Abstract
Introduction
Imaging with 18F-FDG PET/CT represents the cornerstone in identifying ovarian cancer (OC) relapse, granting a significantly higher diagnostic accuracy than conventional imaging with CT or MR. Usually, 18F-FDG PET/CT is performed with a low-dose CT (18F-FDG PET/ldCT). In recent years, 18F-FDG PET integration with full-dose diagnostic CT and contrast medium (18F-FDG PET/ceCT) has been proposed. This approach entails a higher absorbed dose, and its clinical benefits are debated. In this study, a systematic review of the literature with a meta-analysis was carried out to compare 18F-FDG PET/ldCT 18F-FDG PET/ceCT in relapsing OC.
Materials and methods
We performed a systematic review of the literature through the most relevant databases and web sources. Original articles published before September 2020 and concerning a direct comparison of 18F-FDG PET/ceCT and 18F-FDG PET/ldCT in detecting an OC recurrence were considered. A proportion meta-analysis was then performed using a random-effects model.
Results
Out of 111 identified papers, a total of four (296 patients) were selected, all of them representing retrospective analyses. The pooled sensitivity of 18F-FDG PET/ldCT and 18F-FDG PET/ceCT in identifying OC relapse was 84% (95% CI 69–95) and 89% (95% CI 78–97), respectively. The increase of sensitivity when using 18F-FDG PET/ceCT over 18F-FDG PET/ldCT was 6% (95% CI 2–12).
Discussion
18F-FDG PET/CT showed an excellent diagnostic performance in suspected OC recurrence. Given the similar performance between PET/ldCT and PET/ceCT, the low-dose variant could be preferred to reduce absorbed dose and the patients’ discomfort during the examination.
The contrast-enhanced addition could be reserved in the case of PET/ldCT doubtful findings, which could affect the therapeutic management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma (OC), often diagnosed at an advanced stage, has a high likelihood of recurrence, even in case of a complete response after first-line treatment [1, 2], and most patients will relapse within the first two years after diagnosis [3]. OC relapse is often characterized by a peculiar peritoneal spreading associated with lymphatic and hematogenous metastatization [4,5,6]. Peritoneal involvement is the most common sign of disease diffusion, and its identification is particularly specific to confirm disease relapse, regardless of tumor markers (i.e., Ca-125). Although the effectiveness of an early versus delayed treatment of relapse is still under debate [3], the correct diagnosis of a peritoneal recurrence can identify those patients at higher risk of adverse outcome [7]. In this setting, imaging plays an essential role in the detection and quantification of peritoneal carcinomatosis, as well in the identification of lymph node and distant metastases, representing the most reliable and solid evidence on which the therapeutic decision-making is based [8, 9].
18F-FDG positron emission tomography/computed tomography (PET/CT) is a highly accurate imaging procedure in restaging OC. Indeed, 18F-FDG PET/CT has a high sensitivity for detection of OC relapse with a reported pooled sensitivity of 72% when surgical findings have been used as the reference standard [10] and reaching 80–100% when imaging follow-up has been considering the standard of truth [11,12,13,14].
Although CT and magnetic resonance imaging (MRI) are commonly used to identify recurrent ovarian cancer, their reliability is limited, especially in detecting small lesions or metastatic deposits on the visceral surfaces [15]. In particular, CT is burdened by very low sensitivity (25–50%) in detecting peritoneal metastases smaller than 1 cm [12, 16].
18F-FDG PET/CT is commonly performed using a “low-dose” CT (18F-FDG PET/ldCT) without contrast enhancement (Ce). This method grants an adequate CT-based attenuation correction of the PET data and allows the reader to pinpoint the sites of 18F-FDG accumulation while avoiding the high radiation burden that would be associated with a full-dose diagnostic CT [17].
However, some reports suggested that performing a 18F-FDG PET with a diagnostic CT and contrast medium (18F-FDG PET/CeCT) may provide better accuracy when compared with 18F-FDG PET with low-dose CT (18F-FDG PET/CT) [18,19,20]. On the other hand, more recent data, while still confirming the superiority of 18F-FDG PET/CT over CT, could not confirm the diagnostic superiority of 18F-FDG PET/ceCT over 18F-FDG PET/ldCT [21, 22].
The clarification of the usefulness of adding ceCT to the 18F-FDG PET would require a head-to-head comparison between these two PET/CT techniques. Therefore, this study aimed to conduct a systematic review of the literature to find original papers reporting a direct comparison of 18F-FDG PET/CT with 18F-FDG PET/CeCT to detect recurrent OC in the same patient population. Furthermore, a meta-analysis of available data was performed.
Materials and methods
The systematic review was performed in accordance with the PRISMA DTA statement [23].
Search strategy
A four-step search strategy was adopted and the literature search was performed by two authors independently (MM and AP). First, sentinel studies were sought in PubMed using the combinations of the following keywords: 18F-FDG PET/contrast-enhanced CT, 18F FDG PET/CT, contrast-enhanced CT, and ovarian cancer relapse; second, keywords and MeSH terms were identified in PubMed. Third, PubMed, CENTRAL, Scopus, and Web of Science were searched. Fourth, we sought studies evaluating the comparison between 18F-FDG PET/ldCT and 18F-FDG PET/CeCT in identifying relapse in patients with suspected OC recurrence (i.e., PubMed/MEDLINE, Embase and Web of Science). Papers published until August 31st, 2020 were considered. To identify additional studies and expand our search, the references of the articles retrieved were also screened. Furthermore, studies based on preclinical data, phantom studies, case series, case reports, studies including OC patients at the time of first diagnosis and studies with overlapping data were excluded. All remaining articles were screened, and only those reporting a head-to-head comparison between 18F-FDG PET/ldCT and 18F-FDG PET/CeCT in patients with suspected OC relapse were included.
Data extraction
The following information was extracted independently and in duplicate by two investigators (AP, MM) in a piloted form: (1) general information on the study (author, year of publication, country, study type, number of patients); and (2) sensitivity; (3) specificity, (4) accuracy, (5) standard of reference (SOR). For the extraction of data, full papers and supplementary data were searched; if data were missing, the authors were contacted via email. Data were cross-checked and any discrepancy was discussed.
Study quality assessment
The risk of bias of the studies included was assessed independently by two reviewers (AP, PT), according to QUADAS-2. According to the QUADAS-2 recommendations [24], the risk of bias was rated as low, high, or unclear.
Statistical analysis
A proportion meta-analysis was performed using a random-effects model. Pooled data were presented with 95% confidence interval (95% CI). Heterogeneity among studies was assessed utilizing I2, with 50% or higher being regarded as high. Publication bias was evaluated by means of Egger’s test [25].
The StatsDirect statistical software (StatsDirect Ltd.; Altrincham, UK) was used for the statistical analysis.
Results
Literature search
A total of 97 papers were identified after duplicate removal, and their titles and abstracts were analyzed.
Ten articles were excluded because they were case series or did not analyze or mention at least one of the following issues: 18F-FDG PET/CT, ovarian cancer, and relapse. Among the remaining eighty-seven papers, eighty-three had to be excluded because they did not fit the inclusion criteria (see Fig. 1 for details). Therefore, four articles were selected, and 296 patients were finally included (Fig. 1) [26,27,28,29]. These articles were published between 2008 and 2020, had sample sizes ranging from 24 to 132 patients treated with primary debulking surgery and platinum-based first-line chemotherapy.
Qualitative analysis (Systematic review)
All studies had a retrospective design. Two studies were carried out in Japan, one in Austria and one in Italy. The characteristics of the studies, patients and methods are summarized in Tables 1, 2 and 3.
Quality assessment of the studies
The risk of bias was assessed based on four study characteristics; these results are reported in Table 4. In general, the risk of bias ranged from low to non-evaluable. Specifically, in 2 out of 4 studies, the patient selection was unclear. The standard of reference applied in two studies was particularly appropriate to evaluate the sensitivity (i.e., surgical and histological findings), thus limiting the reliability of the diagnostic specificity.
Quantitative analysis (Meta-analysis)
The pooled sensitivity of 18F-FDG PET/ldCT and 18F-FDG PET/CeCT in identifying OC relapse was 84% (95% CI 69–95) and 89% (95% CI 78–97), respectively (Fig. 2). Heterogeneity was found (I2 78.1% and 71.8, respectively), and publication bias was absent (Egger test: p = 0.301 and p = 0.49).
When considering the pooled discrepancy in sensitivity between these two imaging procedures, the sensitivity increased by 6% (95%CI 2–12) using 18F-FDG PET/CeCT (I2 29.1% and Egger test p = 0.415) (Fig. 3).
Discussion
The aim of this systematic review and meta-analysis was to produce evidence-based data on the diagnostic comparison of two important imaging procedures, such as the 18F-FDG PET/ldCT and 18F-FDG PET/CeCT in a particular diagnostic setting as the suspected OC relapse. This issue is of particular interest, considering that the identification of OC relapse can often be challenging, especially in the case of small-sized peritoneal lesions. On the other hand, the confirmation of recurrence has a relevant implication in the patients’ clinical management.
In this field, choosing the most appropriate PET/CT procedure is relevant to offer the patients a personalized diagnostic iter to fit with their needs. To our knowledge, this is the first meta-analysis to focus specifically on this issue. An extensive database search was performed without time restrictions, and inclusion criteria were defined “a priori.”
In the vast majority of PET/CT scans, the CT component is performed with a low current setting and without intravenous contrast. Its purpose is to allow an attenuation correction of the PET dataset and obtain an anatomical correlation of radiotracer distribution. Indeed, the adoption of a low-dose, contrast-free CT protocol has been guided mostly by practical considerations to decrease radiation burden, reduce patient discomfort, and minimize scanning time. However, given the spatial resolution of 4–6 mm of currently available PET systems, and the fact that 18F-FDG is not a tumor-specific tracer, the detection of microscopic lesions remains challenging. This decrease in sensitivity occurs, especially when the anatomic details are unclear, and the delineation of the surrounding organs is hardly evident. To overcome these limitations, PET/CT with intravenous iodine contrast medium and full radiation dose, called PET/CeCT, has been gradually introduced in the clinical setting and was mainly used to reveal abdominal relapse, especially in case of hostile anatomy. In this setting, this hybrid protocol of PET/CeCT has been applied to colorectal [30, 31], ovarian [26,27,28,29], and uterine cancer, showing promising results [32, 33].
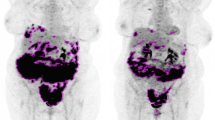
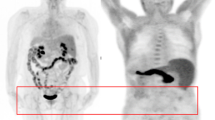
However, when the OC relapse was considered, only a few studies investigated this interesting issue; these articles reported conflicting results. Three studies [26, 27, 29] did not find any significant difference between PET/CT and PET/CeCT at the patient level; on the contrary, one [28] showed a significantly higher sensitivity of 18F-FDG PET/CeCT in detecting OC relapse, especially in the case of peritoneal and retrovesical metastases. Please see Fig. 4 for an example in which the integration of ceCT did not attain an improvement of the diagnostic accuracy; Fig. 5, on the other hand, highlights an example of more accurate diagnostic procedure through the use of PET/ceCT.
Fifty-three-year-old female with suspected OC relapse. 18F-FDG PET/ld CT (a, b) detected a faint but focal tracer uptake with apparent localization on a pelvic portion of the small bowel (red arrows). After injection of contrast enhancement, 18F-FDG PET/CeCT (c, d) showed that the tracer uptake corresponded to a pathological peritoneal nodule adjacent to small intestine (red arrows)
Our study confirmed an irreplaceable role of 18F-FDG PET/CT in the diagnosis of recurrent OC with a pooled sensitivity of 84% that is in line with previous meta-analyses published on this topic [10,11,12,13,14]. In parallel, we found that, although 18F-FDG PET/CeCT is more sensitive than 18F-FDG PET/ldCT, the slight difference in diagnostic performance does not support its routine use in clinical practice.
In light of this evidence, it might be advisable to integrate PET/CT with contrast enhancement on a case-by-case basis in circumstances where an important discrepancy between FDG uptake and low-dose CT findings reduce the interpretation reliability, thus affecting the therapeutic decision-making process. In other words, contrast enhancement could be injected only in the case of doubtful findings on 18F-FDG PET/ldCT (i.e., FDG uptake without any densitometric correlation). This is feasible after a quick evaluation of the images by the on-duty nuclear medicine physician in adequately scheduled hybrid imaging session. Another possibility to manage this tricky imaging interpretation on 18F-FDG PET/ldCT may be to follow, at proper time interval, the patients with 18F-FDG PET/CeCT especially in those for which a consensus is not achieved. This is what is possible to obtain over the years after a profitable collaboration with the radiologists saving cost, time, dose exposure and improving the quality of the PET/CT reports.
This case-by-case approach needs shared acquisition protocols and close cooperation between Nuclear Medicine and Radiology Departments and their Physicians and Technicians.
In all other cases, given the excellent accuracy of PET/CT, the association with contrast medium and diagnostic CT could be discouraged. Indeed, a significant reduction of radiation exposure would be obtained, reducing at the same time the possibility of renal dysfunction, which is not uncommon in ovarian cancer patients [34].
Some limitations, however, should also be mentioned. First, only four papers were included in this meta-analysis with a limited number of patients (i.e., 296 pts). However, this study is based on a direct head-to-head comparison or the two diagnostic procedures performed on the same patient simultaneously. This particular selection of the studies allows a more appropriate interpretation of the data.
A second limitation of our analysis is related to the different truth standards considered in the different studies. Indeed, in the two studies, including most patients, the gold standard was mostly based on follow-up imaging procedures rather than on histopathology. This approach may have overestimated the sensitivity of the PET/CT procedures.
Third, only 3 out of the four studies reported true-negative results. From this point of view, we preferred not to report an unreliable evaluation of the specificity of the two imaging procedures.
Lastly, a significant statistical heterogeneity was found among the included studies about the pooled sensitivity of 18F-FDG PET/ldCT and 18F-FDG PET/CeCT. This heterogeneity could be explained by the different characteristics of patients, index test and comparison in the included studies (see Tables). Unfortunately, the available data were limited to further explore this heterogeneity by using subgroup analyses or meta-regression analysis. Conversely, we did not find a significant publication bias in our analysis. Overall, based on our systematic review, we suggest performing more studies and in particular randomized and large multicentre prospective studies and cost-effectiveness analyses to compare 18F-FDG PET/ldCT and 18F-FDG PET/CeCT in recurrent OC.
Conclusion
18F-FDG PET/ldCT and 18F-FDG PET/CeCT are both sensitive in detecting OC relapse. Furthermore, the discrepancy in sensitivity between the two imaging procedures is 6% in favor of 18F-FDG PET/CeCT. These characteristics should be considered in the clinical context for the management of every patient.
References
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Moufarrij S, Dandapani M, Arthofer E et al (2019) Epigenetic therapy for ovarian cancer: promise and progress. Clin Epigenetics 11:7
Giornelli GH (2016) Management of relapsed ovarian cancer: a review. Springerplus 28(5):1197
Meyers MA (1973) Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med 119:198–206
Berek JS, Kehoe ST, Kumar L, Friedlander M (2018) Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 143(Suppl 2):59–78
Pradeep S, Kim SW, Wu SY et al (2014) Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell 26:77–91
Simojoki M, Santala M, Vuopala S et al (1999) The prognostic value of peritoneal cytology in ovarian cancer. Eur J Gynaecol Oncol 20:357–360
Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM (2003) Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med 44:1407–1412
De Gaetano AM, Calcagni ML, Rufini V, Valenza V, Giordano A, Bonomo L (2009) Imaging of peritoneal carcinomatosis with FDG PET-CT: diagnostic patterns, case examples and pitfalls. Abdom Imaging 34:391–402
Han S, Woo S, Suh CH, Lee JJ (2018) Performance of pre-treatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography for detecting metastasis in ovarian cancer: a systematic review and meta-analysis. J Gynecol Oncol 29:e98
Son H, Khan SM, Rahaman J, Cameron KL, Prasad-Hayes M, Chuang L, Machac J, Heiba S, Kostakoglu L (2011) Role of FDG PET/CT in staging of recurrent ovarian cancer. Radiographics 31:569–583
Pannu HK, Bristow RE, Cohade C, Fishman EK, Wahl RL (2004) PET-CT in recurrent ovarian cancer: initial observations. Radiographics 241:209–223
Kim CK, Park BK, Choi JY, Kim BG, Han H (2007) Detection of recurrent ovarian cancer at MRI: comparison with integrated PET/CT. J Comput Assist Tomogr 316:868–875
Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE, Lee JM (2002) Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. Am J Roentgenol 179:391–395
Cengiz A, Koç ZP, Özcan Kara P, Yürekli Y (2019) The Role of <sup>18</sup>F-FDG PET/CT in detecting ovarian cancer recurrence in patients with elevated CA-125 Levels. Mol Imaging Radionucl Ther 28(1):8–14
Kim HJ, Kim JK, Cho KS (2004) CT features of serous surface papillary carcinoma of the ovary. ARJ Am J Roentgenol 183:1721–1724
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, European Association of Nuclear Medicine (EANM) (2015) Krause BJ FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42(2):328–354
Klumpp BD, Schwenzer N, Aschoff P et al (2013) Preoperative assessment of peritoneal carcinomatosis: intraindividual comparison of 18F-FDG PET/CT and MRI. Abdom Imaging 38:64–71
Pfannenberg C, Konigsrainer I, Aschoff P et al (2009) (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 16:1295–1303
Sebastian S, Lee SI, Horowitz NS et al (2008) PET-CT vs. CT alone in ovarian cancer recurrence. Abdom Imaging 33:112–118
Risum S, Hogdall C, Markova E et al (2009) Influence of 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography on recurrent ovarian cancer diagnosis and on selection of patients for secondary cytoreductive surgery. Int J Gynecol Cancer 19:600–604
Bhosale P, Peungjesada S, Wei W et al (2010) Clinical utility of positron emission tomography/computed tomography in the evaluation of suspected recurrent ovarian cancer in the setting of normal CA-125 levels. Int J Gynecol Cancer 20:936–944
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA-DTA Group (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA Statement. JAMA 319(4):388–396
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 18(155):529–536
Sadeghi R, Treglia G (2017) Systematic reviews and meta-analyses of diagnostic studies: a practical guideline. Clin Transl Imaging 5:83–87
Kitajima K, Murakami K, Yamasaki E et al (2008) Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging 35:1439–1448
Dirisamer A, Schima W, Heinisch M et al (2009) Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol 69:536–541
Kitajima K, Ueno Y, Suzuki K et al (2012) Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT scans for diagnosing ovarian cancer recurrence. Eur J Radiol 81:3557–3562
Gadducci A, Simonetti E, Manca G et al (2020) Positron emission tomography/computed tomography in platinum-sensitive recurrent ovarian cancer: a single-center Italian study. Anticancer Res 40:2191–2197
Dirisamer A, Halpern BS, Flöry D, Wolf F, Beheshti M, Mayerhoefer ME, Langsteger W (2010) Performance of integrated FDG-PET/contrast-enhanced CT in the staging and restaging of colorectal cancer: comparison with PET and enhanced CT. Eur J Radiol 73:324–328
Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE (2007) Non-enhanced CT versus contrast-enhanced CT in integrated PET/CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging 34(10):1627–1634
Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Morita S, Suganuma N, Sugimura K (2009) Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent uterine cancer: comparison with PET and enhanced CT. Eur J Nucl Med Mol Imaging 36:362–372
Kitajima K, Suzuki K, Nakamoto Y, Onishi Y, Sakamoto S, Senda M, Kita M, Sugimura K (2010) Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT studies for the diagnosis of uterine cancer recurrence. Eur J Nucl Med Mol Imaging 37(8):1490–1498
Ozkok A, Edelstein CL (2014) Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014:967826
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study, informed consent is not required.
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Massollo, M., Treglia, G., Trimboli, P. et al. Head-to-head comparison between 18F-FDG PET/low-dose CT and 18F-FDG PET/contrast-enhanced CT in relapsing ovarian carcinoma: a systematic review and meta-analysis. Clin Transl Imaging 9, 73–81 (2021). https://doi.org/10.1007/s40336-020-00403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00403-y