Abstract
Objectives
The need for a unified definition of weight loss (WL) after bariatric surgery has recently been highlighted. We aimed to evaluate the reliability of two clinically common WL indications including percentage of total WL (%TWL) and percentage of excess WL (%EWL) through comparing their performances in predicting metabolic syndrome (MetS) remission 1 year after bariatric surgery.
Methods
A total of 430 individuals with obesity who underwent bariatric surgery were enrolled. Participants were evaluated for changes in anthropometric parameters, metabolic indexes, MetS components and medications before and 1 year after surgery. MetS was defined using the criteria of the National Cholesterol Education Program Adult Treatment Panel III criteria for Asian-Americans.
Results
The prevalence of MetS is 92.3% (397) at baseline. One year after bariatric surgery, 337 individuals (84.9%) were in MetS remission. The multivariate adjusted ORs were 1.16 (95% confidence interval [CI] 1.10–1.22) for each 1% increase in %TWL for MetS remission and 1.18 (95% CI 1.11–1.25) for each 5% increase in %EWL. This association with MetS remission remained statistically significant for %TWL after additional adjustment for %EWL (P for trend 0.029), and disappeared for %EWL. Receiver operating curve (ROC) analyses showed that the %TWL was more predictive than the %EWL (AUC%TWL vs. AUC%EWL, 0.749 vs. 0.700, p = 0.023). The Youden index indicated that the optimal %TWL cutoff point to identify MetS remission was 25%.
Conclusions
We recommend that good responders to bariatric surgery should be defined as those exhibiting %TWL ≥ 25%.
Similar content being viewed by others
Introduction
Over the past decades, increase in prevalence of obesity has become a worldwide major health problem in adults. Behavioral and pharmacotherapeutic treatments for morbid obesity have met with limited long-term success. Bariatric surgery is currently the most effective treatment for substantial and sustained weight loss (WL) in the patients with morbid obesity, markedly improving obesity-related comorbidities [1]. The main goals of bariatric surgery include WL, quality of life improvement, comorbidity risk reduction, and remission in metabolic disease.
Although previous reports have proposed standard definitions for outcome reporting in bariatric surgery [2], there is no clear consensus on what metric should be used to define a good or poor response in relation to WL. In the search for clinically relevant outcome predictors, WL has been characterized using a number of different metrics, including the percentage of total WL (%TWL), the percentage of excess WL (%EWL), and the units body mass index (BMI) loss. In 1998, Oria introduced the arbitrary 50%EWL criterion for a “good” WL result [3]. In another study, patients with obesity from a non-bariatric surgery population with averaged “30% excess weight” exhibited markedly increased mortality [4]. Corcelles et al. suggested that a %TWL > 20% in the first year after surgery indicated a “good responder” [5], based on the evidence that anti-obesity therapy is associated with a reduction in cardiovascular risk factors after WL of 5–10% regardless of the type of the medication taken [6]. Formerly, the %EWL was the most commonly used outcome metric in the bariatric surgery related literature. It reports a percentage of WL relative to an “ideal” BMI, and numerous studies have arbitrarily defined suboptimal WL as %EWL less than 50%. However, recently published studies have suggested that %TWL yield a more accurately reflected outcome without a need to define an ideal body weight [7, 8]. Park et al. found that the WL outcomes expressed in %TWL did not differ statistically between the lower and higher baseline BMI groups, however %EWL showed significant variation with baseline BMI [9]. Van de Laar et al. found that metabolic outcome (T2DM) was no less successful for patients with <50%EWL than those with >50%EWL. The %TWL metric is the best and most commonly used alternative [10].
Nevertheless, all these above criteria or threshold are lack of any direct association with a useful clinical endpoint or support from that. Metabolic syndrome (MetS) usefully identifies patients at increased risk for cardiovascular disease (CVD). In our previous study, the prevalence of MetS in subjects with obesity was almost 90% [11]. Bariatric surgery induces considerable and persistent MetS remission, in parallel with WL. Thus, we propose that the extent of MetS remission might be a good “ruler” to measure which WL indicator is better.
The primary objective of this study was to apply our consecutive series of population with obesity and MetS who underwent bariatric surgery to evaluate the reliability of the %TWL and %EWL. In addition, we also sought to explore optimal cutoff points of these indications.
Materials and methods
Study population
A retrospective review of our institution’s prospective bariatric surgery registry was completed to identify all patients who underwent laparoscopic Roux-en-Y gastric bypass (LRYGB) or laparoscopic sleeve gastrectomy (LSG) from February 2012 through October 2018. Patients with less than 1 year of follow-up or incomplete data or revisional surgery were excluded. Medical history, age, gender, height, weight, BMI, waist circumference, blood pressure (BP), and current medications were recorded before and after surgery. Glucose, HbA1c, and other biochemical items were measured pre- and 12 M post-operatively.
Informed consent was obtained from all participants before the start of the study and the Ethics Committee of our institution approved the study, in accordance with the World Medical Association’s Declaration of Helsinki.
The minimal number of patients is 312 to be included to draw significant conclusions under the conditions of α = 0.05 and power = 80%.
Definitions of MetS and MetS remission
The MetS was defined according to the criteria established by updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian-Americans [12], by the presence of three or more of the following abnormalities: (1) Central obesity (waist circumference ≥90 cm for male and ≥80 cm for female); (2) Elevated triglycerides (TG) (≥1.7 mmol/L), or specific treatment for this lipid abnormality; (3) Reduced high-density lipoprotein cholesterol (HDL-c) (<1.03 mmol/L for male or <1.30 mmol/L for female), or specific treatment for this lipid abnormality; (4) Elevated BP (≥130/85 mmHg or current treatment for hypertension), or previously diagnosed hypertension; and (5) Elevated plasma glucose (FPG ≥ 5.6 mmol/L), or previously diagnosed diabetes mellitus (DM).
We defined patients as having MetS remission if they no longer fulfilled criteria for MetS at the most recent evaluation. MetS non-remission consisted of patients who still had ≥3 criteria of MetS at follow-up [13].
Calculation of percent excess WL and percent total WL
%EWL = [(Initial weight) − (Postoperative weight)]/[(Initial weight) − (Ideal weight)] (in which ideal weight is defined by the weight corresponding to a BMI of 24 kg/m2). This diagnostic standard of BMI 24 kg/m2 is used to define overweight adults in China [14].
Laboratory measurements
Plasma glucose concentrations were measured using the glucose oxidase method. HbA1c levels were determined by high-performance liquid chromatography (Bio-Rad Inc., Hercules, CA, USA). Lipid profiles included serum total cholesterol (TC), TG, HDL-c, and low-density lipoprotein cholesterol (LDL-c), assayed with a 7600–120 Hitachi automatic analyzer (Hitachi, Tokyo, Japan). TC and TG were measured by enzyme assay (Roche Diagnostics GmbH, Mannheim, Germany). HDL-c and LDL-c concentrations were measured via the direct assay method (Sekisui Medical Co. Ltd., Tokyo, Japan).
Statistical analysis
Continuous demographic, clinical, and laboratory variables are expressed as mean ± SD, while categorical variables are expressed as number and percent (%). Differences in continuous variables between groups were assessed using ANOVA. The chi-square method or Fisher’s exact test was used to test the significance of differences between proportions and categorical variables.
Binary logistic regression analysis was used to estimate odds ratios (ORs) for remission of MetS according to different levels of the %TWL and %EWL. The %TWL 1 year after bariatric surgery was evaluated in the following 2 ways: (1) as categories (≤21.9% [First third, reference group], 21.9–28.6% [Middle third], and >28.6% [Last third]) and (2) as a continuous variable (Each 1% %TWL). %EWL 1 year after bariatric surgery was evaluated in the following two ways: (1) as categories (≤76.1% [First third, reference group], 76.1–103.0% [Middle third], and >103.0% [Last third]) and (2) as a continuous variable (Each 5% %EWL). %TWL and %EWL data were included in the models as a dummy variable, and the significance of the trend across categories of the %TWL and %EWL was tested in the same models by giving an ordinal numeric value for each dummy variable. All analyses were carried out in three models. Model 1 adjusted for age and gender; Model 2 adjusted for variables of age, gender, type of surgery, HbA1c and initial BMI; Model 3 additionally adjusted for the %EWL or %TWL 1 year after bariatric surgery, respectively.
The discriminative accuracy of the %EWL and %TWL indications was examined according to the area under the receiver operating characteristic curve (AUC), where a larger AUC indicates better discriminatory ability. In general, an AUC of 0.501–0.699 denotes poor discriminatory ability, 0.700–0.799 denotes acceptable discriminatory ability, 0.800–0.899 denotes to excellent discriminatory ability. The Delong, Delong Clarke-Pearson method is used to determine which curve is significantly better than the other. The optimal AUC thresholds and cutoff scores for each indicator, in terms of sensitivity and specificity, were determined using Youden’s index.
P values < 0.05 (two-tailed tests) were considered statistically significant in this study. All statistical analyses were conducted using IBM SPSS Statistics 18.0 (IBM Corp., Armonk, NY, USA) and MedCalc (version 18.9.1; MedCalc Software, Mariakerke, Belgium).
Results
There were 430 individuals with obesity involved in the present study. The baseline characteristics and metabolic alterations 1 year after bariatric surgery are listed in Table 1. Among those, 397 individuals with obesity have MetS at baseline. Therefore, the prevalence of MetS at baseline is 92.3%. One year after bariatric surgery, BMI, waist circumference, glucose levels, and lipid profiles improved greatly with significantly decreased use of medicines. 337 individuals (84.9%) having 2 or less MetS components, reached MetS remission among 397 individuals with MetS at baseline. However, it should be noted that MetS remission still has the potential to have 1 or 2 risk factors elevated. We have not abolished the elevation in CVD risk but have reduced it slightly—patients still have elevated risk, just not as high as previously. Data on the 397 subjects with MetS before surgery were further analyzed to investigate the association between %EWL and %TWL with MetS remission, respectively.
The clinical characteristics of MetS patients are listed in Table 2. Of the 397 subjects with MetS at baseline, 60 (15.1%) subjects did not attain MetS remission 1 year after bariatric surgery and were considered as the MetS non-remission group. Those who achieved remission underwent more SG surgeries than those who did not exhibit MetS remission. The preoperative SBP, TG, FPG, 2hPG, HbA1c were higher and HDL-c was lower in the non-remission group. Neither the BMI nor waist circumstance differed between these two groups at baseline, but the %TWL and %EWL were much higher in the remission group. The baseline prevalence of MetS components were similar in the two groups. However, the postoperative prevalence of five MetS components were higher in the non-remission group.
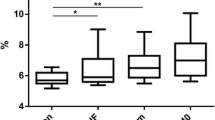
After adjusting for age, sex, surgery type, and the initial HbA1c level and BMI (Model 2), the ORs for MetS remission 1 year after surgery across three different categories were 1.0, 2.5, and 17.4 for the %TWL (p for trend <0.001) and 1.0, 4.4, and 10.1 for the %EWL (p for trend <0.001) (Table 3). When the %TWL and %EWL were considered as continuous variables, the multivariable-adjusted ORs for MetS remission were 1.16 (95% confidence interval [CI] 1.10–1.22) for each 1% increase in the %TWL and 1.18 (95% CI 1.11–1.25) for each 5% increase in the %EWL. This association remained significant for the %TWL after additional adjustment for the %EWL (p for trend 0.029) but disappeared for the %EWL after additional adjustment for the %TWL (p for trend 0.335) (Table 3). We drew receiver operator curves (ROCs), and calculated the areas under the curves (AUCs), to compare the performances of the %EWL and %TWL in terms of predicting MetS remission among the 397 subjects with MetS. The %TWL was better than the %EWL (AUC%TWL vs. AUC %EWL, 0.749 vs. 0.700, p = 0.023) (Fig. 1). The Youden index indicated that a %TWL cutoff of 25% optimally predicted MetS remission.
Receiver operating curve (ROC) analyses showed that the %TWL was more predictive than the %EWL (AUC%TWL vs. AUC%EWL, 0.749 vs. 0.700, p = 0.023). The Youden index indicated that the optimal %TWL cutoff point to identify MetS remission was 25%. %TWL percentage of total weight loss, %EWL percentage of excess weight loss, MetS metabolic syndrome, 12 M 12 months after bariatric surgery.
Discussion
Obesity is a major public health issue worldwide. Management of obesity aims to achieve WL without malnutrition, improvement of body composition and reduce the risk of complications (e.g. type 2 DM or MetS). WL has been reported in many ways to evaluate how a patient responds to bariatric surgery. The best measurement should facilitate accurate comparisons between patients with different weight and population characteristics. Unfortunately, there is no clear consensus as to what definitions (and cutoff values) constitute suboptimal WL after bariatric surgery. Some authors have used the %EWL, %TWL or even simply unit BMI loss.
In clinic, it is a difficult problem to compare the rationality of these parameters. Arnold van de Laar et al. analyzed relative and absolute outcome measurements in 168 female subjects who underwent RYGB and found that the %EWL were not suited for comparing different patients or non-randomized groups [1]. In support, other subsequent published studies have suggested that the TWL can yield a more accurate outcome without the need for defining ideal body weight. Hatoum et al. evaluated outcomes in 846 patients undergoing RYGB in an effort to define the WL measure least associated with the initial BMI. Regression analyses demonstrated that the initial BMI was not significantly associated with the %TWL at 1 year. On the contrary, the initial BMI was negatively strongly associated with the %EWL and positively associated with the BMI units lost at 1 year. It was concluded that the %TWL should be the metric of choice when reporting WL. This was least influenced by the initial BMI, which might be a confounding factor [7, 8]. Sczepaniak et al. assessed the %TWL and %EWL values of patients whose data were logged in the Bariatric Outcomes Longitudinal Database. It was assumed that the lesser the parameter’s variation coefficient (VC), the more reliable the measure is. The %TWL exhibited the lowest VC and therefore more accurately predicted WL after bariatric surgery [15]. Van de Laar et al. found that metabolic outcome (T2DM) was no less successful for patients with <50%EWL than those with >50%EWL. The %TWL metric is the best and most commonly used alternative [10]. However, their conclusions on preferring %TWL above %EWL are mainly based on mathematical evidence, instead of clinical outcomes. A convincing evaluation method or parameter should be linked to clinical endpoints.
MetS is closely associated with morbid obesity, increasing the risk of CVDs and related mortality. In our cohort, the prevalence of MetS was 92.3%, similar to that in a previous study [11]. One year after bariatric surgery, most (84.9%) patients with obesity achieve MetS remission among 397 patients who suffered MetS at baseline. Thus, marked WL reverse all of MetS components [16]. Although predictors for remission of MetS after surgery remain controversial, most studies have found that the extent of WL is associated with MetS remission [11, 17]. A meta-analysis by Buchwald [18] concluded that components of MetS increased markedly with higher BMI and T2DM remission and dyslipidemia (major components of MetS) improvement after RYGB were ultimately depended on WL. Therefore, we propose that MetS remission could be a good “mirror” to reflect whether the extent of WL after surgery is ideal, or at least adequate.
We compared how well the %TWL and %EWL predicted MetS remission after surgery. Regression analyses revealed that the %TWL was significantly associated with MetS remission, even after additional adjustment for the %EWL. On the contrary, the association between %EWL and MetS remission disappeared when the %TWL was co-entered into a multivariable model. Furthermore, ROC analyses revealed that the AUC of the %TWL was significantly greater than that of the %EWL, which demonstrated that the %TWL was more predictive. In line with our study, previous studies have suggested that the %TWL can yield a more accurate outcome without the need for defining ideal body weight [19]. Because the %EWL is based on a somewhat arbitrary target, or “ideal” weight, whether this is defined according to a BMI standard (e.g., a BMI of 25 kg/m2 in Caucasian, 24 kg/m2 in Asian). It is not intrinsically clear what the “right” target should be, and the choice of target can substantially skew the results, particularly if the initial BMI is relatively low. In addition, the %TWL is the standard of reporting WL in nonsurgical studies, and therefore the use of %TWL would facilitate comparison across studies of all types of obesity therapies [6, 20]. In addition, East and South Asians differ in terms of body fat distributions and adiposities even within their lower BMI ranges, rendering %EWL data rather inaccurate. Therefore, the %TWL may be a better and reliable measure than the %EWL, as revealed by most recent studies.
A threshold of 20% in TWL% to define “successful result” after bariatric surgery was firstly proposed by Corcelles in 2016 [5]. This definition was based on improvements in obesity-related comorbidities and cardiovascular risk factors after moderate WL (5–10%TWL) [6, 21]. Therefore, authors artificially presume a threshold of 20% of the TWL% as successful outcome. In our present study, based on the best Youden index, 25% was chosen the optimal cutoff points of the %TWL to identify MetS remission, which is similar to but more objective than the 20% threshold. Van de Laar et al. first proposed 25%TWL as a superior threshold, which aligns with our findings [22].
The limitations of our study include its single-center, retrospective design and a fairly homogenous population. Stratification by surgery type (LRYGB and LSG) were completed only for the multivariate regression model due to relatively limited sample size. Moreover, it is known that a subgroup of individuals with obesity with no typical cardiometabolic risk factors and are at lower risk of CVDs. These individuals are termed as “metabolically healthy obesity” (MHO). The prevalence of MHO ranges from 7 to 74% in both youth and adult populations, depending on the definition employed [23, 24]. We found that the MHO prevalence among our subjects was 7.7%, and such subjects were not involved in subsequent analyses. There is a lot of evidence that nadir WL after RYGB and SG is only reached after 18 months. In our cohort, the follow-up intervals were 6 months and yearly (Y) after surgery, and the maximum WL occurred at 1 year after surgery (Supplementary Table 1 and Fig. 1). It should also be noted that, while important, MetS is only one of the problems associated with severe obesity. Issues like chronic pain, sleep apnea, reflux, fertility, mental health, physical functioning, and/or quality of life also reflect relevant health outcomes. Finally, as Chinese subjects have lower BMIs than westerners, the suggested threshold of 25% %TWL should be verified in larger, multi-center databases of western population.
Patients on conservative drug treatments also gain remission from MetS-related factors. However, patients who chose pharmaceutical intervention alone generally have less severe obesity compared to those who choose metabolic surgery intervention. For patients with less severe obesity, a WL of 10–15% can significantly improve MetS, while patients with severe obesity may require a greater WL, such as the 25% TWL found in our study, to significantly improve MetS components and achieve MetS remission. Thus, our findings apply only to metabolic/bariatric surgical patients who are clinically defined as having severe obesity.
Furthermore, our surgical cohort follow-up study will continue and we anticipate sufficient follow-up time to observe endpoints such as cardiovascular and cerebrovascular events or death and to obtain more accurate indicators of WL. Long-term randomized controlled studies with large sample sizes are urgently required to identify effective metrics between metabolic remission and metabolic/bariatric surgery.
To the best of our knowledge, this is the first study to demonstrate that the %TWL better predicts MetS remission 1 year after bariatric surgery than does the %EWL. Therefore, %TWL is a suitable metric to assess WL across bariatric population. We propose that ≥25 %TWL be considered as an optimal metric to identify individuals that are good responders to bariatric surgery, which warrants further validation in a larger sample.
References
van de Laar A, de Caluwé L, Dillemans B. Relative outcome measures for bariatric surgery. Evidence against excess weight loss and excess body mass index loss from a series of laparoscopic Roux-en-Y gastric bypass patients. Obes Surg. 2011;21:763–7.
Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25:587–606.
Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS). Obes Surg. 1998;8:487–99.
Seltzer CC. Some re-evaluations of the build and blood pressure study, 1959 as related to ponderal index, somatotype and mortality. N Engl J Med. 1966;274:254–9.
Corcelles R, Boules M, Froylich D, Hag A, Daigle CR, Aminian A, et al. Total weight loss as the outcome measure of choice after Roux-en-Y gastric bypass. Obes Surg. 2016;26:1794–8.
Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, et al. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2012;7:e39062.
Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity. 2013;21:1519–25.
van de Laar A. Bariatric outcomes longitudinal database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obes Surg. 2012;22:1843–7.
Park JY, Kim YJ. Validation of the alterable weight loss metric in morbidly obese patients undergoing gastric bypass in Korea. Obes Surg. 2018;28:1704–10.
van de Laar AW, de Brauw LM, Meesters EW. Relationships between type 2 diabetes remission after gastric bypass and different weight loss metrics: arguments against excess weight loss in metabolic surgery. Surg Obes Relat Dis. 2016;12:274–82.
Yu H, Zhang L, Bao Y, Zhang P, Tu Y, Di J, et al. Metabolic syndrome after Roux-en-Y gastric bypass surgery in Chinese obese patients with type 2 diabetes. Obes Surg. 2016;26:2190–7.
Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, et al. Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care. 2006;29:1414–6.
Nora M, Guimarães M, Almeida R, Martins P, Gonçalves G, Santos M, et al. Excess body mass index loss predicts metabolic syndrome remission after gastric bypass. Diabetol Metab Syndr. 2014;6:1.
Zhou B. [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5–10.
Sczepaniak JP, Owens ML, Shukla H, Perlegos J, Garner W. Comparability of weight loss reporting after gastric bypass and sleeve gastrectomy using BOLD data 2008–2011. Obes Surg. 2015;25:788–95.
Shuai X, Tao K, Mori M, Kanda T. Bariatric surgery for metabolic syndrome in obesity. Metab Syndr Relat Disord. 2015;13:149–60.
Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Lopez-Jimenez F. Effect of bariatric surgery on the metabolic syndrome: a population-based, long-term controlled study. Mayo Clin Proc. 2008;83:897–907.
Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56.e5.
Karmali S, Birch DW, Sharma AM. Is it time to abandon excess weight loss in reporting surgical weight loss? Surg Obes Relat Dis. 2009;5:503–6.
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17:565–8.
Aminian A, Jamal M, Augustin T, Corcelles R, Kirwan JP, Schauer PR, et al. Failed surgical weight loss does not necessarily mean failed metabolic effects. Diabetes Technol Ther. 2015;17:682–4.
van de Laar AW, van Rijswijk AS, Kakar H, Bruin SC. Sensitivity and specificity of 50% excess weight loss (50%EWL) and Twelve other bariatric criteria for weight loss success. Obes Surg. 2018;28:2297–304.
Heinzle S, Ball GD, Kuk JL. Variations in the prevalence and predictors of prevalent metabolically healthy obesity in adolescents. Pediatr Obes. 2016;11:425–33.
Messier V, Karelis AD, Prud’homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity. 2010;18:911–7.
Acknowledgements
This study was supported by grants from National Key Research and Development Project of China (2016YFA0502003), National Natural Science Foundation of China (81670791) and Municipal Natural Science Foundation of Shanghai (17ZR1421200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tu, Y., Pan, Y., Han, J. et al. A total weight loss of 25% shows better predictivity in evaluating the efficiency of bariatric surgery. Int J Obes 45, 396–403 (2021). https://doi.org/10.1038/s41366-020-00690-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00690-5
- Springer Nature Limited
This article is cited by
-
Major dietary patterns and their associations with total weight loss and weight loss composition 2–4 years after sleeve gastrectomy
European Journal of Medical Research (2024)
-
Adherence to Dietary Behavior Recommendations Moderates the Effect Between Time Since Metabolic and Bariatric Surgery and Percentage Total Weight Loss
Obesity Surgery (2024)
-
Optimizing Hospital Performance Evaluation in Total Weight Loss Outcomes After Bariatric Surgery: A Retrospective Analysis to Guide Further Improvement in Dutch Hospitals
Obesity Surgery (2024)
-
The Impact of Longer Biliopancreatic Limb Length on Weight Loss and Comorbidity Improvement at 5 Years After Primary Roux-en-Y Gastric Bypass Surgery: A Population-Based Matched Cohort Study
Obesity Surgery (2024)
-
Preoperative Serum Cortisol Level Is Predictive of Weight Loss After Laparoscopic Sleeve Gastrectomy in Men with Severe Obesity but Not Women
Obesity Surgery (2023)