callahan HS, Foster-Schubert KE (2008) Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiol Behav 93:706–712
Levin BE (2004) The drive to regain is mainly in the brain. Am J Physiol Regul Integr Comp Physiol 287:R1297–R1300
Seoane LM, Al-Massadi O, Caminos JE, Tovar SA, Dieguez C, Casanueva FF (2007) Sensory stimuli directly acting at the central nervous system regulate gastric ghrelin secretion. An ex vivo organ culture study. Endocrinology 148:3998–4006
Cummings DE (2006) Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89:71–84
Enomoto M, Nagaya N, Uematsu M et al (2003) Cardiovascular and hormonal effects of subcutaneous administration of ghrelin, a novel growth hormone-releasing peptide, in healthy humans. Clin Sci (Lond) 105:431–435
Crujeiras AB, Casanueva FF (2012) Decreased ghrelin levels: the cause of obesity and weight regain? Expert Rev Endocrinol Metab 7:127–129
Davis JF, Choi DL, Benoit SC (2010) Insulin, leptin and reward. Trends Endocrinol Metab 21:68–74
Gil-Campos M, Aguilera CM, Canete R, Gil A (2006) Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr 96:201–226
Goyenechea E, Parra D, Crujeiras AB, Abete I, Martinez JA (2009) A nutrigenomic inflammation-related PBMC-based approach to predict the weight-loss regain in obese subjects. Ann Nutr Metab 54:43–51
Mutch DM, Pers TH, Temanni MR et al (2011) A distinct adipose tissue gene expression response to caloric restriction predicts 6-mo weight maintenance in obese subjects. Am J Clin Nutr 94:1399–1409
Wang P, Holst C, Andersen MR et al (2011) Blood profile of proteins and steroid hormones predicts weight change after weight loss with interactions of dietary protein level and glycemic index. PLoS ONE 6:e16773
Goyenechea E, Crujeiras AB, Abete I, Martinez JA (2009) Expression of two inflammation-related genes (RIPK3 and RNF216) in mononuclear cells is associated with weight-loss regain in obese subjects. J Nutrigenet Nutrigenomics 2:78–84
Crujeiras AB, Goyenechea E, Abete I et al (2010) Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab 95:5037–5044
Strohacker K, McCaffery JM, Maclean PS, Wing RR (2013) Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes (Lond). doi:10.1038/ijo.2013.118
Crujeiras AB, Parra D, Goyenechea E, Abete I, Martinez JA (2009) Tachyphylaxis effects on postprandial oxidative stress and mitochondrial-related gene expression in overweight subjects after a period of energy restriction. Eur J Nutr 48:341–347
Mera R, Thompson H, Prasad C (1998) How to calculate sample size for an experiment: a case-based description. Nutr Neurosci 1:87–91
Obuchowski NA, Lieber ML, Wians FH Jr (2004) ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem 50:1118–1125
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Elmi A, Hedgire SS, Covarrubias D, Abtahi SM, Hahn PF, Harisinghani M (2013) Apparent diffusion coefficient as a non-invasive predictor of treatment response and recurrence in locally advanced rectal cancer. Clin Radiol 68:e524–e531
Bruns DE, Huth EJ, Magid E, Young DS (2000) Toward a checklist for reporting of studies of diagnostic accuracy of medical tests. Clin Chem 46:893–895
Hlatky MA, Greenland P, Arnett DK et al (2009) Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119:2408–2416
Diaz-Lagares A, Alegre E, Arroyo A et al (2011) Evaluation of multiple serum markers in advanced melanoma. Tumour Biol 32:1155–1161
Popovic V, Leal A, Micic D et al (2000) GH-releasing hormone and GH-releasing peptide-6 for diagnostic testing in GH-deficient adults. Lancet 356:1137–1142
Sturgeon CM, Duffy MJ, Hofmann BR et al (2010) National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem 56:e1–e48
MacLean PS, Higgins JA, Jackman MR et al (2006) Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290:R1577–R1588
Campion J, Milagro FI, Goyenechea E, Martinez JA (2009) TNF-alpha promoter methylation as a predictive biomarker for weight-loss response. Obesity (Silver Spring) 17:1293–1297
Crujeiras AB, Campion J, Diaz-Lagares A et al (2013) Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept 186C:1–6
Altman DG, Bland JM (1994) Diagnostic tests. 1: Sensitivity and specificity. BMJ 308:1552
Larsen TM, Dalskov SM, van Baak M et al (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363:2102–2113
Sandholt CH, Sparso T, Grarup N et al (2010) Combined analyses of 20 common obesity susceptibility variants. Diabetes 59:1667–1673
Sierra-Johnson J, Romero-Corral A, Somers VK et al (2009) IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci (Lond) 116:507–512
Acknowledgments
The authors are grateful to the volunteers of the study. They also wish to thank their physician Blanca E Martínez de Morentin, their nurse Salomé Pérez, their dietician María Hernandez and their technicians Verónica Ciaurriz and Ana Lorente for excellent clinical and technical assistance, respectively. They received no additional compensation for this contribution. This work was supported by Linea Especial about Nutrition, Obesity and Health (University of Navarra LE/97), CIBERobn and RETICS/PREDIMED, ISCIII Initiatives. AB Crujeiras, is funded by the ISCIII through a postdoctoral research contract “Sara Borrell” (CD09/00365). A Díaz-Lagares was supported by the Angeles Alvariño-2009 postdoctoral fellowship from the Xunta de Galicia and European Social Fund (ESF) and at present is the recipient of a “Sara Borrell” postdoctoral contract (CD12/00738) from the ISCIII at the Spanish Ministry of Economy and Competitiveness.
Conflict of interest
The authors A. B. Crujeiras, A. Díaz-Lagares, I. Abete, E. Goyenechea, M. Amil, J. A. Martínez, and F. F. Casanueva declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. B. Crujeiras and A. Díaz-Lagares have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Crujeiras, A.B., Díaz-Lagares, A., Abete, I. et al. Pre-treatment circulating leptin/ghrelin ratio as a non-invasive marker to identify patients likely to regain the lost weight after an energy restriction treatment. J Endocrinol Invest 37, 119–126 (2014). https://doi.org/10.1007/s40618-013-0004-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-013-0004-2