Abstract
Objectives
Frailty has become an independent risk factor for adverse outcomes in critically ill patients. This study aimed to explore the predictive ability of two electronic medical record-based frailty assessment tools, the Hospital Frailty Risk Score (HFRS) and Frailty Index based on physiological and laboratory tests (FI-lab), for long-term adverse prognosis in older critically ill survivors.
Design
Retrospective observational study.
Setting and Participants
9,082 critically ill survivors aged ≥ 65 years.
Measurements
The HFRS and the 33-item FI-lab were constructed based on the published literature. Cox and logistic regression models assessed the association between frailty and 1-year mortality and post-discharge care needs.
Results
2,586 patients died within 1 year of follow-up. In fully adjusted models, frailty assessed using both the HFRS (per point, hazard ratio [HR] 1.06, 95% confidential interval [CI] 1.05–1.06; intermediate frailty risk, HR 2.00, 95% CI 1.78–2.25; high frailty risk, HR 3.06, 95% CI 2.68–3.50) and FI-lab (per 0.01 points, HR 1.03, 95% CI 1.03–1.03; intermediate frailty risk, HR 1.59, 95% CI 1.44–1.76; high frailty risk, HR 2.30, 95% CI 2.06–2.57) was associated with mortality. Addition of frailty indicators improved the predictive validity of the Sequential Organ Failure Assessment score for mortality (HFRS alone Δ C-index 0.034; FI-lab alone Δ C-index 0.016; HFRS and FI-lab combined Δ C-index 0.042). The HFRS but not the FI-lab was associated with higher probability of post-discharge care needs.
Conclusion
Both the HFRS and FI-lab could independently predict 1-year mortality in older critically ill survivors. Adding the HFRS to the SOFA score model improved it more than adding the FI-lab. The greatest improvement was achieved when both frailty indicators were used together. These findings suggest that electronic medical record-based frailty assessment methods can be useful tools for predicting long-term outcomes in older critically ill patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Objectives
With an aging population and increasing life expectancy, there has been a significant increase in the demand for intensive care unit (ICU) admission among older patients (1–3). It is urgent to identify those at high risk of adverse outcomes after critical illness (4). Commonly used scoring systems in the ICU include the Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA) score (5, 6), which are effective in predicting the mortality risk of critically ill patients. However, further exploration is needed to identify other risk factors for adverse outcomes in critically ill patients.
Frailty is a biological syndrome characterized by decreased biological reserves and increased vulnerability to stressors (7). A recent meta-analysis showed that the prevalence of frailty among older patients in the ICU was as high as 45% (8). Compared with non-frail patients, frail patients are less likely to tolerate critical illness, more likely to develop complications and die, and less likely to fully recover (9, 10). It is important to understand the relationship between frailty and adverse outcomes in older critically ill patients.
There is no consensus on the best method for screening frail patients in the ICU (11). The Clinical Frailty Scale (CFS) is the most commonly used in research but may introduce observer bias (12–14). Electronic medical record-based methods offer objectivity and convenience. The Hospital Frailty Risk Score (HFRS) is a validated tool based on International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes and can be easily evaluated using electronic medical records (15). However, studies on its validity in critically ill patients have been inconsistent (16–18). The Frailty Index based on physiological and laboratory tests (FI-lab) is another objective indicator that can be calculated using electronic medical record data; it can be used alone or in combination with other indicators to detect an increased risk of adverse outcomes in community populations (19, 20). However, there are few studies of application of FI-lab in critically ill patients (21). Additionally, information is limited on the impact of frailty status on long-term outcomes in older critically ill survivors.
In this study, we aimed to explore the predictive ability of two electronic medical record-based frailty assessment tools, the HFRS and FI-lab, for long-term mortality in older critically ill survivors.
Methods
Study design and data source
This was a retrospective observational study using the Medical Information Mart for Intensive Care (MIMIC)-IV database 2.0, which is an openly accessible critical care database that has received pre-existing institutional review board approval for data derivation (22, 23). This database comprises 76,943 ICU admission records between 2008 and 2019 from the Beth Israel Deaconess Medical Center in Boston, Massachusetts (USA). Access to this database was granted upon successful completion of the National Institutes of Health’s web-based course on Protecting Human Research Participants (certification number: 11153471).
Study population and data collection
We analyzed only the first-time ICU admission of each patient and excluded those who met any of the following criteria: (1) age less than 65 years; (2) died during the ICU or hospital stay; or (3) missing key variables, including demographic data, admission location, Sequential Organ Failure Assessment (SOFA) scores(6), items necessary to construct the HFRS or FI-lab, and survival status at 1 year after hospital discharge.
We extracted the following data from the database: demographic data (age, gender, and race and ethnicity categorized as White, Black or African American, and other); admission location (emergency department, operating room, peripheral hospital, and other); SOFA scores; comorbidities (cerebrovascular disease, congestive heart failure, dementia, diabetes mellitus, malignancy, myocardial infarction, and kidney disease); discharge location; and survival status at 1 year after hospital discharge.
Assessment of frailty
Hospital Frailty Risk Score (HFRS)
The HFRS score was calculated for each patient included in the study according to their ICD diagnoses documented during their current hospitalization, using methods previously described in the literature (15). Patients were then classified into one of three categories according to their HFRS score: low frailty risk (HFRS < 5), intermediate frailty risk (HFRS 5–15), and high frailty risk (HFRS > 15). Further details on the method used for constructing HFRS and the prevalence of the 109 ICD codes can be found in the supplemental materials (see «Detailed methods of construction of the Hospital Frailty Risk Score» and Table S1).
Frailty Index based on physiological and laboratory tests (FI-lab)
To construct the FI-lab, 33 items were tested or measured during a 24-hour period before and after admission to the ICU (19, 20). The FI-lab included 30 laboratory test items from venous blood, arterial blood gas, and urine samples, as well as three vital signs: systolic blood pressure, diastolic blood pressure, and heart rate. For items with multiple measurements, the mean value was used. Each item was dichotomized using the normal reference intervals provided in the database, and a score of 1 was given to any value lying outside of the reference range, indicating a deficit. The FI-lab score was calculated by summing the deficits present and dividing by the number of items included. The FI-lab score theoretically ranges from 0 to 1; in this study, the score ranged from 0 to 0.82. Only patients with fewer than 20% missing items (n ≥ 27) were included in the study. We divided patients into three frailty risk groups using tertiles: low frailty risk (FI-lab < 0.36), intermediate frailty risk (FI-lab 0.36–0.48), and high frailty risk (FI-lab > 0.48). The reference value, mean/median value, and defect prevalence for each FI-lab item are presented in Table S2.
Outcomes and follow-up
The primary outcome was 1-year mortality after hospital discharge. The secondary outcome was post-discharge care needs (post-discharge care needs including hospice, home care, and other healthcare facilities versus discharge home without further need for nursing care) (24). Follow-up started on the date of discharge and patient deaths occurring more than one year after hospital discharge are censored.
Statistical analyses
Continuous variables are presented as mean with standard deviation (SD) or median with interquartile range (IQR); categorical variables are presented as count and percentage. The Student t-test, analysis of variance, the Mann–Whitney U test, and χ2 test were used for descriptive analysis, as appropriate. Diagnostic consistency of the two frailty assessment tools was checked using Kappa analysis.
For survival analysis, univariate Kaplan–Meier and multivariate Cox regression models were conducted to investigate the relationship between frailty and 1-year mortality after hospital discharge. The Kaplan–Meier curves were compared using the log-rank test. Frailty was assessed as both a continuous and categorical variable in the multivariable Cox model, which was adjusted for age, gender, race and ethnicity, admission location, and SOFA score. Furthermore, five-knot restricted cubic spline curves were used to display the association of each frailty score with 1-year mortality. Logistic regression model was used to examine the association between frailty and post-discharge care needs.
To evaluate the incremental predictive value of change in each frailty score (as a continuous variable) and the combination of HFRS and FI-lab scores beyond the SOFA score-based model for the risk of 1-year mortality, we calculated and compared the C-index, integrated discrimination improvement (IDI), and net reclassification improvement (NRI).
To validate the robustness of our findings, we performed two sensitivity analyses. The first analysis involved repeating the multivariate analyses in 23,668 patients with all variables required to construct the HFRS. The second analysis involved adjusting the simplified Acute Physiology Score (SAPS) II (25), which is one of the most commonly used disease severity scores for critically ill patients and available from the MIMIC database, instead of SOFA to further account for the impact of disease severity.
All statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA) and R version 4.1.2 (The R Project for Statistical Computing, Vienna, Austria). A two-tailed p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
In total, 9,082 first-time ICU admission records for eligible adults aged 65 years or older were analyzed among 76,943 ICU admission records between 2008 and 2019 at Beth Israel Deaconess Medical Center in Boston. Figure S1 shows the selection procedures of the study. Table 1 presents the characteristics of included patients stratified by their survival status within 1 year of follow-up. In brief, the mean age of included patients was 77.2±7.8 years, and 4,753 patients (52.3%) were men. Patients who died within 1 year of discharge were older; more likely to have been admitted to the ICU via the emergency department; had higher SOFA scores; and had a higher proportion of chronic obstructive pulmonary disease, congestive heart failure, dementia, malignancy, myocardial infarction, and kidney disease compared with survivors. The kappa value showed a low diagnostic consistency of two frailty assessment tools (kappa=0.11, p < 0.001; Table S3). The distributions of HFRS and FI-lab scores for the study population are shown in Figure 1. Patients who died had significantly higher HFRS and FI-lab scores and a significantly higher proportion of frailty than survivors. There were no significant differences between groups in terms of gender, race and ethnicity, and prevalence of cerebrovascular disease and diabetes mellitus. Table S4 and S5 provide further detailed characteristics of patients stratified according to each frailty measure.
Association of each frailty measure with 1-year mortality
Spline curves showing the association of each frailty measure with 1-year mortality; a) HFRS and b) FI-lab. The density curve describes the distribution of each frailty measure for more than 99% individuals in the study population. The dotted line indicates the cutoff score for low frailty risk and intermediate frailty risk/ high frailty risk which is also the reference standard for association between frailty and 1-year mortality: 5 points for HFRS and 0.36 points for FI-lab. Spline curves were adjusted for age, gender, race and ethnicity, admission location, and SOFA. None of the patients with FI-lab 0 to 0.06 died within the 1-year follow-up period. The colored background strip of each line represents the confidence interval of the line.
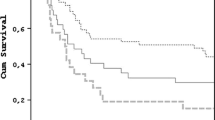
Frailty and 1-year mortality
A total of 2,586 patients died within 1 year of follow-up. The 1-year mortality increased significantly with an increase in frailty severity, as measured using both the HFRS and the FI-lab (Table 2). The cumulative probability of mortality according to the category of frailty as assessed using the HFRS and FI-lab is presented using Kaplan–Meier curves (Figure 2). Univariate Cox regression analysis showed that both HFRS and FI-lab scores (assessed as a continuous variable) were significantly associated with an increased risk of 1-year mortality in surviving patients after discharge (HFRS: per point increase, hazard ratio [HR] 1.06, 95% confidence interval [CI] 1.06–1.07; FI-lab: per 0.01-point increase, HR 1.03, 95% CI 1.03–1.03). Each additional point on the HFRS was associated with a 6% increased risk of mortality (HR 1.06, 95% CI 1.05–1.06) after adjusting for age and gender, and each 0.01-point increase in the FI-lab was associated with a 3% increased risk of mortality (HR 1.03, 95% CI 1.03–1.03). The associations between both frailty measures and mortality remained significant after further adjustment for race and ethnicity, admission location, and SOFA in the multivariable model (Table 2, Figure 1). In the fully adjusted model, intermediate frailty risk patients had a 2-fold higher risk of mortality (HR 2.00, 95% CI 1.78–2.25) and a 1.6-fold higher risk (HR 1.59, 95% CI 1.44–1.76) compared with low frailty risk patients (assessed using the HFRS and FI-lab, respectively). High frailty risk patients had even higher risks of mortality, with HRs of 3.06 (95% CI 2.68–3.50) and 2.30 (95% CI 2.06–2.57) assessed using the HFRS and FI-lab, respectively.
Post-discharge care needs
The multivariate model showed that the HFRS was significantly associated with a higher probability of postdischarge care needs (per point, odds ratio [OR] 1.11, 95% CI 1.10–1.13; intermediate frailty risk, OR 1.79, 95% CI 1.55–2.06; high frailty risk, OR 5.97, 95% CI 4.29–8.30); the FI-lab was not associated with post-discharge care needs (per 0.01 points, OR 1.01, 95% CI 1.00–1.01; intermediate frailty risk, OR 1.08, 95% CI 0.92–1.26; high frailty risk, OR 1.07, 95% CI 0.88–1.29) (Table S6).
Improvement in mortality prediction
The addition of either the HFRS or the FI-lab to risk-adjustment model improved the discrimination for 1-year mortality beyond that provided by the SOFA score-based model. Among the two frailty measures, the HFRS performed better than the FI-lab for predicting 1-year mortality (Table 3, Table S7). Combining the HFRS and the FI-lab into a multivariable model resulted in a further increase in the A C-index to 0.042 (95% CI 0.036–0.047), which was superior to the addition of each frailty assessment alone. Moreover, the combined model showed the greatest improvements in IDI at 0.055 (95% CI 0.048–0.062) and NRI at 0.434 (95% CI 0.401–0.473).
Sensitivity analysis
Table S8 shows the baseline characteristics of 23,668 patients who had all variables needed to construct the HFRS. Sensitivity analyses confirmed the robustness of the associations between frailty, as assessed using the HFRS, and 1-year mortality (Table S9).
Furthermore, multivariate analyses, adjusted for SAPS II instead of SOFA, demonstrated similar associations between frailty and 1-year mortality, assessed using both frailty measures as both a continuous and categorized variable (Table S10).
Discussion
In this large single-center retrospective cohort study, we showed that both the HFRS and FI-lab could independently predict 1-year mortality in critically ill survivors aged 65 years and over in the ICU setting. Using either of these two frailty indicators could provide additional discriminatory value beyond SOFA for long-term survival after discharge, with addition of the HFRS showing greater improvement. The improvement was most evident when both frailty indicators were used in combination. In addition, the HFRS but not the FI-lab was associated with higher probability of post-discharge care needs.
The HFRS was initially developed for hospitalized populations and has been validated in several Western countries (15, 26–29). Limited studies have used the HFRS to assess frailty in ICU patients and predict adverse outcomes, but results have been inconsistent. Redfern and colleges reported that 71.5% of unplanned ICU admissions among hospitalized patients were intermediate frailty risk to high frailty risk, a proportion similar to that found in our study (18) (73.6% for the final study population or 59.1% for the population included in sensitivity analysis). However, Subramaniam et al. found that only 26.2% of patients were assessed as having intermediate frailty risk to high frailty risk using the HFRS (16). Differences in the frailty prevalence may be owing to reliance of the HFRS on counting comorbidities based on ICD-10 codes. Subramaniam et al. only used diagnoses at the time of admission for the current hospitalization to calculate HFRS scores, which may have led to a serious underestimation of frailty in critically ill patients; the prevalence of frailty among patients in the ICU is generally considered to be approximately 45% (8). Redfern and colleagues reported that the HFRS was not associated with in-hospital mortality among ICU patients (18). Bruno et al. also found that the HFRS was not associated with in-ICU mortality in multivariable models adjusted for APACHE II or SAPS II scores (17). Despite potentially underestimating the prevalence of frailty, Subramaniam et al. still found that the HFRS could predict in-hospital and 1-year mortality among critically ill patients (16). Our study found similar results in a larger population; frailty assessed using the HFRS could predict 1-year mortality among older critically ill survivors after discharge. The accuracy and completeness of ICD code records may vary among countries or hospitals of different levels, and a limited sample size may also affect results. Further research is needed to explore application of the HFRS in ICU settings. The HFRS is constructed based on claims data. Similarly, widely used comorbidity indices like the Charlson Index and Elixhauser Index, which are prognosis prediction tools, are also constructed based on claims data and show significant associations with adverse outcomes in critically ill patients (30). However, the construction of HFRS is more complex than that of comorbidity indices. Currently, there is no research comparing the predictive abilities of HFRS and comorbidity indices for adverse outcomes in critically ill patients and warrant further exploration in future study.
The FI-lab was initially developed for community populations and its predictive value for adverse outcomes in both community and hospitalized populations has been reported (19, 20, 31). This study provides the first report on the predictive value of FI-lab for long-term prognosis in critically ill patients with non-specific diseases. When applied in the ICU, FI-lab scores are higher than those in community populations (0.40 vs. 0.27) (20). Owing to the overlap between components of the FI-lab and markers of acute or subacute illness, the FI-lab applied in ICU settings reflects the severity of illness, to some extent. In contrast, the components of the HFRS include more comorbidities associated with long-term outcomes. This may explain why, in this study, the addition of the FI-lab to a multivariate model based on SOFA scores did not improve model performance as much as the addition of the HFRS. Moreover, the HFRS but not the FI-lab was associated with a higher likelihood of post-discharge care needs. Pre-hospital laboratory examination results may be a better choice for constructing the FI-lab but are difficult to implement. Additionally, we found that when two frailty indices were used in combination, model discrimination was improved most significantly. This result suggests that the HFRS and FI-lab may measure different aspects of frailty.
The CFS is the most commonly used frailty assessment tool in research in the ICU. However, the subjectivity of questionnaire-based frailty assessment methods may introduce observer bias. Electronic medical record-based frailty assessment methods can perform simple and rapid assessments for all patients, reducing the workload of physicians and avoiding subjective bias. However, the limitation of using ICD-10 codes is that differences in diagnostic records and coding may result in measurement errors. The FI-lab can more accurately reflect the current state of decline in a patient’s biological reserve. Further research is needed to explore use of the FI-lab among patients in ICU. The findings of our study suggest that utilizing frailty assessment to identify the high-risk population for adverse prognosis among critically ill survivors after discharge can facilitate timely interventions, such as nutritional support, medication management, and functional rehabilitation et al., to improve their long-term prognosis(32). Focusing on whether older critically ill survivors can return home directly after discharge is crucial for reallocating healthcare resources to better meet the growing medical needs of the older adult, especially those who have experienced critical illnesses.
This study has several important advantages. First, this study explored for the first time the predictive value of FI-lab for long-term adverse prognosis in critically ill patients with non-specific diseases. Second, this study has the largest known sample size for frailty assessment using the HFRS in an ICU setting, providing further evidence for validation of the HFRS. Finally, we found that the combined use of different frailty assessment methods may capture different aspects of a patient’s frailty characteristics, which can help guide future practice and research on frailty assessment in ICU settings.
We also acknowledge several limitations in this study. First, the HFRS is based on ICD-10 codes, and both ICD-9 and ICD-10 codes are present in the MIMIC-IV database. We used ICD-9 to ICD-10 conversion to construct the HFRS, which may result in slight inaccuracies in the resultant HFRS scores. Second, pre-hospital laboratory test results may be a better choice for constructing the FI-lab but these are not easily available. Lastly, because APACHE II scores are not available in the MIMIC database, we included SOFA scores in the multivariate analysis and replaced them with SAPS II scores in sensitivity analysis to confirm the robustness of our results.
Conclusion
Both the HFRS and FI-lab could independently predict 1-year mortality in critically ill older survivors aged 65 years and over in the ICU setting. Using either of these two frailty indicators could provide additional discriminatory value beyond SOFA for long-term survival after discharge, with addition of HFRS showing greater improvement. The improvement was most evident when both frailty indicators were used in combination. These findings suggest that electronic medical record-based frailty assessment methods can be useful tools for predicting long-term outcomes in older critically ill patients.
Data availability statement: The MIMIC-IV database are available in PhysioNet (https://physionet.org).
References
Ihra GC, Lehberger J, Hochrieser H, et al. Development of demographics and outcome of very old critically ill patients admitted to intensive care units. Intensive Care Med. 2012;38(4):620–626.
Nguyen YL, Angus DC, Boumendil A, Guidet B. The challenge of admitting the very elderly to intensive care. Ann Intensive Care. 2011;1(1):29.
Bagshaw SM, Webb SA, Delaney A, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care. 2009;13(2):R45.
Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829.
Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710.
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375.
Xia F, Zhang J, Meng S, Qiu H, Guo F. Association of Frailty With the Risk of Mortality and Resource Utilization in Elderly Patients in Intensive Care Units: A Meta-Analysis. Front Med (Lausanne). 2021;8:637446.
Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186(2):E95–102.
Brummel NE, Bell SP, Girard TD, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med. 2017;196(1):64–72.
McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15(1):301.
Bertschi D, Waskowski J, Schilling M, Donatsch C, Schefold JC, Pfortmueller CA. Methods of Assessing Frailty in the Critically Ill: A Systematic Review of the Current Literature. Gerontology. 2022;68(12):1321–1349.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495.
Pugh RJ, Battle CE, Thorpe C, et al. Reliability of frailty assessment in the critically ill: a multicentre prospective observational study. Anaesthesia. 2019;74(6):758–764.
Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782.
Subramaniam A, Ueno R, Tiruvoipati R, Srikanth V, Bailey M, Pilcher D. Comparison of the predictive ability of clinical frailty scale and hospital frailty risk score to determine long-term survival in critically ill patients: a multicentre retrospective cohort study. Crit Care. 2022;26(1):121.
Bruno RR, Wernly B, Flaatten H, Scholzel F, Kelm M, Jung C. The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care. 2019;23(1):239.
Redfern OC, Harford M, Gerry S, Prytherch D, Watkinson PJ. Frailty and unplanned admissions to the intensive care unit: a retrospective cohort study in the UK. Intensive Care Med. 2020;46(7):1512–1513.
Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016;45(4):463–468.
Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171.
Bai W, Hao B, Xu L, Qin J, Xu W, Qin L. Frailty index based on laboratory tests improves prediction of short-and long-term mortality in patients with critical acute myocardial infarction. Front Med (Lausanne). 2022;9:1070951.
Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–220.
Johnson A, Bulgarelli L, Pollard T, Celi LA, Mark R, Horng S. MIMIC-IV (version 2.0). PhysioNet. 2022; Available from: https://doi.org/10.13026/as7t-c445.
Zampieri FG, Iwashyna TJ, Viglianti EM, et al. Association of frailty with short-term outcomes, organ support and resource use in critically ill patients. Intensive Care Med. 2018;44(9):1512–1520.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963.
Nishimura S, Kumamaru H, Shoji S, et al. Assessment of coding-based frailty algorithms for long-term outcome prediction among older people in community settings: a cohort study from the Shizuoka Kokuho Database. Age Ageing. 2022;51(3).
McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf. 2019;28(4):284–288.
Gilbert T, Cordier Q, Polazzi S, et al. External validation of the Hospital Frailty Risk Score in France. Age Ageing. 2022;51(1).
Eckart A, Hauser SI, Haubitz S, et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open. 2019;9(1):e026923.
Ladha KS, Zhao K, Quraishi SA, et al. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open. 2015;5(9):e008990.
Gu JJ, Liu Q, Zheng LJ. A Frailty Assessment Tool to Predict In-Hospital Mortality in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1093–1100.
Montgomery CL, Rolfson DB, Bagshaw SM. Frailty and the Association Between Long-Term Recovery After Intensive Care Unit Admission. Crit Care Clin. 2018;34(4):527–547.
Acknowledgement: We acknowledge the Laboratory for Computational Physiology team from the Massachusetts Institute of Technology (LCP-MIT) who establish and maintain the MIMIC-IV databases.
Funding
Funding: Not available.
Author information
Authors and Affiliations
Contributions
Author contributions: Study concept and design: H-BL, LS and B-CH. Acquisition of data: B-CH, W-HX, W-YG, JQ and T-KH. Analysis and interpretation of data: D-YL, H-NX, H-YL and LL. Drafting of the manuscript: B-CH, W-HX and W-YG. Critical revision of the manuscript for important intellectual content: H-BL, LS, B-CH, W-HX, W-YG, T-KH, LL, D-YL, H-NX, H-YL and JQ.
Corresponding authors
Ethics declarations
Conflicts of interest: The authors declare that they have no Conflicts of interest.
Ethics approval and consent to participate: The establishment of this de-identified database was approved by the Institutional Review Board at the Beth Israel Deaconess Medical Center. Written informed consent for participation was not required for this project in accordance with the national legislation and the institutional requirements.
Electronic Supplementary Material
12603_2023_1961_MOESM1_ESM.docx
Association between frailty assessed using two electronic medical record-based frailty assessment tools and long-term adverse prognosis in older critically ill survivor
Rights and permissions
About this article
Cite this article
Hao, B., Xu, W., Gao, W. et al. Association between Frailty Assessed Using Two Electronic Medical Record-Based Frailty Assessment Tools and Long-Term Adverse Prognosis in Older Critically Ill Survivors. J Nutr Health Aging 27, 649–655 (2023). https://doi.org/10.1007/s12603-023-1961-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-023-1961-6