Abstract
The mitochondrial translocator protein (18 kDa; TSPO) is involved in a wide array of physiological processes importantly including cholesterol transport, steroidogenesis and immunomodulation. In the central nervous system (CNS), TSPO expression regionally increases in glial cells upon brain insult with a differential pattern suggestive of cell-specific functions in inflammation and repair. These properties have made TSPO a valuable marker to assess the state, and progression of diverse neurological and psychiatric conditions, including traumatic brain injury, stroke, neurodegenerative diseases, anxiety, depression and schizophrenia. In the past years, an increasing number of radiolabeled TSPO ligands for the visualization and quantification of TSPO through positron emission tomography (PET), single-photon emission tomography (SPECT) and magnetic resonance imaging (MRI) have been developed in the pursuit of higher sensitivity and specificity for clinical applications. However, TSPO is not the only molecule holding great potential as an imaging marker of neuroinflammation; cell adhesion molecules, such as VCAM-1 and ICAM-1, the myeloperoxidase, matrix metalloproteinases, the cannabinoid receptor 2 (CB2), P2X7, cyclooxygenase 1 (COX-1), free radicals and leukocyte populations have also been subjects of study as targets to image inflammatory processes in the injured or diseased brain. In this review, we present the most relevant aspects of TSPO molecular features that fundament its imaging applications in the context of neuroinflammation, and comment on the development of imaging agents and strategies targeting TSPO as well as other molecules and cells implicated in inflammatory processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mitochondrial translocator protein (18 kDa; TSPO) was previously known as the peripheral-type benzodiazepine receptor (PBR) due to its ability to bind the benzodiazepine diazepam. However, its structure, expression, pharmacology and functions are different from those of the central benzodiazepine receptor [1] leading to renaming the protein TSPO [2]. The availability of high affinity specific TSPO drug ligands allowed for the assessment of the function of the protein. TSPO participates in many physiological processes, including metabolism and cellular respiration, cholesterol transport and steroidogenesis, immunomodulation, porphyrin transport and heme biosynthesis [2, 3]. It has also been proposed that TSPO may play roles in apoptosis and gluconeogenesis [4, 5].
TSPO is expressed in very low levels in the central nervous system (CNS) under physiological conditions; however, its expression levels increase in astrocytes and microglia as a result of brain injury and inflammatory processes [6], and thus it has been a subject of intense study interest, particularly during the past years. The binding of labeled TSPO ligands can be visualized and quantified by in vivo imaging techniques, and has become an important approach to study various neurological and psychiatric conditions. Despite TSPO has received a lot of attention in recent years as a target to image neuroinflammation and is the focus of this review, we will note that other inflammatory cells and molecules have been explored for imaging purposes as well in a diversity of neurological conditions involving inflammatory processes.
TSPO
Expression
The Tspo gene is evolutionarily conserved in most organisms [7]. In human beings, this gene is located on chromosome 22q13.3 and consists of 4 exons encoding a 169 amino acids protein [8, 9]. Although TSPO is expressed in many organs, its highest levels can be found in tissues containing steroid-synthesizing cells, such as adrenals, gonads and the brain [10]. Virtually all immune cells express TSPO [11]. In the brain, TSPO expression was considered to be specific for activated microglia and infiltrating macrophages; nevertheless, currently it is known that reactive astrocytes also express TSPO, although with a different spatiotemporal profile [5]. In addition, certain neuronal cell types have been also shown to express TSPO, such as those of the olfactory bulb [12] and dorsal root ganglia sensory neurons [13], as well as neural stem cells and post-mitotic neuronal precursors in developing or damaged brain regions [14].
Protein structure and binding
TSPO is a ubiquitous protein encoded by nuclear DNA, localized primarily in the mitochondrial outer membrane [15]; however, there is evidence that it may also localize to other subcellular structures such as the nucleus [16] and the plasma membrane [17].
TSPO’s protein structure, as initially suggested by its amino acid sequence, possesses five TM alpha helices with two extra- and two intra-mitochondrial loops. The five TM helices appear to be rigid while the terminal portions of the protein may remain flexible to allow the conformational changes necessary for protein–protein interactions. The N-terminus is located on the inside of the mitochondria while the C-terminus is located towards the cell cytoplasm and highly positively charged. A binding pocket is formed by the TM helices in the upper part of the extra-mitochondrial side, closed by a long loop between TM1 and TM2; nonetheless, additional binding sites may provide a higher level of the protein’s functional regulation [18–21].
It has been proposed that TSPO has a structure that facilitates cholesterol translocation [22–24]. A sequence denoted as the cholesterol recognition amino acid consensus (CRAC) motif is located at the C-terminal region of the protein, comprising residues 147–159 [23]. Within this motif, amino acids Y153 and R156 are believed to be critical for TSPO’s interaction with cholesterol [25].
Evidence exists that TSPO can polymerize by binding to other TSPO proteins or interacting with a range of different molecules. TSPO’s ability to form homopolymers appears to increase with mitochondrial activity and the generation of reactive-oxygen species (ROS) [26]. In support of these findings, recent structure–function studies demonstrated the role of TSPO in ROS generation [21]. Additionally, its protein structure is stabilized by ligand binding, which might mediate cholesterol transport by promoting binding to TSPO polymers [18]. However, polymerization has been found to increase ligand binding but reduce binding to cholesterol, suggesting an important involvement of polymerization in the mediation of TSPO function regarding cholesterol transport [20, 24, 26].
Specific mitochondrial proteins interact with TSPO, suggesting the presence of complexes formed by proteins from both the outer and inner mitochondrial membranes, including the 32 kDa voltage-dependent anion channel (VDAC) and the adenine nucleotide transporter (ANT) [27], together with other cytosolic and mitochondrial proteins [28]. These proteins include the mitochondrial permeability transition pore (MPTP) components, the peripheral benzodiazepine receptor-associated protein 1 (PRAX-1) [29], steroidogenic acute regulatory protein (STAR) and peripheral benzodiazepine receptor-associated protein (PAP7), a member of acyl coenzyme A (acyl-CoA) binding domain-containing proteins [28, 30] and ATPase family AAA domain-containing protein 3A (ATAD3A) [31]. Hence, it is likely that TSPO functions may be determined by the tissue- and cell-specific composition of mitochondrial membranes and mitochondria-associated organelles [32, 33]. The fact that cytosolic proteins can also interact with TSPO suggested a role of TSPO as a mitochondrial anchor transducing intracellular signals to mitochondria. As an example, it is suggested that acyl-CoA, or its binding proteins, may regulate TSPO function in the mitochondria and that TSPO participates in autocrine and paracrine signaling responses of glial cells to injury and pathogenic stimuli, mainly coming from the observations of TSPO interactions with endozepines in the peripheral and central nervous systems [3].
TSPO binds putative endogenous ligands, including cholesterol, protoporphyrin IX, phospholipase A2 (PLA2) and diazepam binding inhibitor (DBI) [34], a member of acyl-CoA binding domain-containing proteins [30], and a range of structurally diverse synthetic ligands including benzodiazepines, such as Ro5-4864 and diazepam, and isoquinoline carboxamide derivatives, such as PK 11195 [4]. In the CNS, PK 11195 reduces microglial activation and production of pro-inflammatory cytokines [35, 36]. How TSPO endogenous ligands, which are present at various levels in the tissues and cells examined, may affect endogenous drug ligand occupancy, affinity and residency time is unknown. This is a research question to be explored, considering the increasing use of radiolabeled TSPO drug ligands as imaging tracers (discussed later in this review) and potential changes in the levels of these endogenous ligands in various disease states.
Cholesterol and porphyrins show high affinities for TSPO, although porphyrins have affinity at the high nanomolar range, compared to the low nanomolar affinity for cholesterol [23, 37]. While cholesterol binds to the C-terminus, other ligands bind mostly to a region within the N-terminus [23, 38], although additional both steroidal and non-steroidal compounds binding at the CRAC motif have been recently reported [39, 40].
The classical/diagnostic synthetic ligands for TSPO are PK 11195 and Ro5-4864 (benzodiazepine 7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one). These ligands have been crucial for the characterization of TSPO’s expression and function and, particularly PK 11195, for the development of new TSPO ligands. For example, novel compounds synthesized to study TSPO’s binding properties have suggested the existence of multiple binding sites with possible allosteric effects in the human TSPO [41].
A single nucleotide polymorphism in the exon 4 of the TSPO gene, rs6971, resulting in the substitution of the amino acid alanine for threonine at position 147 of the TSPO protein (A147T variant), has proven to affect ligand-binding affinity [42, 43] of TSPO and affecting pregnenolone biosynthesis [20, 42, 44], although this might only be true for certain ligands as it was recently demonstrated that the variant shows an affinity to PK 11195 comparable to that observed for the wild-type protein, and retains the structure and dynamic profile [20, 45].
The ability of TSPO to bind cholesterol via the CRAC domain, and the ability of A147T polymorphism to affect cholesterol binding was recently confirmed in a series of structure–function studies where the presence of a cholesterol binding enhancement motif able to induce bacterial TSPO to bind cholesterol was also shown [19, 46].
The synthesis of the TSPO-specific ligand indol-acetamide FGIN-1-27 (2-[2-(4-fluorophenyl)-1H-indol-3-yl]-N,N-dihexylacetamide) [47] advanced the understanding of the TSPO pharmacology and led to the synthesis of a series of ligands which were safe in humans, such as emapuril (XBD-173; N-benzyl-N-ethyl-2-(7-methyl-8-oxo-2-phenyl-purin-9-yl)acetamide), which was shown in a clinical study to be safe and exert anti-anxiety activity while, in contrast to benzodiazepines, did not cause sedation and withdrawal symptoms [48].
Functions
It has been largely accepted that TSPO mediates various mitochondrial functions, including cholesterol transport and steroid hormone synthesis, porphyrin transport and heme biosynthesis, mitochondrial respiration, MPTP opening, calcium homeostasis, oxidation, apoptosis and cellular proliferation and differentiation [2, 49]. However, few of these functions have been directly demonstrated as most TSPO functions have been so far studied through its ligands’ actions. A function for TSPO in normal emotional regulation has also been suggested by the findings of a genetic association of the rs6971 polymorphism with bipolar disorder and adult separation anxiety disorder (ASAD) [50, 51].
TSPO can be found in intracellular locations other than mitochondria, such as the (peri)nuclear region and plasma membrane, playing different functions on a location-dependent manner. Nevertheless, non-mitochondrial TSPO, representing less than 5 % of TSPO [17], has received little attention so far. It is important to note that a Tspo paralogous gene, Tspo2, has been identified encoding an evolutionarily conserved family of proteins that arose by gene duplications [52]. Comparative analysis of Tspo1 and Tspo2 structure and function indicated that TSPO2 was characterized by the loss of diagnostic drug ligand-binding but retention of cholesterol-binding properties, and is involved in cholesterol redistribution during erythropoiesis [52].
The complex formed by mitochondrial TSPO in association with VDAC and ANT has been suggested to have a role in apoptosis, possibly through MPTP opening [34]. However, treatment with TSPO ligands has shown the ability to provide neuroprotection [53–55]. In fact, ligands such as PK 11195 and Ro5-4864 possess both pro- and anti-apoptotic properties, making PK 11195 and other TSPO ligands interesting targets for cancer therapies. Although the pro-apoptotic effects may or may not involve TSPO, the anti-apoptotic effects shown by these molecules are likely to take place through inhibition of TSPO’s apoptotic function, for which TSPO remains as a potential therapeutic target [11].
TSPO is thought to be important for tissue development and function. It may also participate in the biogenesis of mitochondrial membranes during cell proliferation and/or repair [3]. Furthermore, resulting from studies in animal models of neurodegenerative diseases, the observations that TSPO up-regulation in microglia and astrocytes associates with deleterious and beneficial effects, respectively [56], not only raise the possibility for a role of TSPO in regenerative processes but also suggest cell-specific functions. It has been shown that TSPO can modulate steroid production and, in turn, steroids are also able to affect TSPO’s ligand-binding properties [57]. A series of studies have supported a role for TSPO in inflammatory processes in peripheral tissues and the nervous system as a response to injury and disease, possibly through the regulation of steroid production [11, 58]. Nevertheless, recent studies have challenged the view of TSPO’s role in steroidogenesis, viability [59, 60] and MPTP [61], showing that knock-down/-out of TSPO in animal models does not affect steroid hormone biosynthesis, cell viability or MPTP activation even when induced by TSPO-binding molecules, suggesting that the mechanisms for these actions may not involve TSPO, as previously believed, and thus providing evidence to refute the major previously proposed functions for TSPO. Nonetheless, conditional knockout mice lacking TSPO in steroidogenic cells recently suggested that TSPO is indeed necessary for embryonic development during the pre-implantation phase and has a role in the mediation of stress responses [62]. Interestingly, similar discrepancy in genetic models has been reported for mitochondrial VDAC, where deletion of Vdac1 has been reported as both lethal and viable with minor phenotype [63]. Taken together these findings suggest that genetic models have to be analyzed with caution when dealing with evolutionary conserved proteins and conclusion should be reached when combining with biochemical, pharmacological and structural studies.
The TSPO-mediated pharmacology of cholesterol transport and steroidogenesis is well defined [24, 64], and supported by the biochemical and recent structural studies [2, 3, 19–21, 24, 45]. Indeed, TSPO drug ligands offered pharmacological means to regulate neurosteroid formation both in vitro and in vivo [3, 65]. This field expanded to neuropsychiatric and neurodegenerative disorders as well as neurotrauma [34, 66] and led to the use of TSPO drug ligands to alleviate neuropsychiatric disease symptoms mediated by increased neurosteroid formation in brain [3].
TSPO seems to be a sensitive biomarker of brain damage and neurodegeneration, particularly of inflammation and reactive gliosis. In the CNS, up-regulation of TSPO in response to damage is delayed in astrocytes as compared to microglia; however, the up-regulation in astrocytes is long-lasting, suggesting it may be crucial for its functions in neuronal survival and regeneration [67, 68]. Hence, TSPO expression can be expected to result modified in response to stressful stimuli and show alteration in diverse neurological and psychiatric conditions. A schematic representation of TSPO topology, effectors and functions is depicted in Fig. 1.
Schematic representation of TSPO topology, effectors and functions. TSPO is localized in the outer mitochondrial membrane, where it is found either alone or as part of a multiprotein complex together with VDAC and ATAD3. Cytosolic proteins, such as ACBD3, can also associate with TSPO. In active steroidogenic cells, this complex also contains the CYP11A1 enzyme responsible for the metabolism of cholesterol to pregnenolone, precursor of androgen, estrogen, mineralocorticoids, glucocorticoids and neurosteroids. TSPO drug ligands (e.g., PK 11195, Ro5-4864, FGIN-1-27, DAA1106, AC5216/XBD173, etifoxine), endogenous ligands (e.g., protoporhyrin IX, DBI and its metabolite TTN, PLA2), as well as associated proteins (e.g., ACBD3, PRAX1), could act as effectors and sometimes regulators of TSPO function. TSPO drug ligands could also serve as imaging agents to assess TSPO protein levels as related to various disease states (e.g., neurodegeneration, traumatic brain injury, cancer). TSPO functions, as assessed by the effects of its ligands, in mitochondrial respiration, cholesterol binding, import and steroidogenesis, porphyrin binding for heme biosynthesis, protein import for membrane biogenesis, control of free radical production, regulation of MPTP, apoptosis, cell proliferation, microglia activation and immune function
Radiolabeled ligands and neuroimaging
TSPO is expressed at low levels in the normal brain but is locally up-regulated in sites of injury, possibly even before evident pathological and structural changes can be observed. This has provided a sensitive approach to accurately localize lesions and active disease processes [3] through the in vivo visualization and quantification of the binding of radiolabeled TSPO ligands as imaging agents for, mainly, positron emission tomography (PET) and, in a lesser extent, single-photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI).
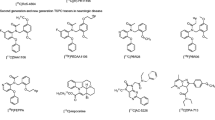
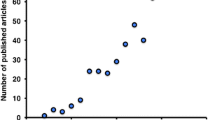
While Ro5-4864 and PK 11195 are the prototype diagnostic ligands for TSPO and have been long used to characterize the protein’s function, the development of new synthetic ligands has opened the doors for a wider array of applications which include, importantly, in vivo imaging of activated microglia and macrophage infiltration in the CNS, important markers of ongoing inflammation, in different pathological conditions. A range of synthetic TSPO ligands of diverse structural classes has emerged in past years, such as isoquinoline carboxamides, benzothiazepines and benzoxazepines, indoleacetamides, pyrazolopyrimidines, vinca alkaloids and aryloxyanilides, among others [69]. Examples of such compounds include DPA-713 and DPA-714 [70], DAA1106 [71] and its derivative, FEDAA1106 [72], PBR28 [73] and PBR111 [74], AC-5216 [75], CLINDE [76] and vinpocetine [77] (Table 1). Nevertheless, several characteristics need to be taken into account when evaluating these compounds, such as sensitivity, specificity, stability, clearance, species-specific metabolism and even the variable binding affinity in humans resulting from the rs6971 genetic polymorphism, for which new and improved TSPO ligands continue to be developed and evaluated for clinical purposes.
Some of the developed compounds may as well hold therapeutic potential for a number of neurological and psychiatric conditions, whether they selectively bind to TSPO or also bind to gamma-aminobutyric acid A (GABAA), or other types of receptors, which adds value to research in this field. For example, etifoxine, a benzoxazine, binds to TSPO and GABAA receptors and, while its anxiolytic effects have been suggested to involve the GABAA receptors [78], its neuroregenerative effects have been mainly attributed to TSPO [79].
TSPO neuroimaging applications
Up-regulation of TSPO expression in glial cells in response to injury and inflammation is associated with brain pathology [6] and its timing can track glial cell activation also during regenerative processes, which makes TSPO imaging a valuable tool to assess state, progression and repair in heterogeneous brain lesions, such as those resulting from traumatic brain injury (TBI) [66] and stroke [80]. Although up-regulation of TSPO has also been observed in the normal ageing brain using tracers such as [11C]PK11195 and [11C]vinpocetine [77, 81], suggesting that the activation of glial cells as well develops as part of the ageing process, the association between ageing and TSPO up-regulation remains controversial. A more recent PET study using the second-generation tracer [18F]FEPPA examined this association, finding no differences in TSPO expression related to normal ageing [82]. It is possible that the discrepancies between studies might be the result of not only the binding properties of different TSPO radioligands, but also of varying outcome measures and methods of analysis.
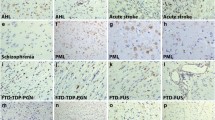
Up-regulation of TSPO at sites of neurodegeneration, and even more remote brain regions, has been observed in patients and animal models of diseases such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and frontotemporal dementia (FTD) [3, 83–85]. It is important to note that, in some cases (e.g., AD), the use of a specific TSPO tracer failed to confirm the increases in TSPO reported using other tracers [86]. In contrast to neurodegenerative diseases, and consistent with the role of neurosteroids as modulators of depression and anxiety [87], decreases in TSPO have been reported in a number of psychiatric disorders with anxious or depressive symptoms, including adult separation anxiety, post-traumatic stress disorder (PTSD) and schizophrenia [3, 88].
TSPO levels are increased in several types of cancer, including brain tumors, and thus it has been gaining attention in this area not only as an imaging agent but also as a target for the development of anti-cancer treatments [89–92]. In the peripheral nervous system, up-regulation of TSPO in Schwann cells, macrophages and neurons occurs in response to peripheral nerve injury, and TSPO ligands have shown to promote repair and provide neuroprotection [79, 93–96], in many cases likely to be mediated by increased neurosteroid production [3, 97].
Ten studies are currently registered using TSPO as a marker of neuroinflammation, mainly through PET imaging, in clinical trials for TBI, ALS, MS, PD, AD, mild cognitive impairment (MCI), schizophrenia, psychosis, major depressive disorder (MDD) and brain metastasis [ClinicalTrial.gov; accessed in June, 2015].
Other targets for imaging neuroinflammation
Besides TSPO, different types of molecules are also under investigation as targets to image ongoing neuroinflammatory processes in animal models and patients of a variety of neurological and psychiatric disorders using common and emerging imaging technologies (Table 1). One type of such is the cell adhesion molecules. Cell adhesion is essential for the migration of immune-competent cells to sites of injury, including leukocyte entry into the brain [98]. These molecules thus play an important role in inflammatory processes and have been targeted to observe infiltrating neutrophils, macrophages and T lymphocytes as well as activated platelets and endothelial cells using, for example, antibodies directed against the vascular cell adhesion molecule-1 (VCAM-1) and the intracellular adhesion molecule 1 (ICAM-1) conjugated with micron particles of iron oxide (MPIO) or paramagnetic liposomes [99–102], or a glyconanoparticle conjugated with syalil Lewisx (GNP-sLex) for the E- and P-selectins [103].
Strategies to measure oxidative stress-induced mitochondrial dysfunction and proteolytic activity have also been a subject of study to shed light on the inflammatory processes resulting from brain injury and disease. For example, approaches targeting free radicals through electron paramagnetic resonance imaging (EPRI), computerized electron spin resonance tomography (ESR-CT) or Overhauser magnetic resonance imaging (OMRI) using agents (or its precursors) with unpaired electrons, such as nitroxide radicals and methoxycarbonyl-PROXYL (MC-PROXYL) probes [104–107], have been developed. The myeloperoxidase (MPO) has been targeted using the MRI probe Gd-bis-5-HT-DTPA (MPO-Gd) [108] and luminol [109, 110]. Furthermore, a method has been proposed in which a combination of luminol and lucigenin bioluminescence enables the specific detection of acute (MPO-dependent) and chronic (NADPH oxidase-dependent) inflammation, respectively [111].
In response to CNS insult, infiltrating leukocytes, microglia and endothelial cells show increases in the expression of matrix metalloproteinases (MMPs) [112], whose activity is believed to play important roles in inflammation and blood–brain barrier breakdown. MMP activity has been visualized through non-invasive near-infrared fluorescence (NIRF) and, more recently, optical imaging, using activatable probes [113–115]. Imaging of MMP activity has been proposed as a useful measure to monitor anti-inflammatory effects [116] and, as well due to the important implication of MMPs in tumor formation, new combinatorial imaging methods are currently under exploration.
Much attention has been given to the cannabinoid receptor 2 (CB2), for which a diversity of PET tracers [117–124] and NIRF imaging probes [125, 126] have been developed. However, additional molecules implicated in a range of inflammatory processes have been targeted to image neuroinflammation, some examples include: cathepsin B, imaged using NIRF agents [127]; the cyclooxygenase 1 (COX-1), targeted using the PET tracer [11C]ketoprofen methyl ester [128]; P2X7, which has been gaining attention, targeted using the recently synthesized PET tracer [11C]A-740003 [129, 130]; I2-imidazoline receptors (I2Rs) detected using the PET tracer [11C]FTIMD [131]; and β-glucuronidase activity, visualized using [18F]FEAnGA for PET [132]. Toll-like receptors, receptor for advanced glycation end products, cytokines and chemokines [133] may represent good targets to image inflammatory status in the injured or diseased brain.
Finally, radiolabels such as technetium-99m (99mTc), indium-111 (111In), [18F]FDG and 64Cu enable the visualization of infiltrating leukocytes and may be used for labeling of specific leukocyte subpopulations. Phagocytic cells can be labeled using perfluorocarbons 19F and gadofluorine M. Another approach is to use iron oxide particles, such as ultra-small superparamagnetic iron oxide (USPIO), superparamagnetic iron oxide (SPIO) and MPIO, for in vitro or in vivo labeling [134].
Conclusions
Studies in animal models and early trials in humans suggest that the mitochondrial TSPO may be a sensitive biomarker of neuroinflammation and reactive gliosis. Although changes in TSPO expression are likely indicative of changes in mitochondrial function, the pathophysiological significance of increased TSPO expression in these processes is not well understood. However, the availability of specific imaging probes for TSPO makes this target attractive to assess the evolution and response to treatment of diseases with a major neuroinflammatory component.
In recent years, different types of molecules involved in inflammatory processes, other than TSPO, have been targeted for neuroimaging purposes. However, these studies have been relatively limited, thus rendering difficult the objective comparison of their advantages and disadvantages over TSPO imaging in the assessment of the inflammatory status of the injured CNS. Further studies on the most promising targets should shed light into their specificity, and the safety of the imaging molecules used to label them, compared to TSPO. Considering the complexity and dynamic nature of neuroinflammation, it is likely that more than one target may be required to assess its onset and progression.
References
Casellas P, Galiegue S, Basile AS (2002) Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 40:475–486
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M (2006) Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27(8):402–409
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9(12):971–988
Liu GJ, Middleton RJ, Hatty CR, Kam WW, Chan R, Pham T, Harrison-Brown M, Dodson E, Veale K, Banati RB (2014) The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol 24(6):631–653
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 35(3):306–328
Chen MK, Guilarte TR (2008) Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther 118(1):1–17
Fan J, Lindemann P, Feuilloley MG, Papadopoulos V (2012) Structural and functional evolution of the translocator protein (18 kDa). Curr Mol Med 12(4):369–386
Riond J, Mattei MG, Kaghad M, Dumont X, Guillemot JC, Le Fur G, Caput D, Ferrara P (1991) Molecular cloning and chromosomal localization of a human peripheral-type benzodiazepine receptor. Eur J Biochem 195(2):305–311
Lin D, Chang YJ, Strauss JF 3rd, Miller WL (1993) The human peripheral benzodiazepine receptor gene: cloning and characterization of alternative splicing in normal tissues and in a patient with congenital lipoid adrenal hyperplasia. Genomics 18(3):643–650
Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 51(4):629–650
Veenman L, Papadopoulos V, Gavish M (2007) Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des 13(23):2385–2405
Bolger GT, Mezey E, Cott J, Weissman BA, Paul SM, Skolnick P (1984) Differential regulation of ‘central’ and ‘peripheral’ benzodiazepine binding sites in the rat olfactory bulb. Eur J Pharmacol 105(1–2):143–148
Karchewski LA, Bloechlinger S, Woolf CJ (2004) Axonal injury-dependent induction of the peripheral benzodiazepine receptor in small-diameter adult rat primary sensory neurons. Eur J Neurosci 20(3):671–683
Varga B, Markó K, Hádinger N, Jelitai M, Demeter K, Tihanyi K, Vas A, Madarász E (2009) Translocator protein (TSPO 18 kDa) is expressed by neural stem and neuronal precursor cells. Neurosci Lett 462(3):257–262
Anholt RR, Pedersen PL, De Souza EB, Snyder SH (1986) The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem 261(2):576–583
Marangos PJ, Patel J, Boulenger JP, Clark-Rosenberg R (1982) Characterization of peripheral-type benzodiazepine binding sites in brain using [3H]Ro 5-4864. Mol Pharmacol 22(1):26–32
Oke BO, Suarez-Quian CA, Riond J, Ferrara P, Papadopoulos V (1992) Cell surface localization of the peripheral-type benzodiazepine receptor (PBR) in adrenal cortex. Mol Cell Endocrinol 87(1–3):R1–R6
Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M (2014) Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343(6177):1363–1366
Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S (2015) Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science 347(6221):555–558
Li F, Liu J, Garavito RM, Ferguson-Miller S (2015) Evolving understanding of translocator protein 18 kDa (TSPO). Pharmacol Res. pii S1043–6618(15):00062–00066
Guo Y, Kalathur RC, Liu Q, Kloss B, Bruni R, Ginter C, Kloppmann E, Rost B, Hendrickson WA (2015) Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 347(6221):551–555
Bernassau JM, Reversat JL, Ferrara P, Caput D, Lefur G (1993) A 3D model of the peripheral benzodiazepine receptor and its implication in intra mitochondrial cholesterol transport. J Mol Graph 11(4):236–244
Li H, Papadopoulos V (1998) Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 139(12):4991–4997
Lacapère JJ, Papadopoulos V (2003) Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 68(7–8):569–585
Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V (2001) Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci USA 98(3):1267–1272
Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Péranzi G, Yao ZX, Maccario J, Lacapère JJ, Papadopoulos V (2003) In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochemistry 42(15):4506–4519
McEnery MW, Snowman AM, Trifiletti RR, Snyder SH (1992) Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A 89(8):3170–3174
Liu J, Rone MB, Papadopoulos V (2006) Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 281(50):38879–38893
Galiègue S, Jbilo O, Combes T, Bribes E, Carayon P, Le Fur G, Casellas P (1999) Cloning and characterization of PRAX-1. A new protein that specifically interacts with the peripheral benzodiazepine receptor. J Biol Chem 274(5):2938–2952
Fan J, Liu J, Culty M, Papadopoulos V (2010) Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog Lipid Res 49(3):218–234
Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V (2012) Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol 26(11):1868–1882
Issop L, Rone MB, Papadopoulos V (2013) Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol Cell Endocrinol 371(1–2):34–46
Issop L, Fan J, Lee S, Rone MB, Basu K, Mui J, Papadopoulos V (2015) Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: role of ATAD3. Endocrinology 156(1):334–345
Veenman L (2012) Gavish M (2012) The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr Mol Med 12(4):398–412
Choi HB, Khoo C, Ryu JK, van BE, Kim SU, McLarnon JG (2002) Inhibition of lipopolysaccharide- induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem 83:546–555
Veiga S, Carrero P, Pernia O, Azcoitia I, Garcia-Segura LM (2007) Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia 55(14):1426–1436
Verma A, Nye JS, Snyder SH (1987) Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc Natl Acad Sci U S A 84(8):2256–2260
Farges R, Joseph-Liauzun E, Shire D, Caput D, Le Fur G, Ferrara P (1994) Site-directed mutagenesis of the peripheral benzodiazepine receptor: identification of amino acids implicated in the binding site of Ro5-4864. Mol Pharmacol 46(6):1160–1167
Midzak A, Akula N, Lecanu L, Papadopoulos V (2011) Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem 286(11):9875–9887
Midzak AS, Akula N, Rone MB, Papadopoulos V (2015) Computational modeling and biological validation of novel non-steroidal ligands for the cholesterol recognition/interaction amino acid consensus (CRAC) motif of the mitochondrial translocator protein (TSPO). Pharmacol Res. pii: S1043-6618(15)00064-X
Scarf AM, Luus C, Da Pozzo E, Selleri S, Guarino C, Martini C, Ittner LM, Kassiou M (2012) Evidence for complex binding profiles and species differences at the translocator protein (TSPO) (18 kDa). Curr Mol Med 12(4):488–493
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP (2012) An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32(1):1–5
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB; Biomarkers Consortium PET Radioligand Project Team (2013) A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 33(1):53–58
Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C (2009) The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology 150(12):5438–5445
Jaremko M, Jaremko L, Giller K, Becker S, Zweckstetter M (2015) Structural integrity of the A147T polymorph of mammalian TSPO. ChemBioChem. doi:10.1002/cbic.201500217
Li F, Liu J, Valls L, Hiser C, Ferguson-Miller S (2015) Identification of a key cholesterol binding enhancement motif in translocator protein 18 kDa. Biochemistry 54(7):1441–1443
Romeo E, Auta J, Kozikowski AP, Ma D, Papadopoulos V, Puia G, Costa E, Guidotti A (1992) 2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR). J Pharmacol Exp Ther 262(3):971–978
Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K (2009) Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 325(5939):490–493
Gut P, Zweckstetter M, Banati RB (2015) Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol Metab. pii S1043–2760(15):00080–00086
Costa B, Pini S, Abelli M, Gabelloni P, Da Pozzo E, Chelli B, Calugi S, Lari L, Cardini A, Lucacchini A, Cassano GB, Martini C (2012) Role of translocator protein (18 kDa) in adult separation anxiety and attachment style in patients with depression. Curr Mol Med 12(4):483–487
Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N, Young AH (2013) Bipolar Disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa Translocator Protein (TSPO). Psychoneuroendocrinology 38(11):2826–2829
Fan J, Rone MB, Papadopoulos V (2009) Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J Biol Chem 284(44):30484–30497
Itzhak Y, Norenberg MD (1994) Attenuation of ammonia toxicity in mice by PK 11195 and pregnenolone sulfate. Neurosci Lett 182:251–254
Veenman L, Leschiner S, Spanier I, Weisinger G, Weizman A, Gavish M (2002) PK 11195 attenuates kainic acid-induced seizures and alterations in peripheral-type benzodiazepine receptor (PBR) protein components in the rat brain. J Neurochem 80(5):917–927
Ryu JK, Choi HB, McLarnon JG (2005) Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol Dis 20:550–561
Ji B, Maeda J, Sawada M, Ono M, Okauchi T, Inaji M, Zhang MR, Suzuki K, Ando K, Staufenbiel M, Trojanowski JQ, Lee VM, Higuchi M, Suhara T (2008) Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer’s and other CNS pathologies. J Neurosci 28(47):12255–12267
Gavish M, Weizman R (1997) Role of peripheral-type benzodiazepine receptors in steroidogenesis. Clin Neuropharmacol 20:473–481
Girard C, Liu S, Adams D, Lacroix C, Sinéus M, Boucher C, Papadopoulos V, Rupprecht R, Schumacher M, Groyer G (2012) Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. J Neuroendocrinol 24(1):71–81
Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V (2014) Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155(1):89–97
Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V (2014) Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem 289(40):27444–27454
Šileikytė J, Blachly-Dyson E, Sewell R, Carpi A, Menabò R, Di Lisa F, Ricchelli F, Bernardi P, Forte M (2014) Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor [Translocator Protein of 18 kDa (TSPO)]. J Biol Chem 289(20):13769–13781
Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V (2015) Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A 112(23):7261–7266
Raghavan A, Sheiko T, Graham BH, Craigen WJ (1818) Voltage-dependant anion channels: novel insights into isoform function through genetic models. Biochim Biophys Acta 6:1477–1485
Papadopoulos V, Aghazadeh Y, Fan J, Campioli E, Zirkin B, Midzak A (2015) Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol 408:90–98
Costa E, Auta J, Guidotti A, Korneyev A, Romeo E (1994) The pharmacology of neurosteroidogenesis. J Steroid Biochem Mol Biol 49(4–6):385–389
Papadopoulos V, Lecanu L (2009) Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol 219(1):53–57
Kuhlmann AC, Guilarte TR (2000) Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem 74(4):1694–1704
Chen MK, Guilarte TR (2006) Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol Sci 91(2):532–539
Wang M, Gao M, Hutchins GD, Zheng QH (2009) Synthesis of [11C]FEDAA1106 as a new PET imaging probe of peripheral benzodiazepine receptor expression. Eur J Med Chem 44(6):2748–2753
Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EF (2009) [11C]-DPA-713 and [18F]-DPA-714 as new PET tracers for TSPO: a comparison with [11C]-(R)-PK11195 in a rat model of herpes encephalitis. Mol Imaging Biol 11(6):386–398
Chaki S, Funakoshi T, Yoshikawa R, Okuyama S, Okubo T, Nakazato A, Nagamine M, Tomisawa K (1999) Binding characteristics of [3H]DAA1106, a novel and selective ligand for peripheral benzodiazepine receptors. Eur J Pharmacol 371(2–3):197–204
Zhang MR, Maeda J, Ogawa M, Noguchi J, Ito T, Yoshida Y, Okauchi T, Obayashi S, Suhara T, Suzuki K (2004) Development of a new radioligand, N-(5-fluoro-2-phenoxyphenyl)-N-(2-[18F]fluoroethyl-5-methoxybenzyl)acetamide, for pet imaging of peripheral benzodiazepine receptor in primate brain. J Med Chem 47(9):2228–2235
Imaizumi M, Briard E, Zoghbi SS, Gourley JP, Hong J, Fujimura Y, Pike VW, Innis RB, Fujita M (2008) Brain and whole-body imaging in nonhuman primates of [11C]PBR28, a promising PET radioligand for peripheral benzodiazepine receptors. Neuroimage 39(3):1289–1298
Fookes CJ, Pham TQ, Mattner F, Greguric I, Loc’h C, Liu X, Berghofer P, Shepherd R, Gregoire MC, Katsifis A (2008) Synthesis and biological evaluation of substituted [18F]imidazo[1,2-a]pyridines and [18F]pyrazolo[1,5-a]pyrimidines for the study of the peripheral benzodiazepine receptor using positron emission tomography. J Med Chem 51(13):3700–3712
Zhang MR, Kumata K, Maeda J, Yanamoto K, Hatori A, Okada M, Higuchi M, Obayashi S, Suhara T, Suzuki K (2007) 11C-AC-5216: a novel PET ligand for peripheral benzodiazepine receptors in the primate brain. J Nucl Med 48(11):1853–1861
Mattner F, Mardon K, Katsifis A (2008) Pharmacological evaluation of [123I]-CLINDE: a radioiodinated imidazopyridine-3-acetamide for the study of peripheral benzodiazepine binding sites (PBBS). Eur J Nucl Med Mol Imaging 35(4):779–789
Gulyás B, Vas A, Tóth M, Takano A, Varrone A, Cselényi Z, Schain M, Mattsson P, Halldin C (2011) Age and disease related changes in the translocator protein (TSPO) system in the human brain: positron emission tomography measurements with [11C]vinpocetine. Neuroimage 56(3):1111–1121
Verleye M, Akwa Y, Liere P, Ladurelle N, Pianos A, Eychenne B, Schumacher M, Gillardin JM (2005) The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol Biochem Behav 82(4):712–720
Girard C, Liu S, Cadepond F, Adams D, Lacroix C, Verleye M, Gillardin JM, Baulieu EE, Schumacher M, Schweizer-Groyer G (2008) Etifoxine improves peripheral nerve regeneration and functional recovery. Proc Natl Acad Sci USA 105(51):20505–20510
Lartey FM, Ahn GO, Shen B, Cord KT, Smith T, Chua JY, Rosenblum S, Liu H, James ML, Chernikova S, Lee SW, Pisani LJ, Tirouvanziam R, Chen JW, Palmer TD, Chin FT, Guzman R, Graves EE, Loo BW Jr (2014) PET imaging of stroke-induced neuroinflammation in mice using [18F]PBR06. Mol Imaging Biol 16(1):109–117
Kumar A, Muzik O, Shandal V, Chugani D, Chakraborty P, Chugani HT (2012) Evaluation of age-related changes in translocator protein (TSPO) in human brain using (11)C-[R]-PK11195 PET. J Neuroinflammation 9:232
Suridjan I, Rusjan PM, Voineskos AN, Selvanathan T, Setiawan E, Strafella AP, Wilson AA, Meyer JH, Houle S, Mizrahi R (2014) Neuroinflammation in healthy aging: a PET study using a novel Translocator Protein 18 kDa (TSPO) radioligand, [(18)F]-FEPPA. Neuroimage 84:868–875
Winkeler A, Boisgard R, Martin A, Tavitian B (2010) Radioisotopic imaging of neuroinflammation. J Nucl Med 51(1):1–4
Ching AS, Kuhnast B, Damont A, Roeda D, Tavitian B, Dollé F (2012) Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 3(1):111–119
Varley J, Brooks DJ, Edison P (2014) Imaging neuroinflammation in Alzheimer’s and other dementias: recent advances and future directions. Alzheimers Dement. pii S1552–5260(14):02820–02829
Varrone A, Mattson P, Forsberg A, Takano A, Nag S, Gulyás B, Borg J, Boellaard R, al-Tawil N, eriksdotter M, Zimmermann T, Schultze-Mosgau M, Thiele A, Hoffmann A, Lammertsma AA, Halldin C (2013) In vivo imaging of the 18-kDa translocator protein (TSPO) with [18F]FEDAA1106 and PET does not show increased binding in Alzheimer’s disease patients. Eur J Nucl Med Mol Imaging 40(6):921–931
Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R (2011) Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience 191:55–77
Da Pozzo E, Costa B, Martini C (2012) Translocator protein (TSPO) and neurosteroids: implications in psychiatric disorders. Curr Mol Med 12(4):426–442
Batarseh A, Papadopoulos V (2010) Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol 327(1–2):1–12
Wu C, Yue X, Lang L, Kiesewetter DO, Li F, Zhu Z, Niu G, Chen X (2014) Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-Alfatide II and differentiation with tumors. Theranostics 4(5):546–555
Daniele S, Taliani S, Da Pozzo E, Giacomelli C, Costa B, Trincavelli ML, Rossi L, La Pietra V, Barresi E, Carotenuto A, Limatola A, Lamberti A, Marinelli L, Novellino E, Da Settimo F, Martini C (2014) Apoptosis therapy in cancer: the first single-molecule co-activating p53 and the translocator protein in glioblastoma. Sci Rep 4:4749
Su Z, Roncaroli F, Durrenberger PF, Coope DJ, Karabatsou K, Hinz R, Thompson G, Turkheimer FE, Janczar K, Du Plessis D, Brodbelt A, Jackson A, Gerhard A, Herholz K (2015) The 18-kDa mitochondrial translocator protein in human gliomas: an 11C-(R)PK11195 PET imaging and neuropathology study. J Nucl Med 56(4):512–517
Mills C, Makwana M, Wallace A, Benn S, Schmidt H, Tegeder I, Costigan M, Brown RH Jr, Raivich G, Woolf CJ (2008) Ro5-4864 promotes neonatal motor neuron survival and nerve regeneration in adult rats. Eur J Neurosci 27(4):937–946
Giatti S, Pesaresi M, Cavaletti G, Bianchi R, Carozzi V, Lombardi R, Maschi O, Lauria G, Garcia-Segura LM, Caruso D, Melcangi RC (2009) Neuroprotective effects of a ligand of translocator protein-18 kDa (Ro5-4864) in experimental diabetic neuropathy. Neuroscience 164(2):520–529
Miller TR, Wetter JB, Jarvis MF, Bitner RS (2013) Spinal microglial activation in rat models of neuropathic and osteoarthritic pain: an autoradiographic study using [3H]PK11195. Eur J Pain 17(5):692–703
Liu X, Li W, Dai L, Zhang T, Xia W, Liu H, Ma K, Xu J, Jin Y (2014) Early repeated administration of progesterone improves the recovery of neuropathic pain and modulates spinal 18 kDa-translocator protein (TSPO) expression. J Steroid Biochem Mol Biol 143:130–140
Schumacher M, Sitruk-Ware R, De Nicola AF (2008) Progesterone and progestins: neuroprotection and myelin repair. Curr Opin Pharmacol 8(6):740–746
Man S, Ubogu EE, Ransohoff RM (2007) Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol 17(2):243–250
Sipkins DA, Gijbels K, Tropper FD, Bednarski M, Li KC, Steinman L (2000) ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol 104(1):1–9
McAteer MA, Sibson NR, von Zur Muhlen C, Schneider JE, Lowe AS, Warrick N, Channon KM, Anthony DC, Choudhury RP (2007) In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med 13(10):1253–1258
Montagne A, Gauberti M, Macrez R, Jullienne A, Briens A, Raynaud JS, Louin G, Buisson A, Haelewyn B, Docagne F, Defer G, Vivien D, Maubert E (2012) Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage 63(2):760–770
Deddens LH, van Tilborg GA, van der Toorn A, van der Marel K, Paulis LE, van Bloois L, Storm G, Strijkers GJ, Mulder WJ, de Vries HE, Dijkhuizen RM (2013) MRI of ICAM-1 upregulation after stroke: the importance of choosing the appropriate target-specific particulate contrast agent. Mol Imaging Biol 15(4):411–422
van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG (2009) Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci U S A 106(1):18–23
Yokoyama H, Itoh O, Aoyama M, Obara H, Ohya H, Kamada H (2002) In vivo temporal EPR imaging of the brain of rats by using two types of blood-brain barrier-permeable nitroxide radicals. Magn Reson Imaging 20(3):277–284
Lee MC, Shoji H, Miyazaki H, Yoshino F, Hori N, Miyake S, Ikeda Y, Anzai K, Ozawa T (2003) Measurement of oxidative stress in the rodent brain using computerized electron spin resonance tomography. Magn Reson Med Sci 2(2):79–84
Yamato M, Shiba T, Yamada K, Watanabe T, Utsumi H (2009) Noninvasive assessment of the brain redox status after transient middle cerebral artery occlusion using Overhauser-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab 29(10):1655–1664
Yamato M, Shiba T, Naganuma T, Ichikawa K, Utsumi H, Yamada K (2011) Overhauser-enhanced magnetic resonance imaging characterization of mitochondria functional changes in the 6-hydroxydopamine rat model. Neurochem Int 59(6):804–811
Chen JW, Querol Sans M, Bogdanov A Jr, Weissleder R (2006) Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology 240(2):473–481
Zhang N, Francis KP, Prakash A, Ansaldi D (2013) Enhanced detection of myeloperoxidase activity in deep tissues through luminescent excitation of near-infrared nanoparticles. Nat Med 19(4):500–505
Alshetaiwi HS, Balivada S, Shrestha TB, Pyle M, Basel MT, Bossmann SH, Troyer DL (2013) Luminol-based bioluminescence imaging of mouse mammary tumors. J Photochem Photobiol B 127:223–228
Tseng JC, Kung AL (2013) In vivo imaging method to distinguish acute and chronic inflammation. J Vis Exp. doi:10.3791/50690
Yong VW (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 6(12):931–944
Klohs J, Baeva N, Steinbrink J, Bourayou R, Boettcher C, Royl G, Megow D, Dirnagl U, Priller J, Wunder A (2009) In vivo near-infrared fluorescence imaging of matrix metalloproteinase activity after cerebral ischemia. J Cereb Blood Flow Metab 29(7):1284–1292
Liu N, Shang J, Tian F, Nishi H, Abe K (2011) In vivo optical imaging for evaluating the efficacy of edaravone after transient cerebral ischemia in mice. Brain Res 1397:66–75
Barber PA, Rushforth D, Agrawal S, Tuor UI (2012) Infrared optical imaging of matrix metalloproteinases (MMPs) up regulation following ischemia reperfusion is ameliorated by hypothermia. BMC Neurosci 13:76
Schwenck J, Griessinger CM, Fuchs K, Bukala D, Bauer N, Eichner M, Röcken M, Pichler BJ, Kneilling M (2014) In vivo optical imaging of matrix metalloproteinase activity detects acute and chronic contact hypersensitivity reactions and enables monitoring of the antiinflammatory effects of N-acetylcysteine. Mol Imaging. doi:10.2310/7290.2014.00044
Horti AG, Gao Y, Ravert HT, Finley P, Valentine H, Wong DF, Endres CJ, Savonenko AV, Dannals RF (2010) Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). Bioorg Med Chem 18(14):5202–5207
Evens N, Muccioli GG, Houbrechts N, Lambert DM, Verbruggen AM, Van Laere K, Bormans GM (2009) Synthesis and biological evaluation of carbon-11- and fluorine-18-labeled 2-oxoquinoline derivatives for type 2 cannabinoid receptor positron emission tomography imaging. Nucl Med Biol 36(4):455–465
Evens N, Vandeputte C, Muccioli GG, Lambert DM, Baekelandt V, Verbruggen AM, Debyser Z, Van Laere K, Bormans GM (2011) Synthesis, in vitro and in vivo evaluation of fluorine-18 labelled FE-GW405833 as a PET tracer for type 2 cannabinoid receptor imaging. Bioorg Med Chem 19(15):4499–4505
Evens N, Vandeputte C, Coolen C, Janssen P, Sciot R, Baekelandt V, Verbruggen AM, Debyser Z, Van Laere K, Bormans GM (2012) Preclinical evaluation of [11C]NE40, a type 2 cannabinoid receptor PET tracer. Nucl Med Biol 39(3):389–399
Turkman N, Shavrin A, Paolillo V, Yeh HH, Flores L, Soghomonian S, Rabinovich B, Volgin A, Gelovani J, Alauddin M (2012) Synthesis and preliminary evaluation of [18F]-labeled 2-oxoquinoline derivatives for PET imaging of cannabinoid CB2 receptor. Nucl Med Biol 39(4):593–600
Mu L, Slavik R, Müller A, Popaj K, Cermak S, Weber M, Schibli R, Krämer SD, Ametamey SM (2014) Synthesis and preliminary evaluation of a 2-oxoquinoline carboxylic acid derivative for PET imaging the cannabinoid type 2 receptor. Pharmaceuticals (Basel) 7(3):339–352
Saccomanni G, Pascali G, Carlo SD, Panetta D, De Simone M, Bertini S, Burchielli S, Digiacomo M, Macchia M, Manera C, Salvadori PA (2015) Design, synthesis and preliminary evaluation of (18)F-labelled 1,8-naphthyridin- and quinolin-2-one-3-carboxamide derivatives for PET imaging of CB2 cannabinoid receptor. Bioorg Med Chem Lett 25(12):2532–2535
Slavik R, Grether U, Müller Herde A, Gobbi L, Fingerle J, Ullmer C, Krämer SD, Schibli R, Mu L, Ametamey SM (2015) Discovery of a high affinity and selective pyridine analog as a potential positron emission tomography imaging agent for cannabinoid type 2 receptor. J Med Chem 58(10):4266–4277
Ling X, Zhang S, Shao P, Li W, Yang L, Ding Y, Xu C, Stella N, Bai M (2015) A novel near-infrared fluorescence imaging probe that preferentially binds to cannabinoid receptors CB2R over CB1R. Biomaterials 57:169–178
Zhang S, Shao P, Ling X, Yang L, Hou W, Thorne SH, Beaino W, Anderson CJ, Ding Y, Bai M (2015) In vivo inflammation imaging using a CB2R-targeted near infrared fluorescent probe. Am J Nucl Med Mol Imaging 5(3):246–258
Eaton VL, Vasquez KO, Goings GE, Hunter ZN, Peterson JD, Miller SD (2013) Optical tomographic imaging of near infrared imaging agents quantifies disease severity and immunomodulation of experimental autoimmune encephalomyelitis in vivo. J Neuroinflammation 10:138
Ohnishi A, Senda M, Yamane T, Sasaki M, Mikami T, Nishio T, Ikari Y, Nishida H, Shukuri M, Takashima T, Mawatari A (2014) Doi H, Watanabe Y, Onoe H Human whole-body biodistribution and dosimetry of a new PET tracer, [(11)C]ketoprofen methyl ester, for imagings of neuroinflammation. Nucl Med Biol 41(7):594–599
Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L (2012) P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y2 receptor interactions in neuroinflammation. Mol Neurobiol 46(1):96–113
Janssen B, Vugts DJ, Funke U, Spaans A, Schuit RC, Kooijman E, Rongen M, Perk LR, Lammertsma AA, Windhorst AD (2014) Synthesis and initial preclinical evaluation of the P2X7 receptor antagonist [11C]A-740003 as a novel tracer of neuroinflammation. J Labelled Comp Radiopharm 57(8):509–516
Kawamura K, Kimura Y, Yui J, Wakizaka H, Yamasaki T, Hatori A, Kumata K, Fujinaga M, Yoshida Y, Ogawa M, Nengaki N, Fukumura T, Zhang MR (2012) PET study using [11C]FTIMD with ultra-high specific activity to evaluate I2-imidazoline receptors binding in rat brains. Nucl Med Biol 39(2):199–206
Antunes IF, Doorduin J, Haisma HJ, Elsinga PH, van Waarde A, Willemsen AT, Dierckx RA, de Vries EF (2012) 18F-FEAnGA for PET of β-glucuronidase activity in neuroinflammation. J Nucl Med 53(3):451–458
Jacobs AH, Tavitian B, INMiND consortium (2012) Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab 32(7):1393–1415
Pulli B, Chen JW (2014) Imaging Neuroinflammation—from Bench to Bedside. J Clin Cell Immunol 5:226. doi:10.4172/2155-9899.1000226
Acknowledgments
This work was supported by funds of the INMiND Project of the European Union to M.T.H. and a grant from the Canadian Institutes of Health Research (MOP125983) as well as a Canada Research Chair in Biochemical Pharmacology to V.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M Herrera-Rivero and MT Heneka declare no conflict of interest. V Papadopoulos is named inventor in patents and patent applications reporting TSPO drug ligands.
Rights and permissions
About this article
Cite this article
Herrera-Rivero, M., Heneka, M.T. & Papadopoulos, V. Translocator protein and new targets for neuroinflammation. Clin Transl Imaging 3, 391–402 (2015). https://doi.org/10.1007/s40336-015-0151-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-015-0151-x