Abstract
Objective
To evaluate the role of Doppler ultrasonography in the assessment of splanchnic circulation’s hemodynamic changes in septic preterms at risk of necrotizing enterocolitis.
Methods
A total of 51 septic preterms were divided into two groups: 25 preterms with clinical signs of necrotizing enterocolitis (NEC) and 26 preterms with no clinical signs of NEC. Both groups were assessed with Doppler ultrasonography of the celiac and superior mesenteric arteries, and each septic preterm’s peak systolic velocity (PSV), end-diastolic velocity (EDV), resistivity index (RI), and pulsatility index (PI) was calculated and recorded.
Results
These included a statistically significant lower PSV (p: 0.001) and a lower EDV (p: 0.001) in the superior mesenteric artery in the septic group with clinical signs of NEC in comparison with the septic group with no clinical signs of NEC. A statistically significant (p < 0.001) higher PSV celiac (CA)/PSV superior mesenteric (SMA) ratio was found for the group of septic preterms with clinical signs of NEC when compared to the other group.
Conclusion
The study results showed that Doppler ultrasonography of the splanchnic circulation can be a tool for the early identification of NEC cases among septic preterms.
Sommario
Obiettivo
valutare il ruolo del Doppler nella valutazione dei cambiamenti emodinamici del circolo splancnico in pz pretermine settiche e a rischio di enterocolite necrotizzante.
Metodi
51 pazienti pretermine settiche divise in due gruppi sia con segni clinici di enterocolite necrotizzante (NEC) o asintomatiche, entrambi valutati con Doppler a ultrasuoni delle arterie celiaca e mesenterica superiore, con velocità di picco sistolico (PSV), fine della velocità diastolica (EDV), indice di resistività (RI), indice di pulsatilità (PI) il calcolo e la registrazione.
Risultati
il più basso PSV è risultato statisticamente significativo (p: 0.001), il valore inferiore di EDV (p: 0.001) campionati a livello dell’arteria mesenterica superiore nel gruppo settico con segni clinici di NEC, a confronto con il gruppo delle settiche senza segni clinici di NEC. Statisticamente significativa (p < 0.001) l’aumento del rapporto PSV celiaco (CA)/PSV a livello della mesenterica superiore (SMA) nel gruppo di sepsi con segni clinici di NEC in confronto con l’altro gruppo.
Conclusione
I risultati dello studio hanno mostrato che il Doppler del circolo splancnico può essere uno strumento di identificazione precoce dei casi NEC tra le pazienti pretermine settiche.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
NEC is one of the most common acquired, life-threatening gastrointestinal diseases in newborns, affecting 1–5% of neonatal intensive care unit admissions and up to 10% of neonates under 1500 g [1]. Mortality rates secondary to NEC are as high as 20–30%, making it one of the leading causes of death in premature infants [2]. Although the exact etiology of necrotizing enterocolitis (NEC) remains unknown, researchers suggest that it is multifactorial. Prematurity (with an immature GIT and immature host defenses) is the primary risk factor [3]; ischemia and/or reperfusion injury, exacerbated by the activation of pro-inflammatory intracellular cascades, may play a significant role [4]. Neonatal sepsis is considered a risk factor for NEC [5].
There is a significantly high NEC incidence (up to 50%) in neonates with sepsis or septic shock who are admitted to neonatal intensive care units (ICUs) [6]. Due to non-specific clinical signs and symptoms, which resemble neonatal sepsis in many cases, the diagnosis of NEC is very difficult. Although the pathogenesis of NEC is not definitely known, several factors, including intestinal trauma and damage due to sepsis and intestinal ischemia and the colonization of various bacteria can initiate and induce the disease [7]. Bowel ultrasonography provides an opportunity for the evaluation of bowel dynamics as well as free intra-abdominal fluid. Many studies have emphasized the higher diagnostic accuracy of gray-scale and Doppler ultrasonography over plain abdominal radiography for the detection of some bowel wall changes, especially those in bowel wall thickness, echogenicity, and perfusion, as well as portal venous gas and focal or free intra-abdominal fluid [7,8,9,10,11,12,13,14].
Coombs et al. demonstrated that neonates at risk of developing necrotizing enterocolitis had abnormal gut blood-flow velocities. They provided evidence that an alteration in the splanchnic circulation may be an important factor in the final common pathway that links diverse risk factors for necrotizing enterocolitis to clinical disease [15].
Since necrotizing enterocolitis is one of the most serious disorders of the gastrointestinal tract during the neonatal period, early diagnosis and adequate treatment are essential in determining whether clinical suspicion of NEC is present [16]. In this study, we aimed to evaluate the splanchnic circulation of the septic preterm with clinical signs of NEC using Doppler ultrasonography.
Methodology
Patient population
Fifty-one patients from the neonatal intensive care unit at Cairo Pediatric Hospital were eligible for this cross-sectional study. They were eligible if they had a gestational age of less than 37 weeks and suffered from neonatal sepsis. Sepsis was defined as proven sepsis with clinical signs and the isolation of bacterial infective agent from blood or probable sepsis with the presence of clinical signs, two positive screening parameters, and sterile blood culture [(1) CRP; (2) TLC (total leucocytic count) increase/decrease (=5000 or 25,000, 30,000, and 21,000/mm3 at birth, at 12–24 h, and from day 2 onwards, respectively); (3) band cell count <1800 cells/mm3; (4) ANC (absolute neutrophilic count); (5) I/T (immature to total neutrophil) ratio >0.2; and (6) platelets = 100,000/mm3] [17].
We included infants with or without feeding intolerance in our study. Feeding intolerance was defined as the inability to digest feedings, presented as a gastric residual volume of more than 50%, abdominal distension or emesis or both, and the disruption of the patient’s feeding plan [18]. We excluded infants if they exhibited congenital gut anomalies, other congenital malformations, unstable hemodynamic conditions, or catheters in their umbilical arteries or had previously undergone abdominal surgery.
Informed oral consent was obtained from all patients’ parents before involving them in the study as there was no interventional process, nor was there a perceived risk. The steps of the study, its aims, and the procedure were discussed with the parents of the study group. Moreover, the confidentiality of all data was ensured. The ethics committee of the Pediatric Department of Cairo University Hospitals approved all the human studies. Therefore, the studies were performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its subsequent amendment.
Our patients were divided into two groups: Group 1 included sepsis cases with clinical signs of NEC and group 2 included sepsis cases with no clinical signs of NEC.
Group 1 cases
NEC was classified according to Bell’s staging criteria with Walsh’s and Kliegman’s modifications [19]. Briefly, stage 1 was defined as the presence of systemic signs (temperature instability, apnea, and bradycardia); intestinal signs of feeding intolerance (elevated pregavage residuals, mild abdominal distension, and occult blood in stool); and a normal X-ray or one showing mild distension. Stage II was defined as stage I plus absent bowel sound, abdominal tenderness and radiological features of ileus, and intestinal pneumatosis (IIA) plus portal vein gas with or without ascites (IIB). Stage III was an advanced stage, showing the radiological features of IIA plus definite ascites or those of IIB plus pneumoperitoneum.
Group 2 cases
This group included septic premature neonates who tolerated enteral feeding well and had no gastrointestinal symptoms.
Clinical and laboratory examinations
All patients were subjected to full histories and clinical examinations with emphasis on whether they had feeding intolerance and abdominal distension and their onset if any. “Small for gestational age” (SGA) was defined as a birth weight below the 10th percentile [20].
Laboratory investigations
These included complete blood count, Na, K, Ca, random blood sugar, C-reactive protein, blood culture, and arterial blood gases.
Imaging
Abdominal radiographs, abdominal ultrasonography, and the Doppler evaluation of the splanchnic arteries were performed for all patients. The abdominal radiographs performed before each sonogram were assessed if they were acquired within 24 h of the US.
Abdominal X-ray
Abdominal X-rays were categorized according to the abdominal gas pattern (normal gas pattern, mild-moderate diffuse distension, severe diffuse distension, gasless abdomen, and asymmetrical distension), intramural gas, intraperitoneal gas, and portal venous gas [21].
High-resolution abdominal ultrasonographic assessment
A single experienced sonographer performed all ultrasound examinations with the Toshiba Nemio Powervision Software 6000 SSA-370A (Toshiba Corporation, Tokyo, Japan) with a 5–10 MHz linear transducer to scan all four abdominal quadrants transversely and longitudinally.
The resolution in the near, middle, and far fields was optimized independently by adjusting the focal zone of the US beam. Select images of the bowel and peritoneal free fluid, if present, were taken. Measurement of the bowel wall thickness was undertaken.
We used a categorization made by a previous study to predict the outcome of NEC according to ultrasound findings: We used focal fluid collections, echogenic free fluid, increased bowel wall echogenicity, and increased bowel wall thickness (2.7 mm or more) to predict an unfavorable outcome. Other features that predicted poor outcomes follow: free peritoneal gas, pneumatosis intestinalis, aperistalsis, and bowel wall thinning (1 mm or less). Anechoic free peritoneal fluid predicted a good outcome [21].
Doppler sonography
The same experienced sonographer performed color Doppler examination of the SMA and CA using the Toshiba Nemio Powervision Software 6000 SSA-370A (Toshiba Corporation, Tokyo, Japan) with a 5–10 MHZ curvilinear transducer.
Examination was performed and provided that there was a period of cardiorespiratory stability (defined as a mean blood pressure >30 mm Hg and continuous oxygen saturation monitoring at 90/95%). The neonate was kept in a supine position with the transducer positioned in the epigastric region, immediately below the xiphoid process, to scan the aorta and detect the celiac trunk and of the superior mesenteric artery a few millimeters after their emergence from the aorta in the sagittal plane. A clear image in real-time ultrasonography of the artery was obtained and correction made for the angle of insonation. A Doppler sample volume was placed in the proximal portion of the artery near its origin from the aorta, and pulsed wave Doppler traces were acquired. Peak systolic velocity, end-diastolic velocity, resistivity, and pulsatility indices were obtained from the recorded Doppler tracings. At each timepoint, three sets of measurements were recorded from each artery, and the final measurements were the mean of these three readings.
The ratio of the celiac artery peak systolic velocity to the superior mesenteric artery peak systolic velocity was calculated as the systolic velocity ratio. This parameter was used to compensate for patient-to-patient hemodynamic variables. The results of the SMA Doppler flow indices were not disclosed to the attending neonatologist, so the clinical management of the cases, including the timing and volumes of feeding and the diagnosis of NEC and comorbidities, was performed with the neonatologist blinded to the Doppler results.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (±SD), median and range, or frequencies (number of cases) and percentages when appropriate. The comparison of numerical variables between the study groups was done using Student’s t test for independent samples to compare the two groups when normally distributed and the Mann–Whitney U test for independent samples when not normally distributed. To compare categorical data, the Chi-squared (χ 2) test was performed. The exact test was used instead when the expected frequency was less than 5. p values lower than 0.05 were considered statistically significant. All statistical calculations were carried out using SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) Release 15 for Microsoft Windows.
Results
Patients
We detected SGA infants in ten of our NEC cases (group 1) (40%) and ten of our sepsis cases (group 2) (40%). There was a tendency for infants in group 2 to achieve full enteral feeds and better progression of feeds (93.3%) compared to those group 1 (68%). Group 1 showed more infants on nil per os (NPO) (32% vs. 7.7%) than group 2. The p value was 0.07. The median age of feeding intolerance was 12 days for group 1, while the median age for our examination was day 16 for group 2. The demographic and laboratory characteristics of the enrolled neonates on the day of examination are shown in Table 1.
There were two cases with positive blood cultures in group 1 compared to five cases with positive blood cultures in group 2. There was no statistical difference between them. There were three MRSA positive and one Enterobacter positive cultures in group 2 and one Klebsiella positive and one MRSA positive culture in group 1 (Table 2).
Radiology
Plain radiographic findings for the cases in group 1 showed free intraperitoneal air (gas under the diaphragm) in 1 case and abdominal distension in 24 cases (of which 21 cases showed mild diffuse distension and 3 cases showed severe diffuse distension). The group 2 cases were found to have rather normal bowel gas distribution patterns.
Sonographic findings
The predominant sonographic finding for group 1 was non-specific abdominal gaseous distension. One patient showed focal fluid collection; his abdominal X-ray showed severe diffuse distension. Bowel wall thickening on ultrasound was found in three cases. The patient with gas under the diaphragm on plain radiography was shown to have free intraperitoneal gas on ultrasound. The abdominal sonographic findings for group 2 were rather unremarkable.
Doppler findings
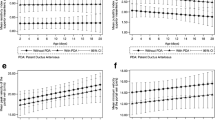
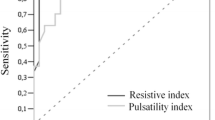
Doppler flow velocimetry revealed a statistically significant reduction of the SMA PSV and EDV and an increase in the CA PSV/SMA PSV ratio in group 1 cases compared to group 2 cases. There was no statistically significant difference in the CA Doppler indices between the two groups (Table 1).
Prospective data
On the basis of the plain radiography, of the 25 cases in group 1, we diagnosed 21 cases of stage I, 3 cases of stage II, and 1 case of stage III NEC. Through ultrasonography of the group 1 cases, we predicted an unfavorable outcome in four cases (one with focal fluid collection and three with bowel wall thickening, and a poor outcome in the case with intraperitoneal gas). The demographic and radiological features of the four group 1 cases with abnormal sonographic findings are presented in Table 3.
Cases 2, 3
Ultrasonography revealed bowel wall thickening, all had low SMA PSV, EDV, and the one that had an increased CA PSV/SMA PSV ratio exceeding 2 (case 3) had a poor outcome and died from septic shock (Figs. 1, 2, 3).
Case 4
The focal fluid collection on ultrasound indicated marked a reduction in the SMA BFV (PSV, EDV), with increased CA PSV/SMA PSV exceeding 2, with a poor outcome (death from septic shock).
SGA
We detected SGA infants in about 40% of each group. Comparing their laboratory and radiological features, we found a statistically significant increase in the incidence of thrombocytopenia, metabolic acidosis, and hyponatremia in the SGA case group. Moreover, there was a statistically significant decrease in SMA PSV and SMA EDV and an increase in the CA PSV/SMA PSV ratio in the NEC infants.
Outcome
There was a statistically significant increase in the mortality of the group 1 (NEC) cases (36%) compared to the group 2 cases (3.8%). p = 0.01. There were nine deaths in the NEC group: seven from septic shock and two from DIC. Moreover, only one neonate with pneumoperitoneum was operated upon, but he died from septic shock. There was one death from septic shock among group 2 cases.
Discussion
The results of the Doppler velocity measurements in the SMA showed reduction in the flow velocities in the NEC group in comparison with the sepsis cases with no feeding intolerance or clinical suspicion of NEC. Mostly, unremarkable Doppler changes were found in the celiac circulation in these cases compared to those associated with the septic cases. Coombs et al. also described such a reduction in the SMA PSV in neonates at risk of necrotizing enterocolitis. They speculated that the decreased blood-flow velocities in the SMA represented a compromised splanchnic circulation [15]. The significant reduction in the SMA EDV in the NEC cases in our study matched that in Murdoch et al.’s study, which found that the risk of NEC significantly decreased with the rise in the end-diastolic flow velocity and that infants who developed NEC later in the neonatal period had a high resistance pattern in the SMA, or the celiac artery, on their first day of life. That may be attributed to the fact that the vasoconstriction of the circulation in neonatal life has a role in the etiology of NEC [22].
Few of our patients had received enteral feeding at any stage in their lives. Enteral feeding has been shown to increase SMA velocity even in premature infants [23], so this may also have contributed to the reduction of the SMA flow velocities in our NEC group. We found no statistically significant difference in celiac artery blood-flow velocity indices between the 2 patient groups. Our findings agreed with an observation, made in the previous studies, that the risk of NEC was not associated with resistance in the celiac artery [15, 23]. The origins of the CA and SMA from the descending aorta are almost contiguous, yet they supply very different vascular beds. The flow within each vessel is subject to virtually identical upstream influences, such that differences in flow velocity between the two vessels measured at their origins from the aorta must reflect differences in their downstream vascular resistance [15].
The CA PSV/SMA PSV ratio was higher in our clinically suspected NEC cases; that was statistically significant. The CA PSV/SMA PSV ratio was = 1.59 ± 0.47 in NEC cases and 0.96 ± 0.22 in sepsis cases. Coombs et al. described an increase in the ratio of the celiac to the SMA systolic BFV in a heterogeneous group of patients at risk of necrotizing enterocolitis [15]. They ascribed the Doppler changes to a lower PSV in the SMA and a higher downstream vascular resistance of the SMA compared to that of the CA.
Among our clinically suspected NEC cases, a subgroup was predicted to have an unfavorable outcome of NEC based on the ultrasound findings, which took the form of bowel wall thickening (three cases) and focal fluid collection (one case) [21]. Among these four cases, only one improved: the case that had the lowest CA PSV/SMA PSV ratio. This finding may suggest a correlation between the CA PSV/SMA PSV ratio and the clinical outcome of the patient. An increase in the peak flow velocity within the SMA could represent an increase in the splanchnic flow volume or splanchnic vasoconstriction. However, the lack of an increase in diastolic flow velocities and the increase in the RI constitute a very strong argument for splanchnic vascular constriction [24]. Our results agree with previous research that states that conditions, such as established neonatal necrotizing enterocolitis, are associated with increased superior mesenteric artery (SMA) blood-flow velocity [23, 25]. Moreover, in an animal model of NEC, animals with severe NEC showed increases in PSV and RI values, which may suggest that profound bowel ischemia is a trigger for NEC in susceptible animals [24]. It seems obvious from our results that infants in the early stages of NEC had evidence of ischemia, while those in the later stages reflected splanchnic hyperemia, possibly with some element of vasoconstriction.
Our data accord with prospective data that showed low SMA in one infant before the onset of symptoms, suggesting that an ischemic insult to the gut may precede the onset of NEC with increased SMA velocities later, representing post-ischemic hyperemia [23]. Moreover, in a rabbit model, it was found that an insult in a susceptible animal may lead to early profound bowel ischemia, which may trigger NEC. However, in later stages, there are compensations for alterations in the splanchnic flow, and they return to the same level in animals without NEC [24]. Clearly, changes in perfusion cannot be the sole factor behind the development of necrotizing enterocolitis but are among several acting in concert. However, altered perfusion links some of the diverse known risk factors [15].
Our subjects were a heterogeneous group, and the insult that led to the abnormalities in perfusion may have occurred prenatally, perinatally, or postnatally. Although our patients were comparable in weight and age, we had ten SGA infants in each group. SGA neonates were at increased risk of NEC compared to AGA neonates [26]. The pathogenesis of NEC in SGA neonates is not clear. Several hypotheses have been postulated, focusing mostly on the link between hypoxic-ischemic injury to the intestinal tract, the physiological immaturity of the gastrointestinal tract, and alterations in the normal microbiological flora of the intestines [27].
Our study results showed increased mortality among NEC cases in comparison with sepsis cases. Four of the nine infants who died in the NEC group may have died from NEC or its complications. One of the advantages of assessing SMA Doppler flow velocimetry is that it should be possible to obtain the relevant measurements in all infants admitted for neonatal intensive care.
Our study had several limitations: it was a cross-sectional study with a small sample size. Moreover, antenatal Doppler for assessing the splanchnic circulation of infants at risk was not available. The absence of a confirmed diagnosis of NEC in all of the cases was a relative weakness of the present study, but the diagnosis was made independently of the Doppler results, precluding the possibility of bias.
Conclusion
This study indicates that SMA Doppler flow velocimetry may be clinically useful for identifying infants at increased risk of NEC. Reduction in the SMA PSV and the CA/SMA PSV ratio can be used to predict the presence of early NEC and can be used clinically to manage such patients as early as possible.
References
Stoll BJ (1994) Epidemiology of necrotizing enterocolitis. Clin Perinatol 21:205–218
Luedtke SA, Yang JT, Wild HE (2012) Probiotics and necrotizing enterocolitis: finding the missing pieces of the probiotic puzzle. J Pediatr Pharmacol Ther 17(4):308–328
Moss RL, Kalish LA, Duggan C et al (2008) Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: a multi-institutional study. J Perinatol 28(10):665–674
Lin PW, Nasr TR, Stoll BJ (2008) Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 32(2):70–82
Kempley ST, Murdoch E (2000) Splanchnic hemodynamic disturbances in neonatal sepsis. Arch Dis Child Fetal Neonatal 83:F139–F142
Grosfeld JL, Chaet M, Molinari F et al (1996) Increased risk of necrotizing Enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann Surg 224(3):350–355
Kim WY, Kim WS, Kim IO et al (2005) Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol 35(11):1056–1061
Faingold R, Daneman A, Tomlinson G et al (2005) Necrotizing Enterocolitis: Assessment of Bowel Viability with Color Doppler US. Radiology 235(2):587–594
Silva CT, Daneman A, Navarro OM et al (2007) Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 37(3):274–282
Miranda FC, Sameshima YT, Deutsch ADA et al (2009) Ultrasonography in diagnosis of necrotizing enterocolitis. Einstein 7(1):91–95
Dördelmann M, Rau GA, Bartels D et al (2009) Evaluation of portal venous gas detected by ultrasound examination for diagnosis of necrotising enterocolitis [abstract]. Arch Dis Child Fetal Neonatal Ed 94(3):F183–F187
Kim WY, Kim WS, Kim IO, Kang GH (2011) Bowel sonography in sepsis with pathological correlation: an experimental study. Pediatr Radiol 41(7):237–243
Dilli D, Suna Oğuz S, Erol R et al (2011) Does abdominal sonography provide additional information over abdominal plain radiography for diagnosis of necrotizing enterocolitis in neonates? [abstract]. Pediatr Surg Int 27(3):321–327
Shebrya NH, Amin SK, El-Shinnawy MA, Imam SS (2012) Abdominal ultrasonography in preterm necrotizing enterocolitis. Is it superior to plain radiography? Egypt J Radiol Nucl Med 43:457–463
Coombs RCM, Morgan EI, Durbin GM, Booth IW, McNeish AS (1992) Abnormal gut blood flow velocities in neonates at risk of necrotising enterocolitis. J Pediatr Gastroenterol Nutr 15:13–19
Staryszak J, Stopa J, Kucharska-Miąsik I, Osuchowska M, Guz W, Błaż W (2015) Usefulness of ultrasound examinations in the diagnostics of necrotizing enterocolitis. Pol J Radiol 80:1–9
Kar SS, Dube R, Mahapatro S (2013) The Role of Clinical Signs in the Diagnosis of Late-onset Neonatal Sepsis and Formulation of Clinical Score. Indian J Clin Pract 23(10):654–660
Carter BM (1992) Feeding intolerance in preterm infants and standard of care guidelines for nursing assessments. http://www.medscape.com/viewarticle/775632_4. Accessed Feb 2015
Kliegman RM, Walsh MC (1987) Walsh and Kliegman’s modifications. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 17(4):213–288
Battaglia FC, Lubchenco LO (1967) A practical classification of newborn infants by weight and gestational age. J Pediatr 71:159
Muchantef K, Epelman M, Darge K, Kirpalani H, Laje P, Anupindi SA (2013) Sonographic and radiographic imaging features of the neonate with necrotizing enterocolitis: correlating findings with outcomes. Pediatr Radiol. 43(11):1444–1452
Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GC, Kempley ST (2006) Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics 118:1999
Kempley ST, Gamsu HR (1992) Superior mesenteric artery blood flow velocity in necrotising enterocolitis. Arch Dis Child 67(7 Spec No):793–796
Choi YH, Kim IO, Cheon JE et al (2010) Doppler sonographic findings in an experimental rabbit model of necrotizing enterocolitis. J Ultrasound Med 29(3):379–386
Deeg KH, Rupprecht T, Schmid E (1993) Doppler sonographic detection of increased flow velocities in the celiac trunk and superior mesenteric artery in infants with necrotizing enterocolitis. Pediatr Radiol 23:578–582
Ree IM, Smits-Wintjens VE, Rijntjes-Jacobs EG et al (2014) Necrotizing enterocolitis in smallfor-gestational-age neonates: a matched case-control study. Neonatology 105:74–78
Lin PW, Stoll BJ (2006) Necrotising enterocolitis. Lancet 368:1271–1283
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hashem, R.H., Mansi, Y.A., Almasah, N.S. et al. Doppler ultrasound assessment of the splanchnic circulation in preterms with neonatal sepsis at risk for necrotizing enterocolitis. J Ultrasound 20, 59–67 (2017). https://doi.org/10.1007/s40477-016-0228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-016-0228-z