Abstract
Background
Little is known about frailty in Chinese nursing home residents.
Aims
(1) To evaluate the prevalence of frailty in nursing home residents according to the FI-Lab or FRAIL-NH; and (2) to compare the predictive validity of these two tools for mortality.
Methods
We conducted a prospective study in four nursing homes in China. Frailty was assessed using the fatigue, resistance, ambulation, illness, loss of weight, nutrition, and help with dressing questionnaire (FRAIL-NH) and frailty index based on common laboratory tests (FI-Lab), respectively. The survival status was collected via medical records or telephone interviews. Receiver-operating characteristic (ROC) curves were calculated to estimate the area under the ROC curves (AUCs) for FI-Lab and FRAIL-NH in relation to mortality. Cox proportional hazard models were applied to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality by FRAIL-NH and FI-Lab, separately.
Results
We included 329 participants. The FI-Lab score was significantly and strongly associated with the FRAIL-NH score (r = 0.799, p < 0.001). Frailty was defined as the FI-Lab score ≥ 0.3 or the FRAIL-NH score ≥ 6, and the prevalence of frailty was 56.2% and 58.7%, respectively. Seventy-three participants (22.7%) died during the 1-year follow-up. The FI-Lab (AUC 0.700, 95% CI 0.647–0.750) was slightly better than the FRAIL-NH (AUC 0.676, 95% CI 0.622–0.727) for predicting mortality (p = 0.025). After adjusted for age and gender, the increment of the FI-Lab score was associated with mortality (adjusted HR per 0.01 increment in score 1.07, 95% CI 1.05–1.09), the increment of the FRAIL-NH score was also associated with mortality (adjusted HR per 1 increment in score 1.28, 95% CI 1.19–1.46).
Conclusion
The FI-Lab and FRAIL-NH are valuable for predicting mortality in Chinese nursing home residents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty generally refers to a clinical condition of decreased reserve and resistance to stressors, causing increased vulnerability to adverse health outcomes, although there is currently no unique definition [1, 2]. It has been proven to be related to many adverse outcomes, such as poor quality of life, functional disability, falls, hospitalization, institutionalization, and even death [2,3,4,5,6]. Frailty is highly prevalent in elderly adults [7]. Therefore, a recent consensus emphasized the importance of using a brief and rapid screening for frailty in elderly adults aged 70 years and older [2].
Elderly nursing home residents are more prone to frailty than those living in communities [3]. A recent systematic review of nine studies indicated that the prevalence of frailty in nursing homes ranged from 19.0 to 75.6% [8], whereas another systematic review of 21 studies found that the prevalence of frailty in community-dwelling older adults 4.0–59.1% [7]. To manage elderly adults with frailty, the first step is to identify frailty accurately as early as possible in this specific population.
Currently, the diagnosis of frailty is mainly based on two concepts, namely the cumulative deficit model and the underlying phenotype model [9]. Frailty index (FI) is a classic diagnostic tool for frailty according to the cumulative deficit model [2, 10]. Recent reports suggest that frailty index based on common laboratory tests (FI-Lab) may serve as a diagnostic tool for frailty and is associated with mortality [11,12,13]. The FI-Lab has been validated in Canadian elderly adults living in long-term care facilities [14]. On the other hand, the fatigue, resistance, ambulation, illness, loss of weight, nutrition, and help with dressing questionnaire (FRAIL-NH), a novel frailty screening tool, has recently been developed for nursing home residents according to the phenotype model [15]. Current evidence suggests that the FRAIL-NH is also a valid tool for identifying frailty in nursing homes [16, 17].
However, both the FI-Lab and FRAIL-NH have not been validated in Chinese nursing home residents. Furthermore, whether these two tools have a comparable value for predicting mortality remains unclear. Therefore, we conducted a prospective study (1) to evaluate the prevalence of frailty in a study population of Chinese nursing home residents according to the FI-Lab and FRAIL-NH, respectively; and (2) to compare the predictive validity of these two tools for mortality in this population.
Methods
Study design and study population
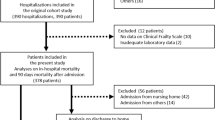
A prospective study was conducted in four nursing homes in Chengdu, China, from September 2016 to November 2017. Elderly adults aged 70 years or older who lived in these nursing homes were invited to participate in this study. Individuals with the following conditions were excluded: (1) living in nursing homes less than 2 weeks; (2) unable to communicate with interviewers; and (3) refusing to participate in this study. Each participant had a baseline investigation through a face-to-face interview, anthropometry, and a fasting blood sample. The Research Ethics Committee of Sichuan University approved the study protocol. A signed informed consent was obtained from each participant.
Frailty measures
According to the recent international consensus, we defined frailty in this study as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function” [2]. We evaluated frailty using the FRAIL-NH questionnaire and the FI-Lab, respectively. The study nurses completed the FRAIL-NH through face-to-face interviews. The FRAIL-NH consists of seven items (fatigue, transferring, mobility, incontinence, weight loss, nutrition, and dressing) [15]. The total score of FRAIL-NH ranged from 0 (best) to 14 (worst). The detailed information of FRAIL-NH is shown in Supplementary Table 1. Because the cutoffs of FRAIL-NH to define frailty remain uncertain, we applied the cutoff of ≥ 7 according to the original FRAIL-NH study [15] and the cutoff of ≥ 5 according to Luo’s study [18]. We also categorized the participants as nonfrail (0–1 score), frail (2–5 scores), and most frail (6–14 scores) according to Theou’s study [17]. We further tried to find out the optimal cutoff of the FRAIL-NH score to define frailty according to its capability to predict mortality in our study population. Moreover, we treated the FRAIL-NH score as a continuous variable.
According to the principle of selecting the individual parameters to construct the frailty index [19, 20], we created the 30-item FI-Lab from common blood tests including total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase, total serum protein, serum albumin, serum globulin, alkaline phosphatase, gamma-glutamyl transpeptidase, urea nitrogen, creatinine, cystatin C, uric acid, fasting glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, red blood cells, hemoglobin, hematocrit value, mean corpuscular volume, mean corpuscular hemoglobin, platelets, white blood cells, neutrophils, lymphocytes, monocytes, and eosinophils. The FI-Lab was constructed by scoring each item as 0 or 1. For each item, any value outside of the normal range is scored “1”, as a deficit, whereas “0” indicates the value was within the normal range. The parameters that constitute the FI-Lab and the relevant normal ranges are presented in Supplementary Table 2. The FI-Lab score was calculated using the sum of the number of deficits divided by the number of potential deficits evaluated. As a result, the FI-Lab score ranges from 0 to 1. For example, an individual with a deficit in eight variables of the 30-item FI-Lab would have an FI-Lab score of 0.27 (8 divided by 30). Because the cutoff of the FI-Lab score to define frailty remains uncertain, we categorized the participants into five groups as reported by previous studies [11, 21]: < 0.1, 0.1–0.2, 0.2–0.3, 0.3–0.4, and > 0.4. We also tried to find out an optimal cutoff of the FI-Lab score to define frailty according to its capability to predict mortality based on our study population. Furthermore, we treated the FI-Lab score as a continuous variable as reported by previous studies [11, 20].
Other measures
Trained study nurses also collected the following information from the medical records of the nursing homes: age, gender, education level, smoking status, alcohol drinking status, body mass index (BMI), and comorbidities (hypertension, ischemic heart disease, chronic heart failure, chronic obstructive pulmonary disease, diabetes, stroke, cancer of any type, osteoarthritis, Parkinson’s disease, cognitive impairment, and depression).
Mortality
The mortality data were collected from the medical records in the nursing homes by the study nurses at the 12th month after the baseline. For those who left the nursing homes during the follow-up, the survival status was collected via telephone interviews.
Statistical analysis
Data were analyzed using the SPSS version 20.0 (IBM Inc., Chicago, IL, USA) and the MedCalc Statistical Software 15.2 (MedCalc Software bvba, Ostend, Belgium). The level of statistical significance was a priori at p < 0.05. We present descriptive statistics as mean values ± standard deviations (SD) or percentages. We applied ANOVA for continuous data and Chi square for categorical data to compare participant characteristics according to survival status.
The mean of each FI-Lab or FRAIL-NH score at each age was plotted to examine the age association of FI-Lab or FRAIL-NH. Pearson correlation analyses were performed to explore the association between FI-Lab and FRAIL-NH and their relationship with age.
Receiver-operating characteristic (ROC) curves were calculated to estimate the area under the ROC curve (AUCs) for FI-Lab and FRAIL-NH in relation to mortality. The comparison of the AUCs was performed using the DeLong method [22]. We applied the Youden index method to determine the optimal cutoffs of FI-Lab or FRAIL-NH for predicting mortality [23].
We applied Cox proportional hazard models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality by FRAIL-NH and FI-Lab, separately. In addition to the unadjusted model, we also adjusted age and gender. As stated above, both FRAIL-NH and FI-Lab were treated as continuous variables and categorical variables in different models. To examine the increased risk by a 0.01 increment in the FI-Lab score, we multiplied each FI-Lab score by 100 and then converted the scores to integers (0–100). Furthermore, we applied the Kaplan–Meier method to estimate the survival curves and applied the log-rank test to compare the difference between the survival curves.
Results
We included 329 participants (105 men and 224 women) in the baseline investigation. The mean age of the participants was 85.2 ± 3.4 year. The characteristics of the study population are shown in Table 1.
The FI-Lab score was significantly and moderately associated with age (r = 0.371, p < 0.001). Similarly, the FRAIL-NH score was also significantly and moderately associated with age (r = 0.329, p < 0.001). Furthermore, the FI-Lab score was significantly and strongly associated with the FRAIL-NH score (r = 0.799, p < 0.001).
Using the Youden index method, we set the cutoffs of FI-Lab and FRAIL-NH as ≥ 0.3 and ≥ 6, respectively, to define frailty (Table 2). Based on these cutoffs, the prevalence of frailty was 56.2% according to the FI-Lab and 58.7% according to the FRAIL-NH. Table 3 shows the prevalence of frailty according to other cutoffs.
During the 1-year follow-up, seven individuals were lost. As a result, the final study population included 322 participants. Seventy-three participants (22.7%) died during the follow-up. The mean score of FI-Lab was significantly lower in the survival group than in the decreased group (0.28 versus 0.36, p < 0.001). The mean score of FRAIL-NH was also significantly lower in the survival group than in the deceased group (6.01 versus 7.62, p < 0.001).
Supplementary Fig. 1 shows the ROC curves of the FI-Lab and FRAIL-NH for predicting 1-year mortality. The FI-Lab (AUC 0.700, 95% CI 0.647–0.750) was slightly better than the FRAIL-NH (AUC 0.676, 95% CI 0.622–0.727) for predicting mortality (p = 0.025, Table 2).
Table 4 shows the results of Cox proportional hazard models of FI-Lab and FRAIL-NH for predicting time-to-death during 1-year follow-up. After adjusted for age and gender, the increment of the FI-Lab score was associated with an increased risk of mortality (adjusted HR per 0.01 increment in score 1.07, 95% CI 1.05–1.09). Similarly, the increment of the FRAIL-NH score was also associated with an increased risk of mortality (adjusted HR per 1 increment in score 1.28, 95% CI 1.19–1.46). Using the cutoff of FI-Lab score ≥ 0.3, frailty was related to an increased risk of mortality (adjusted HR 2.00, 95% CI 1.18–3.42). Using the cutoff of FRAIL-NH score ≥ 6, frailty was also associated with an increased risk of mortality (adjusted HR 2.26, 95% CI 1.32–3.85). However, when using the cutoffs of FRAIL-NH score as 2 and 6, individuals with frail (FRAIL-NH score: 2–5) or with severe frailty (FRAIL-NH score: 6–14) were not significantly associated with mortality (frailty: adjusted HR 1.33, 95% CI 0.18–10.09; severe frailty: adjusted HR 2.64, 95% CI 0.35–19.67).
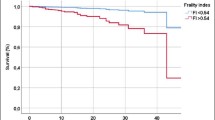
The survival curves of FI-Lab and FRAIL-NH according to various cutoffs for predicting time-to-death during 1-year follow-up are shown in Fig. 1.
The survival curves of FI-Lab and FRAIL-NH for predicting time-to-death during 1-year follow-up: a frailty is defined as the FRAIL-NH score ≥ 7 (log-rank test: p < 0.001); b frailty is defined as the FRAIL-NH score ≥ 5 (log-rank test: p < 0.001); c frailty is defined as the FRAIL-NH score ≥ 6 (log-rank test: p = 0.007); d frailty and severe frailty are defined as the FRAIL-NH score of 2–5 and 6–14, respectively (log-rank test: p = 0.026); e frailty is defined as the FI-Lab score ≥ 0.3 (log-rank test, p = 0.002); f using the quintiles of the FI-Lab score (log-rank test, p = 0.001)
Discussion
This study is the first to compare the applications of the FI-Lab and FRAIL-NH in Chinese nursing homes. Our results suggest that both FI-Lab and FRAIL-NH can be successfully applied to identify frailty in Chinese nursing home residents. Both frailty tools are valuable for predicting 1-year mortality in our study population, although the FI-Lab appears to be slightly better than FRAIL-NH. Our study also demonstrates that frailty can be defined by the FI-Lab score ≥ 0.3 or the FRAIL-NH score ≥ 6, respectively. Based on these cutoffs, frailty is associated with an increased risk of mortality.
Our study indicated that the FI-Lab was feasible to identify frailty and the increment of the FI-Lab score was associated with 1-year mortality in nursing home residents. The FI-Lab in our study is based on common laboratory tests that are regularly performed in Chinese nursing home residents. It is easy to be calculated and does not need the collection of further information through self-report procedures or face-to-face interviews like traditional questionnaires, such as the FRAIL-NH. However, it may be time-consuming to determine the abnormalities of the 30 items. If the FI-Lab can be coded into a program (or an app) and integrated into hospital information systems (HIS) and if the FI-Lab score can be calculated automatically, the performance of the FI-Lab would be significantly simplified and time-saving. Therefore, the FI-Lab may serve as a potential screening tool for frailty in nursing homes.
The cutoff of FI-Lab for defining frailty has not been established. Previous studies usually applied the quintiles of the FI-Lab score to group the individuals into different levels [11, 21]. However, our study found the HRs of different quintile groups for predicting mortality were not significant. Our results suggest using the cutoff of FI-Lab ≥ 0.3 to define frailty. However, this cutoff needs to be further validated in the future. In fact, frailty index (including the FI-Lab) is usually treated as a continuous variable instead of a categorical variable [19, 20, 24]. In our study, when administered as a continuous variable, each 0.01 increment in the FI-Lab score increased the HRs for 1-year mortality by 7%. This finding is very similar to a previous study conducted in geriatric wards, in which, the corresponding increment of HRs for 1-year mortality was 7.1% [20].
Since being developed in 2015, the FRAIL-NH has been validated in various ethnic populations, such as French [25] and American [16]. In addition, a recent Hong Kong study validated a modified FRAIL-NH (that using the FRAIL-NH model but the items were from the Minimum Data Set) in 2380 Chinese elderly nursing home residents and found that the modified FRAIL-NH could predict 9-year mortality. Our study also supports the application of FRAIL-NH as a simple frailty screening tool in Chinese nursing homes. However, it is noteworthy that the contents of FRAIL-NH in previous studies were slightly different [15,16,17, 25]. Therefore, it should be cautious when comparing the results of these studies.
The cutoff of FRAIL-NH for determining frailty has not been established yet. In this study, we applied four different cutoffs of FRAIL-NH to define frailty. Three of them were reported in previous studies, whereas the other was determined using the Youden index method. Our research supports the cutoffs of FRAIL-NH ≥ 6 or ≥ 7 to define frailty. Theou and colleagues suggested using the cutoffs of FRAIL-NH ≥ 2 and 6 to define frailty and severe frailty, respectively [17]. However, our study found that frailty or severe frailty defined by these cutoffs could not predict 1-year mortality in our study population. These findings indicated that the cutoffs of FRAIL-NH to define frailty might vary for populations with different clinical features. In fact, frailty is considered as “a transitional state in a dynamic process” instead of an “all-or-nothing” state [26]. Therefore, it may be reasonable to treat the FRAIL-NH score as a continuous variable (like the FI-Lab score) instead of a categorical variable. Our study indicated that each one-score increment in the FRAIL-NH score increased the HRs for 1-year mortality by 32%.
We failed to find any previous study which compares the FI-Lab and FRAIL-NH in nursing home residents. However, a recent cross-sectional study compared the FI based on clinical information and FRAIL-NH in Australian residential aged care facilities and reported that both tools were suitable to measure frailty in their study population [17]. Our study demonstrated that both FI-Lab and FRAIL-NH had value for predicting 1-year mortality in our study population, although the AUC of FI-Lab was slightly larger than that of FRAIL-NH for predicting mortality (AUC = 0.70 and 0.68, respectively). However, it is noteworthy that an AUC of 0.68 or 0.70 is not high. Generally speaking, an AUC of > 0.9, 0.7–0.9, and 0.5–0.7 indicates high, moderate, and low predictive value, respectively [27]. In fact, the sensitivity and specificity of both tools for predicting 1-year mortality were low, therefore, other unmeasured components, such as functional disability and acute medical events, might also contribute to the mortality. For clinical practice, this finding implies that the assessment of frailty is important but not enough for distinguishing nursing home residents who might be at higher risk of death. A comprehensive geriatric assessment that integrates the evaluation of frailty and other geriatric syndromes is therefore required.
In our study, the prevalence of frailty ranged from 40.7 to 82.1% when using different cutoffs of FI-Lab or FRAIL-NH that were reported in previous studies. We also defined frailty using the cutoffs of FI-Lab and FRAIL-NH as ≥ 0.3 and ≥ 6 according to the Youden index method, respectively. The relevant prevalence of frailty was then 56.2% and 58.7%, respectively, which were similar to the pooled estimate of the prevalence of frailty (52.3%) according to a recent systematic review [8].
The association between frailty and mortality has been well documented [2]. However, evidence regarding this relationship in nursing home residents is relatively limited. It is notable that the relationship between frailty and mortality may be influenced by the diagnostic criteria of frailty. For example, a recent study in French nursing homes demonstrated that severe frailty defined by the FRAIL-NH score from 6 to 14 was associated with 1-year mortality but frailty (FRAIL-NH score: 2–5) was not [25]. Our study demonstrated that frailty (FRAIL-NH score: 2–5) and severe frailty (FRAIL-NH score: 6–14) were not associated with 1-year mortality, but frailty defined by the FRAIL-NH score ≥ 6 or ≥ 7 was significantly associated with mortality.
Our study has some limitations. First, we only included Chinese nursing home residents; it should be cautious when generalizing our findings (especially the cutoffs of FI-Lab and FRAIL-NH) to clinical practice. Second, the sample size of our study is relatively small; third, some critical outcomes (such as disability, falls, hospitalization, and quality of life), some important confounder (such as acute medical events), and the reasons explaining the nursing home institutionalization (such as loneliness and mood disorders) were not analyzed in our study. Fourth, it is noteworthy that cognitive function and social function evaluations have been increasingly integrated into recent frailty instruments [28, 29]. In this study, cognitive impairment was determined only based on the medical records of the nursing homes. The social function was not evaluated. We did not address cognitive frailty and social frailty but focused on physical frailty because we applied the definition of frailty according to the recent international consensus on frailty which also focused on physical frailty [2]. Fifth, we did not have the data regarding the reason of death, therefore, we could not analyze the association between frailty and the disease-specific mortality.
Conclusion
Our study supports the application of both the FI-Lab and FRAIL-NH in Chinese nursing home residents. More than half of our study population are frailty according to the FI-Lab or FRAIL-NH. Both frailty screening tools are comparable for predicting 1-year mortality, although the FI-Lab has slightly higher predictive validity. However, whether these findings can be generalized to clinical practice needs to be further evaluated. Prospective studies addressing the predictive value of the FI-Lab and FRAIL-NH for other important outcomes (e.g., disability and quality of life) are also needed.
References
Rodriguez-Manas L, Feart C, Mann G et al (2013) Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 68:62–67
Morley JE, Vellas B, van Kan GA et al (2013) Frailty consensus: a call to action. J Am Med Dir Assoc 14:392–397
De Silva T, Ahip S, Theou O et al (2017) Frailty in nursing home residents: a scoping review protocol. JBI Database Syst Rev Implement Rep 15:2040–2046
Buckinx F, Reginster JY, Gillain S et al (2017) Prevalence of frailty in nursing home residents according to various diagnostic tools. J Frailty Aging 6:122–128
Buckinx F, Reginster JY, Petermans J et al (2016) Relationship between frailty, physical performance and quality of life among nursing home residents: the SENIOR cohort. Aging Clin Exp Res 28:1149–1157
Kojima G, Tanabe M (2016) Frailty is highly prevalent and associated with vitamin D deficiency in male nursing home residents. J Am Geriatr Soc 64:e33–e35
Collard RM, Boter H, Schoevers RA et al (2012) Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60:1487–1492
Kojima G (2015) Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc 16:940–945
Walston JD, Bandeen-Roche K (2015) Frailty: a tale of two concepts. BMC Med 13:185
Rockwood K, Abeysundera MJ, Mitnitski A (2007) How should we grade frailty in nursing home patients? J Am Med Dir Assoc 8:595–603
Blodgett JM, Theou O, Howlett SE et al (2016) A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing 45:463–468
Howlett SE, Rockwood MR, Mitnitski A et al (2014) Standard laboratory tests to identify older adults at increased risk of death. BMC Med 12:171
Mitnitski A, Collerton J, Martin-Ruiz C et al (2015) Age-related frailty and its association with biological markers of ageing. BMC Med 13:161
Rockwood K, McMillan M, Mitnitski A et al (2015) A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc 16:842–847
Kaehr E, Visvanathan R, Malmstrom TK et al (2015) Frailty in nursing homes: the FRAIL-NH scale. J Am Med Dir Assoc 16:87–89
Kaehr EW, Pape LC, Malmstrom TK et al (2016) FRAIL-NH predicts outcomes in long term care. J Nutr Health Aging 20:192–198
Theou O, Tan EC, Bell JS et al (2016) Frailty levels in residential aged care facilities measured using the frailty index and FRAIL-NH scale. J Am Geriatr Soc 64:e207–e212
Luo H, Lum TY, Wong GH et al (2015) Predicting adverse health outcomes in nursing homes: a 9-year longitudinal study and development of the FRAIL-minimum data set (MDS) quick screening tool. J Am Med Dir Assoc 16:1042–1047
Searle SD, Mitnitski A, Gahbauer EA et al (2008) A standard procedure for creating a frailty index. BMC Geriatr 8:24
Ritt M, Jager J, Ritt JI et al (2017) Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging 12:1029–1040
Blodgett JM, Theou O, Howlett SE et al (2017) A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geoscience 39:447–455
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Perkins NJ, Schisterman EF (2005) The Youden index and the optimal cut-point corrected for measurement error. Biometr J Biometrische Zeitschrift 47:428–441
Tabue-Teguo M, Kelaiditi E, Demougeot L et al (2015) Frailty index and mortality in nursing home residents in France: results from the INCUR Study. J Am Med Dir Assoc 16:603–606
De Silva TR, Theou O, Vellas B et al (2018) Frailty screening (FRAIL-NH) and mortality in French nursing homes: results from the incidence of pneumonia and related consequences in nursing home residents study. J Am Med Dir Assoc 19:411–414
Lang PO, Michel JP, Zekry D (2009) Frailty syndrome: a transitional state in a dynamic process. Gerontology 55:539–549
Linden A (2006) Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract 12:132–139
Azzopardi RV, Beyer I, Vermeiren S et al (2018) Increasing use of cognitive measures in the operational definition of frailty—a systematic review. Ageing Res Rev 43:10–16
Bunt S, Steverink N, Olthof J et al (2017) Social frailty in older adults: a scoping review. Eur J Ageing 14:323–334
Funding
This work was supported by the Health and Family Planning Commission of Sichuan Province (Grant number ZH2018-102) and the Sichuan Medical Association (Grant number S17054). The sponsors had no role in the design, methods, data collection, analysis, and preparation of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest related to this work.
Human and animal rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any study with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all the participants in this study or their legal proxies.
Additional information
Ming Yang and Yan Zhuo are contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Zhuo, Y., Hu, X. et al. Predictive validity of two frailty tools for mortality in Chinese nursing home residents: frailty index based on common laboratory tests (FI-Lab) versus FRAIL-NH. Aging Clin Exp Res 30, 1445–1452 (2018). https://doi.org/10.1007/s40520-018-1041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-018-1041-7