Abstract
Background
Postprandially induced oxidative stress can cause damage to mitochondrial components and initiate cellular degradative processes; which are related to obesity comorbidities.
Aim of the study
This trial sought to determine whether weight loss induced by caloric restriction provides antioxidant protection to reduce the postprandial response of mitochondrial function and oxidative stress markers.
Methods
A group of overweight/obese volunteers (n = 17; 39 ±7 years, 32.5 ± 4.8 kg/m2) followed an 8-week hypocaloric diet. Volunteers provided blood samples at fasting and 2-h after a test drink (CHO: 95% E, PROT: 5% E and containing antioxidants) and these were examined for postprandial oxidative stress responses, before and after the nutritional intervention. The expression of four mitochondrial-related genes, COX15, NDUFS2, MGST2 and TNF-alfa, was measured in peripheral blood mononuclear cells (PBMC) by quantitative RT-PCR. Lipid peroxidation and nitrosative stress biomarkers, total antioxidant capacity (AOP), uric acid and glutathione peroxidase were also determined.
Results
Before nutritional treatment, the test drink induced a postprandial increase in lipid peroxidation and nitrosative stress biomarkers with a concomitant increase in the AOP. The increase in postprandial oxidative stress biomarkers was accompanied by a decrease in PBMC COX15 mRNA levels. Interestingly, after the weight loss period (−5.8 ± 2.3%), the postprandial-induced changes were lower than at the beginning of the study and involved oxidative stress biomarkers and the COX15 and MGST2 transcripts. This finding suggests the occurrence of a tachyphylactic process.
Conclusions
We demonstrate for the first time that the well-known effect of energy restriction on oxidative stress is accompanied by a tolerance mechanism on the postprandial oxidative stress response and mitochondrial function-related genes. Indeed, the COX15 and MGST2 gene expression assays in PBMC emerged as valuable nutrigenomic biomarkers of the oxidative response under energy-restriction conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a complex multifactorial disease, in which genetic and environmental-related factors are involved [21]. Among the causative agents, inadequate eating habits in genetically predisposed subjects are an important contributor to the development of obesity, such habits have been repeatedly linked to a number of metabolic impairments and associated with oxidative stress disturbances such as atherosclerosis and insulin resistance [13].
In this context, defective cellular energy metabolism has been suggested as a relevant cause of obesity [22, 33]. This impairment appears to be originated, in some cases, by increased levels of free radicals, which cause damage to mitochondrial components and initiate cellular degradative processes [6].
Chronic hypernutrition occurring in obesity could be a key cause of the increase in the risk of oxidative damage, which could trigger mitochondrial dysfunction [34]. Indeed, a recent study demonstrates that mitochondrial dysfunction is a complication of hyperglycemia- and hyperlipidemia-induced reactive oxygen species (ROS) production in skeletal muscle, which induces a decrease in cytochrome c oxidase (COX)-related genes mRNA levels [5].
Reducing these undesirable oxidative processes could be a part of the strategy for the prevention of diseases such as obesity and associated comorbidities [17]. Thus, supplementation with antioxidants may reduce some risks associated with such postprandial effects [29, 35]. Moreover, caloric restriction has been shown to be involved in slowing the progression of a variety of diseases [12], likely acting by decreasing oxidative stress [8].
Therefore, the aim of this study was to investigate, apparently for the first time, if weight loss induced by caloric restriction could specifically influence postprandial free radical production in healthy obese subjects by measuring specific markers of oxidative stress as well as the gene expression of selected proteins such as COX15, NDUFS2, MGST2 and TNFAIP8L1 involved in mitochondrial-related oxidative processes.
Materials and methods
Subjects
Caucasian overweight/obese subjects with a mean age of 39 ± 7 years and a mean body mass index (BMI) of 32.5 ± 4.8 kg/m2, were recruited through local advertisements. The participants in this translational research (n = 17; 10 men and 7 women) were in apparent good health as determined by medical history, physical examination, and routine laboratory tests. They reported neither consumption of supplemental vitamin or minerals nor regular prescription of medications during the previous 3 months, and changes in body weight were less than ±3 kg during this time. A written informed acceptance to participate in the trial was obtained before the start of the study. This consent, as well as the study protocol, was previously approved by the Ethical Committee of the University Clinic of Navarra (Ref. 54/2006), in agreement with the Helsinki Declaration.
Study design
The trial was a controlled nutritional intervention supervised by trained dieticians from the Department of Nutrition and Food Sciences, Physiology and Toxicology of the University of Navarra. The acute experimental protocol was performed at rest after an overnight fast. Obese volunteers were given a test drink (200 mL) in the form of commercial orange juice (Kasfruit, Vitoria, Spain) supplying 84 kcal with 95% energy from carbohydrates and 5% energy from proteins of which 90% corresponded to leucine. The test drink was selected based on the carbohydrate and protein properties to enhance ROS production [27, 28, 32].
The day after the acute intake experiment, the subjects began the 8-week energy-restricted dietary treatment to lose weight. After the energy-restricted intervention treatment to lower weight, the experimental protocol was repeated, so the volunteers consumed a test drink with the same composition as at the beginning. Before and after the nutritional intervention, selected anthropometric measurements were made.
Blood samples were drawn at fasting and 2 h after the test drink intake to determine changes in postprandial oxidative stress before and after the weight loss process. The EDTA-plasma and serum of volunteers were separated from whole blood, peripheral blood mononuclear cells (PBMC) were extracted as described below and the samples were immediately frozen at −80°C until assay.
Weight loss intervention protocol
Weight loss was induced by means of a hypocaloric diet devised to produce a 30% caloric restriction with respect to the subject’s baseline energy expenditure, which was individually measured by indirect calorimetry (Deltratac, Datex Ohmeda, Finland), following conventional protocols described elsewhere [18] and adjusting for the physical activity level according to WHO criteria [30]. The macronutrient distribution of the hypocaloric diet was 18% energy as proteins, 52% energy as carbohydrates and 30% energy as lipids and was prescribed according to a food exchange system, in which the menu plans were individually designed as described elsewhere [1]. Dietary compliance was assessed through 3-day weighted food records [1, 7, 11], calculating the energy and nutrient intake by using Medisystem software (Sanocare Human Systems LS, Spain) based on recognized Spanish food composition tables.
Biochemical parameters assessment
Plasma levels of glucose, total cholesterol and triacylglycerol were measured by specific colorimetric assays (Horiba ABX Diagnostics, Montpellier, France) using an automatized system (COBAS MIRA, Roche, Basel, Switzerland), while circulating insulin was determined by using a commercially available ELISA kit (Mercodia, AB, Uppsala, Sweden). The HOMA index (HOMA-IR) was calculated according to Mathews et al. [24] in order to assess insulin resistance.
Measurement of oxidative stress markers
Analyses of antioxidant status in blood
Total antioxidant capacity (AOP) and malondialdehyde (MDA) were evaluated in serum by colorimetric assay kits (OXIS International, OR, USA). Uric acid (UA) was measured by a specific colorimetric assay (Horiba ABX Diagnostics) using an automatized system (COBAS MIRA). Plasma levels of circulating oxidized LDL (ox-LDL) were assessed using an ELISA kit (Mercodia, AB). Plasma nitrotyrosine (NT) and glutathione peroxidase (GPx) values were determined by commercially available ELISA kits, respectively (HyCult Biotechnology b.v., Uden, The Netherlands and OXIS International). Nitric oxide (NO) levels were evaluated with a nitrate/nitrite colorimetric assay kit (Oxford Biomedical Research, MI, USA). Absorbance was spectrophotometrically read at appropriate wavelengths using the Multiskan Spectrum (Thermo Electron Corporation, Vantaa, Finland).
Gene expression assessment
The gene expression quantification was performed as previously described [9]. PBMC were isolated by differential centrifugation using Polymorphprep medium (Axis Shield PoC AS, Oslo, Norway). The extraction of total RNA from the PBMC was based on the Trizol reagent method according to the manufacturer’s instructions (Invitrogen, CA, USA). Quantitative real-time PCR was performed using an ABI PRISM 7000 HT Sequence Detection System as described by the provider (Applied Biosystems, CA, USA).
The genes to be explored were selected from microarray analyses on PBMC after the caloric restriction carried out in our laboratory [10] due to their putative relationships with free radicals production and modulation.
Statistical analysis
The sample size for this translational investigation was calculated by the equation published by Mera et al. [25], taking into account reported values for the MDA standard deviation [3]. This condition required seven subjects as the minimum sample size of participants that completed the nutritional intervention. The sample size recruited in this study was twenty-three subjects; seventeen obese volunteers (10 men, 7 women) completed both baseline and after weight loss testing and were included in the analyses. Gene expression parameters were analyzed in 14 volunteers due to insufficient blood sampling. Statistical analysis was initially performed in men and women, separately. Then, given that no differences were found, the data were pooled.
The normal distribution was explored through the Kolmogorov-Smirnov and the Shapiro Wilk tests. The paired student test was applied to detect differences before and after the intake and before and after the hypocaloric treatment to evaluate global nutritional status variables. Since oxidative stress markers, including gene expression were not normally distributed, the Wilcoxon signed ranks test was applied. Sequential comparisons of nitrotyrosine levels were made with log-transformed values [4]. Postprandial changes from fasting were calculated as the difference between 2 h after the intake and fasting measures to determine whether the postprandial measurements at baseline and at endpoint of the nutritional intervention were different. Sequential comparisons of postprandial changes in the oxidative stress biomarkers before and after nutritional treatment were performed with data normalized to a baseline of 100% and endpoint values expressed as percentage of baseline because these biomarkers changes varied markedly from one person to another. Untransformed data on circulating oxidative stress markers are reported as the mean ± standard deviation. The fold change in gene expression was calculated using the 2−ΔΔCt relative quantitation method [20, 26] according to the manufacturer’s guidelines (Applied Biosystems) reporting data as the mean (SEM). The Spearman coefficient was used to evaluate the potential association between gene expression and AOP. A P value ≤ 0.05 was considered statistically significant and a p value ≤ 0.1 as a trend for significance [36]. Statistical analysis was performed by SPSS 15.0 software (SPSS Inc, IL, USA) for Windows XP (Microsoft, WA, USA).
Results
Biochemical and oxidative stress changes
The 8-week caloric restriction (CR) was associated with a reduction of −5.8 ± 2.3% (−5.46 ± 2.08% for men vs. −6.43 ± 2.64% women; p = 0.475) in body weight, a decrease in BMI and other variables related to nutritional status (Table 1). As expected, these responses were accompanied by a decrease in total plasma cholesterol and insulin fasting levels (Table 2), although no relevant changes in plasma glucose and triacylglycerol concentrations as compared with baseline were detected (Table 2). When the postprandial changes were evaluated after CR, differences in such biochemical variables were not found (Table 2).
As for the oxidative stress markers after the nutritional treatment, MDA and ox-LDL fasting plasma levels fell with no statistical changes in NT, when compared with baseline point (Table 3). Values of NO and AOP significantly increased with no changes in UA, while GPx protein levels fell significantly (Table 3). At the beginning of the intervention study, the acute intake of the test drink induced increases of different degree in circulating MDA (p = 0.044), ox-LDL (p = 0.287), and NT (p = 0.022) when compared with the fasting state (Table 3). Also, an increase in NO (p = 0.019) and AOP (p = 0.001), and a trend for decrease in uric acid (p = 0.068) with no changes in GPx protein levels (p = 0.382) were observed (Table 3). After the caloric restriction period, only the postprandial increase in MDA was maintained. Interestingly, the postprandial changes from fasting after CR in MDA (−2 ± 25%), NT (−52 ± 104%) and NO (−14 ± 19%) were statistically lower than those before CR, while no statistically differences were found in ox-LDL (0.62 ± 29%), AOP (−15 ± 41%), UA (−2 ± 21%) and GPx (34 ± 297%) after treatment (Table 3).
Oxidative stress-related gene expression changes
After a caloric restriction period to lose weight, no relevant changes were found in fasting mRNA levels of COX15, MGST2, TNFAIP8L1, while, the NDUFS2 transcripts at fasting slightly increased with a trend for the significance (fold change 2.92(0.30); p = 0.096) after dieting.
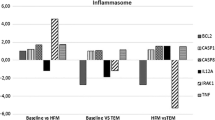
Interestingly, at the beginning, the test drink intake induced a significant decrease in COX15 mRNA levels (Fig. 1a), with no statistical changes in MGST2 (Fig. 1b), when compared with fasting state, while TNFAIP8L1 tended to diminish (Fig. 1c), and NDUFS2 showed no marked changes (Fig. 1d). These postprandial changes in the COX15 transcripts were not observed after the caloric restriction period (Fig. 1a), while MGST2 mRNA levels increased dramatically (Fig. 1b).
Fold change of each subject with regard to their fasting mRNA levels (2−ΔΔCt) in PBMC (mean and SEM as range; n = 14) concerning (1A) COX15 (Cytochrome c oxidase assembly protein 15), (1B) MGST2 (microsomal glutathione S-transferase), (1C) NDUFS2 (NADH-Coenzyme Q reductase) and (1D) TNFAIP8L1 (tumor necrosis factor alfa-related gene). &Statistical differences in the fold change induced by test drink in gene expression after the nutritional intervention as compared with the fold change 2 h after the intake at baseline
Indeed, the postprandial fold change in gene expression after the weight loss tended to be higher in COX15 (5.18(3.26); p = 0.084) and TNFAIP8L1 (2.16(0.45); p = 0.056). It was statistically higher in MGST2 (2.64(0.76); p = 0.010) than before the nutritional intervention. While no changes were found in NDUFS2 (3.05(1.06); p = 0.198). Moreover, the baseline COX15 and MGST2 mRNA levels were directly associated with baseline AOP circulating levels (Fig. 2).
Discussion
The relationship between an excess in body weight and oxidative stress could be partially explained by the overeating characteristically found in obesity, since that inflammatory and postprandial oxidative stress is greater and more prolonged in obese than in normal subjects. As a result, the reduction of undesirable postprandial oxidative stress processes could form part of the strategy for prevention of diseases such as obesity and its associated comorbidities [31].
In the current study, weight loss decreased lipid peroxidation and nitrosative stress markers and raised the antioxidant defence, as has been found in previous studies [7, 11, 14, 15]. In consequence, the postprandial changes from fasting in the studied markers, in both circulating levels and gene expression in PBMC, after the weight loss period were less marked than those at the beginning of the study. This suggests the existence of a tachyphylactic effect on the antioxidant defences after the weight loss treatment, which attenuates the postprandial oxidative response. In other words, some kind of tolerance or accommodation is accompanying the effects of a hypoenergetic diet on the oxidative stress response to an acute intake of a test drink containing antioxidants and a macronutrient mixture. Indeed, a major finding of this research was that the improvement in the antioxidant status induced by the energy restriction tended to tone down the postprandial reduction in COX15 gene expression induced by the nutrient intake, while a marked increase in the MGST2 postprandial gene expression was produced in relation to fasting. COX15 is an essential protein for the biogenesis of COX [16], which is an important marker of aerobic metabolism, whereas MGST2 is an important factor against oxidative injury [19]. Reinforcing the link of these genes with oxidative stress, COX15 and MGST2 gene expression was directly associated with AOP at baseline.
It has been suggested that the hyperglycemia- and hyperlipidemia-induced ROS generation mediated by the downregulation of pathways involved in the mitochondrial respiratory chain appears to be an initial key event triggering high-fat diet induced insulin resistance [5, 23]. Therefore, this downregulation of mitochondrial function appears to be prevented by caloric restriction devised to lose weight, while a postprandial up-regulation of an antioxidant enzyme-related gene is induced.
Taking these data together, this study showed that in obese subjects a hypocaloric diet to lose weight per se seems to be an effective strategy to minimize the oxidative processes related to obesity and its comorbidities. Thus, the novelty of this research relies on the fact that a process of tachyphylaxis concerning oxidative stress is occurring after an energy-restricted regime has been followed. Moreover, COX15 and MGST2 gene expression in PBMC could constitute novel markers for the application of nutrigenomic studies to personalize dietary treatment in obesity [2, 10].
Abbreviations
- AOP:
-
Serum total antioxidant capacity
- BMI:
-
Body mass index (kg/m2)
- COX15:
-
Cytochrome c oxidase assembly protein
- CR:
-
Caloric restriction
- GPx:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
- MGST2:
-
Microsomal glutathione S-transferase
- NDUFS2:
-
NADH-Coenzyme Q reductase
- NO:
-
Nitric oxide
- NT:
-
Nitrotyrosine
- Ox-LDL:
-
Oxidized-LDL
- PBMC:
-
Peripheral blood mononuclear cell
- TNFAIP8L1:
-
Tumor necrosis factor alfa-related gen
- UA:
-
Uric acid
- UV:
-
Ultraviolet visible
References
Abete I, Parra D, Martinez JA (2008) Energy-restricted diets based on a distinct food selection affecting the glycemic index induce different weight loss and oxidative response. Clin Nutr 27:545–551
Bergmann MM, Görman U, Mathers JC (2008) Bioethical considerations for human nutrigenomics. Annu Rev Nutr 28:447–467
Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L (2002) Factors associated with oxidative stress in human populations. Am J Epidemiol 156:274–285
Bo S, Gambino R, Guidi S, Silli B, Gentile L, Cassader M, Pagano GF (2005) Plasma nitrotyrosine levels, antioxidant vitamins and hyperglycaemia. Diabet Med 22:1185–1189
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Crujeiras AB, Parra D, Abete I, Martinez JA (2007) A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res 41:498–506
Crujeiras AB, Parra D, Goyenechea E, Abete I, González-Muniesa P, Martínez JA (2008) Energy restriction in obese subjects impact differently two mitochondrial function markers. J Physiol Biochem 64:211–220
Crujeiras AB, Parra D, Goyenechea E, Martínez JA (2008) Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest 38:672–678
Crujeiras AB, Parra D, Milagro FI, Goyenechea E, Larrarte E, Margareto J, Martinez JA (2008) Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: a nutrigenomics study. OMICS 12:251–261
Crujeiras AB, Parra MD, Rodriguez MC, Martinez de Morentin BE, Martinez JA (2006) A role for fruit content in energy-restricted diets in improving antioxidant status in obese women during weight loss. Nutrition 22:593–599
Chung HY, Sung B, Jung KJ, Zou Y, Yu BP (2006) The molecular inflammatory process in aging. Antioxid Redox Signal 8:572–581
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R (2005) Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448–1454
Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R (2001) The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86:355–362
Fenster CP, Darley-Usmar VM, Landar AL, Gower BA, Weinsier RL, Hunter GR, Patel RP (2004) Weight loss and race modulate nitric oxide metabolism in overweight women. Free Radic Biol Med 37:695–702
Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E (2000) Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic Biol Med 29:211–221
Kay CD, Holub BJ (2003) The postprandial effects of dietary antioxidants in humans. Curr Atheroscler Rep 5:452–458
Labayen I, Diez N, Parra D, Gonzalez A, Martinez JA (2004) Basal and postprandial substrate oxidation rates in obese women receiving two test meals with different protein content. Clin Nutr 23:571–578
Li X, Liu Z, Luo C, Jia H, Sun L, Hou B, Shen W, Packer L, Cotman CW, Liu J (2008) Lipoamide protects RPE cells from oxidative stress and mitochondrial dysfunction. Free Radic Biol Med 44:1465–1474
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Marti A, Martinez-Gonzalez MA, Martinez JA (2008) Interaction between genes and lifestyle factors on obesity. Proc Nutr Soc 67:1–8
Martinez JA (2006) Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J Physiol Biochem 62:303–306
Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S (2008) Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 57:1071–1077
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Mera RTH, Prasad C (1998) How to calculate sample size for an experiment: a case-based description. Nutr Neurosci 1:87–91
Milagro FI, Campion J, Martinez JA (2007) 11-beta Hydroxysteroid dehydrogenase type 2 expression in white adipose tissue is strongly correlated with adiposity. J Steroid Biochem Mol Biol 104:81–84
Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P (2002) Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr 75:767–772
Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P (2000) Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 85:2970–2973
Natella F, Ghiselli A, Guidi A, Ursini F, Scaccini C (2001) Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radic Biol Med 30:1036–1044
OMS-WHO (2000) Obesity: preventing and managing the global epidemic. Report of WHO consultation. Word Health Organ Tech Resp Ser 894:1–253
Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P (2007) Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 92:4476–4479
Perez de Obanos MP, Lopez-Zabalza MJ, Arriazu E, Modol T, Prieto J, Herraiz MT, Iraburu MJ (2007) Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim Biophys Acta 1773:1681–1688
Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E (2006) TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116:2791–2798
Vincent HK, Taylor AG (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 30:400–418
Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H (2004) Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med 37:411–421
Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM (2007) Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 85:385–391
Acknowledgments
Thanks are given to Linea Especial about Nutrition, Obesity and Health (University of Navarra LE/97) and IBERCAJA for financial support as well as to Friends of University of Navarra and CAIXANOVA that supports AB Crujeiras with a fellowship. We also wish to thank our physician Blanca E Martínez de Morentin, our nurse Salomé Pérez, our dietician María Hernandez and our technicians Ana Lorente and Verónica Ciaurriz, for excellent clinical and technical assistance. We also thank Paul Miller from the Foreign Language Institute at the University of Navarra for careful reading of the last version of this manuscript for English grammar and style.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crujeiras, A.B., Parra, D., Goyenechea, E. et al. Tachyphylaxis effects on postprandial oxidative stress and mitochondrial-related gene expression in overweight subjects after a period of energy restriction. Eur J Nutr 48, 341–347 (2009). https://doi.org/10.1007/s00394-009-0019-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0019-9