Abstract
Introduction
Mental fatigue (MF) is a psychobiological state that impairs cognitive as well as physical performance in different settings. Recently, numerous studies have sought ways to counteract these negative effects of MF. An overview of the explored countermeasures for MF is, however, lacking.
Objectives
The objective of this review is to provide an overview of the different MF countermeasures currently explored in literature. Countermeasures were classified by the timing of application (before, during or after the moment of MF) and type of intervention (behavioural, physiological and psychological).
Methods
The databases of PubMed (MEDLINE), Web of Science and PsycINFO were searched until March 7, 2022. Studies were eligible when MF was induced using a task with a duration of at least 30 min, when they assessed MF markers in at least two out of the three areas wherein MF markers have been defined (i.e., behavioural, subjective and/or [neuro]physiological) and used a placebo or control group for the countermeasure.
Results
A total of 33 studies investigated one or more countermeasures against MF. Of these, eight studies assessed a behavioural countermeasure, 22 a physiological one, one a psychological countermeasure and two a combination of a behavioural and psychological countermeasure. The general finding was that a vast majority of the countermeasures induced a positive effect on behavioural (e.g., task or sport performance) and/or subjective MF markers (e.g., visual analogue scale for MF or alertness). No definitive conclusion could be drawn regarding the effect of the employed countermeasures on (neuro)physiological markers of MF as only 19 of the included studies investigated these measures, and within these a large heterogeneity in the evaluated (neuro)physiological markers was present.
Discussion
Within the physiological countermeasures it seems that the use of odours during a MF task or caffeine before the MF task are the most promising interventions in combating MF. Promising behavioural (e.g., listening to music) and psychological (e.g., extrinsic motivation) countermeasures of MF have also been reported. The most assumed mechanism through which these countermeasures operate is the dopaminergic system. However, this mechanism remains speculative as (neuro)physiological markers of MF have been scarcely evaluated to date.
Conclusion
The present systematic review reveals that a wide range of countermeasures have been found to successfully counteract MF on a subjective, (neuro)physiological and/or behavioural level. Of these, caffeine, odours, music and extrinsic motivation are the most evidenced for countering MF. To provide in-detail practical guidelines for the real-life application of MF countermeasures, more research must be performed into the underlying mechanisms and into the optimal dosage and time of application/intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The cognitive and/or physical impairments caused by mental fatigue can be partially countered by using a physiological, behavioural or psychological countermeasure. |
Caffeine, odours, music and extrinsic motivation seem promising countermeasures against mental fatigue. However, the heterogeneity in the protocols to induce and determine mental fatigue makes it difficult to draw firm conclusions. |
Future studies should evaluate the impact of the most promising countermeasures on (neuro)physiological markers of mental fatigue and on optimizing mental fatigue-combating interventions by combining multiple strategies. |
1 Introduction
Mental fatigue (MF), which can be defined as a psychobiological state caused by prolonged episodes of cognitive exertion, has already been demonstrated to decrease accuracy and increase reaction time during cognitive tasks [1] and to deteriorate endurance performance [2,3,4], as well as technical and tactical aspects in team sports [5, 6]. Subsequently, in real-world settings such as car driving, within the workplace or in a sport setting, this leads to an elevated risk for accidents, injuries and/or incidents [7,8,9]. As such, research to counteract, avoid or habituate against MF has soared in recent years.
Countermeasures against MF have been evaluated within multiple settings, such as non-physical cognitive performance [10], as well as sport-specific settings [11, 12]. One example of this is the use of creatine to combat the MF-associated decrements in both cognitive and psychomotor performance [13, 14]. Van Cutsem et al. [13] argued that the occurrence of MF is associated with a drop in the phosphocreatine concentration in the brain; as such, creatine supplementation might be able to increase brain energy resources, postpone the drop in phosphocreatine and subsequently also the occurrence of MF. They discovered that creatine supplementation successfully counteracted the MF-associated decrease in cognitive performance, but not psychomotor performance [13]. Another example of a potential MF countermeasure is the adenosine receptor antagonist caffeine [15, 16]. Caffeine is a natural xanthine alkaloid that is able to cross the blood–brain barrier and block the adenosine receptors to alter central nervous system (CNS) function [15, 16]. Adenosine inhibits the release of brain excitatory neurotransmitters (e.g., dopamine) and reduces arousal and spontaneous behavioural activity. Indeed, the interplay between adenosine and dopamine may play an important mechanistic role in the MF-associated decrease in performance [17, 18]. Martin et al. [17] argued that mental exertion could result in an accumulation of cerebral adenosine, an inhibitory neuromodulator, increasing the excitatory stimulus required (leading to a higher perception of effort), as well as directly impacting both dopamine release and binding to its receptor. If true, this would provide a clear neurophysiological pathway through which caffeine can reduce MF and provide an easy-to-implement MF countermeasure within multiple contexts (e.g., work and sport) [11, 12, 19]. Moreover, non-nutritional interventions, for example the use of music, have also been found to counteract the negative impact of MF [20]. It has been proposed that music may act through increasing important neurotransmitter levels and, as such, attenuating feelings of pain and physical exertion experienced during physical performance [21, 22]. Therefore, the use of music is another promising countermeasure against MF which could be easily implemented in multiple settings.
Despite recent scientific interest in MF countermeasures, investigations into the methods, routines or substances are still in their infancy. Some studies [2, 11, 13, 14] have provided preliminary insight in the search for optimal MF countermeasures, but further understanding of the MF mechanisms is necessary to develop new and/or optimize already existing interventions. As no systematic review on possible MF countermeasures has been published, a possible consequence is that counter-, or preventative measures for MF are either being neglected or sub-optimally employed [23, 24]. Therefore, to optimise their use, the purpose of this review is to create an overview of the available MF countermeasures and their effectiveness.
To create a clear overview of the currently available MF countermeasures, we restricted this review to acute countermeasures. For the sake of clarity, we use the word ‘countermeasure’ to refer to an intervention or action taken to counteract or compensate for the effect(s) of something else. In this regard, the word countermeasure also includes measures that can work preventively or curatively against the negative consequences of MF on human functioning (i.e., subjectively, behaviourally and/or [neuro]physiologically). MF countermeasures can be considered to be acute, working within a few days after implementation (e.g., caffeine, creatine etc.) or chronic, with longer implementation periods (e.g., Brain Endurance Training, mindfulness etc.) [11, 13, 25, 26]. In the current review, we focused on interventions applied up to 7 days before a mentally fatiguing activity, i.e., acute countermeasures. The arbitrary choice for a maximum time interval of 7 days between the application/intake of a countermeasure and the actual activity that might trigger MF was based on the assumption that MF countermeasures that require more than 7 days to positively impact MF will be more challenging to implement in most settings (e.g., time consuming, not worth the investment) and therefore are less applicable. Within the acute countermeasures we further classified the interventions based on (1) the timing of application of the countermeasure (before/during/after engaging in a task that possibly triggers MF); and on (2) the nature of the countermeasure (behavioural, e.g., taking a break [27], psychological, e.g., increase of motivation [28] or physiological, e.g., nutritional, pharmacological interventions [11]). These distinctions are likely to further support understanding and the real-world implications of such interventions. This classification should, in the end, serve to apply the available MF countermeasures more easily and optimally in the relevant context.
2 Methods
This systematic review was completed using the PRISMA (Preferred Reporting Items for Systematic review and Meta-analyses) guidelines [29].
2.1 Eligibility Criteria
To establish the keywords for this systematic review, PICOS categories (Population, Intervention, Comparison, Outcome and Study design) were used (see Table 1). For the population, studies using healthy adults (aged 18–50 years) were included in this review. In terms of the intervention, the focus was on acute (up to 7 days prior) MF countermeasures with a physiological, behavioural or psychological basis. Additionally, a placebo or control group had to be included in the design in order to obtain studies of high quality. Concerning the main outcomes, studies were only found eligible if they assessed at least two out of the three areas in which markers of MF have been defined: behavioural (e.g., performance on a cognitive/physical task), subjective (e.g., perception of MF using a visual analogue scale [VAS]) or (neuro)physiological MF markers (e.g., brain activity through electroencephalography), after performing a MF-inducing task with a duration of at least 30 min. In one of our previous systematic reviews on the effects of mental fatigue on physical performance [2], studies from ego depletion literature (i.e., where prior cognitive exertion most often lasts < 30 min) were excluded. Although two recent meta-analyses have demonstrated decrements in physical performance subsequent to shorter (< 30 min) and longer (> 30 min) cognitive tasks [3, 30], this time cut-off was maintained. When task difficulty is similar, task duration negatively affects subsequent task performance [31, 32] with 30 min appearing to be a consistent threshold for MF-related performance impairments [2, 33, 34]. In addition, the vigilance decrement that typically occurs after 20–30 min of continuous work in the literature on sustained attention [2, 33], and the typical 30-min time range in which an increase in perceived mental fatigue is often observed in the literature on mental fatigue [2, 34], further supports the 30-min time cut-off. Lastly, in terms of study design, only randomized controlled trials (RCTs) or non-randomized controlled trials (nRCTs) were included for further review.
2.2 Information Sources and Search Strategy
Three electronic databases, PubMed, Web of Science (WoS) (all databases) and PsycINFO were searched until March 7, 2022. Medical subject heading (MeSH) terms, if available in PubMed, were used for a qualitative literature search. See Table 2 for an overview of the keywords that were used in the different databases. In addition, the reference lists of included studies, and studies that cited the included studies, were screened.
2.3 Study Selection
After executing the search strategy, all studies from the different databases were gathered and imported into Rayyan [35]. After removing duplicates, two authors (MP and JH) screened the studies, independently and blinded from each other, on title and abstract. Studies were included and excluded following the inclusion and exclusion criteria (see Table 3). Conflicts concerning the included or excluded studies at this stage of screening were resolved, then full texts of the remaining studies were screened by the same two authors (MP and JH). In addition, the reference lists of included studies (i.e., backward screening), and studies that cited the included studies (i.e., forward screening) were screened to make the search as complete as possible.
2.4 Data Extraction
To answer the research question, all details concerning the effect on MF (subjectively, behaviourally or [neuro]physiologically), type, timing of application and possible effects of the countermeasures were extracted from the included studies and are summarized in Table 4. Other extracted information included study design, participant demographics, sample size, control/placebo groups and MF intervention. As already mentioned in Sect. 1, included studies were further classified based on (1) the timing of application of the countermeasure (before/during/after engaging in a task that possibly triggers MF); and on (2) the nature of the countermeasure (behavioural, psychological or [neuro]physiological). We are well aware that this classification is arbitrary and that, certainly in terms of the classification based on the nature of the countermeasures, multiple evaluated countermeasures could fit in multiple subdivisions. For example, the countermeasure ‘taking a break’ was classified as a behavioural countermeasure because it requires a behavioural change by the individual to take a break. However, classifying ‘taking a break’ as a psychological countermeasure because a motivational effect is expected might also be considered. In general, countermeasures that primarily required an individual to undertake or undergo a specific activity that is different from the MF-inducing task were classified as ‘behavioural countermeasures’. Countermeasures that were primarily developed to impact an individual’s perception were classified as ‘psychological countermeasures’. Countermeasures that primarily aimed to impact the body’s physiology, via the intake of some kind of nutritional/pharmacological agent or the application of stimulation techniques, were classified as ‘physiological countermeasures’.
2.5 Risk of Bias Assessment
All included studies were individually assessed for risk of bias (RoB). Since both within- (i.e., crossover) and between-subject study designs were included, separate RoB tools were utilized dependent on the study design. For studies using a within-subject design, the revised Cochrane risk-of-bias tool for randomized crossover trials (RoB 2 for crossover trials) was utilised. The revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [36] was used for between-subject studies. Two authors (MP and JH) performed this RoB assessment. Finally, conflicts between the authors were resolved through discussion until consensus was reached and an overall risk of bias, following the recommended protocol, was made for each study.
3 Results
3.1 Study Selection
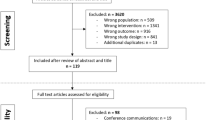
The systematic literature search yielded 4690 studies from all selected databases. Due to duplicate records, 735 studies were removed resulting in a total of 3955 studies for further evaluation. Figure 1 displays a flow diagram of the selection process. After the first round, 86 studies were selected for further screening. The full-text evaluation resulted in 30 included studies. Forward and backward screening provided three additional papers, resulting in a total of 33 studies that were included in the present systematic review. The level of blinded agreement for title plus abstract and for the full-text screening were 97.1% and 98.1%, respectively.
3.2 Risk of Bias
All 33 included studies, of which ten are between-subject and 23 within-subject design studies, were screened for risk of bias (see Figs. 2, 3). This assessment determined that 25 studies [12, 14, 20, 26, 27, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] had a high risk of bias, three studies [57,58,59] had some concerns of bias and five studies [11, 13, 60,61,62] had a low risk of bias.
Risk of bias in a individual crossover studies; b individual randomized controlled studies. a D1 bias arising from the randomization process, D2 bias arising from period and carryover effects, D3 bias due to deviations from intended intervention, D4 bias due to missing outcome data, D5 bias in measurement of the outcome, D6 bias in selection of the reported result, X high risk of bias, − some concerns regarding bias, + low risk of bias. b D1 bias arising from the randomization process, D2 bias due to deviations from intended intervention, D3 bias due to missing outcome data, D4 bias in measurement of the outcome, D5 bias in selection of the reported result, X high risk of bias, − some concerns regarding bias, + low risk of bias
3.3 Study Characteristics
A summary of included study characteristics can be found in Table 4. All studies included healthy individuals, covering a total population of 1012 participants (53% male when sex reported). Nine studies used a male-only population [11, 12, 43, 47,48,49, 52, 58, 62], one study a female population [55] and three studies did not report the sex of their participants (93 participants of the total) [44, 45, 61]. The average age of all participants was 26 years old, ranging between 18 and 45 years old. A total of six studies included a physical task whereby information on the physical fitness of the participants was mentioned [11, 13, 43, 49, 61, 62]. Table 5 provides an overview of the different types and timing of application of the evaluated countermeasures.
3.4 Type of Countermeasure
3.4.1 Behavioural
Ten studies used a behavioural countermeasure (see Sect. 2 for a definition) [20, 26, 27, 40, 45,46,47, 54, 55, 59]. Of these, five studies used a physical sensation-based intervention (e.g., physical activity and massage) [40, 45, 47, 54, 59], four other studies utilized an auditory intervention (e.g., listening to music) [20, 26, 40, 45], and two studies [27, 46] assessed the effect of a nap on MF. As well as utilising a nap, Hayashi et al. [27] incorporated a moment of rest in the middle of the task as an extra countermeasure in their study. Tanaka and colleagues [55] provided the only investigation into the effect of sitting in an open space connected to nature on the restoration of performance after MF. In a combined approach, Lim et al. [45] sought to understand if the use of a mechanical massage chair in conjunction with binaural beats could act as a countermeasure against MF (see Table 4 for an overview of the evaluated countermeasures).
Nine of the ten studies reported significant differences on a behavioural marker (e.g., flanker task performance or a physical task performance; see Table 4 for further details on this matter and Table 6 for an overview of the effects) with a MF countermeasure [20, 26, 27, 40, 45, 47, 54, 55, 59]. Eight studies used subjective reports of MF, with a visual analogue scale for MF (M-VAS) most commonly employed, but only four found that the intervention had an effect [20, 27, 54, 55] (see Table 4). Nine studies employing behavioural countermeasures assessed (neuro)physiological indices (e.g., heart rate, electroencephalography or accelerated plethysmography). However, only four of them observed benefits due to the countermeasure on their outcomes [20, 45, 47, 54] (see Table 4). Only Loch et al. [46] failed to observe an impact of their countermeasure(s) (a powernap, systematic breathing and combined systematic breathing plus mental imagery intervention) on any marker of MF in the studies collated. Further details of the intervention and study details are presented in Table 4.
3.4.2 Physiological
Twenty-two studies that met the inclusion criteria investigated a physiological countermeasure against MF (see Sect. 2 for a definition) [11,12,13,14, 38, 39, 41,42,43,44, 48,49,50,51,52,53, 56,57,58, 60,61,62], of which five were not substances administered orally. Of the 17 oral-consumption-based interventions, five studies focused on caffeine as a countermeasure [11, 12, 44, 56, 61]. Kennedy and Scholey [44] trialled caffeine in combination with glucose, Van Cutsem et al. [12] used it as a mouth rinse combined with maltodextrin, and Ataka et al. [56] compared caffeine with a D-ribose supplement and placebo in a 1-week intervention. Four other 1-week interventions included either creatine [13, 14] or chicken essence [48, 58]. Similar to Van Cutsem et al. [12], Brietzke et al. [43] and Bailey et al. [42] used a mouth rinse countermeasure, with carbohydrates as the only active ingredient. Brietzke et al. [43] used it after the MF-inducing task, while Bailey et al. [42] applied it during the task. Five other included studies assessed the effect of less common oral-consumption-based interventions. Of these, Reay et al. [50, 51] conducted two studies with Panax Ginseng, Kennedy et al. [60] and Kennedy et al. [57] investigated a commercial vitamin complex and a specific type of essential oil, respectively, and Scholey et al. [53] researched the effects of cocoa flavanols on MF. Lastly, Rattray et al. [62] were the only investigators using a pharmacological intervention (i.e., modafinil). The five studies that used non-orally administered countermeasures employed a non-invasive brain stimulation or odour-based intervention. Ishihara et al. [38] evaluated the effect of a magnetic field caused by liquid crystal display, while Li et al. [41] used magnitopuncture, a more localised magnetic-based intervention. Penna et al. [49] employed a transcranial direct current stimulation (tDCS) protocol during the MF task in an attempt to counter its negative effects. Two studies made use of odour as an intervention against MF [39, 52].
Regarding the effects on the different indicators of MF, of which an overview can be found in Table 6, 20 of the physiological countermeasures were evaluated against a behavioural marker (performance on a specific battery of cognitively demanding tasks). Of these, 16 studies reported improvements in performance following use of the countermeasure [11,12,13, 41, 43, 44, 50,51,52,53, 56,57,58, 60,61,62] (see Table 4 for further details on this matter). Only Bailey et al. [42], Nagai et al. [48], Penna et al. [49] and Watanabe et al. [14] found no effect on the behavioural marker. All studies except Watanabe et al. [14] used subjective markers of MF. Of these 21 studies, 14 [11, 12, 41, 42, 44, 48, 50, 51, 53, 57, 58, 60,61,62] showed a significant difference following the physiological countermeasure (see Table 4 for further details on this matter). Also (neuro)physiological markers were monitored in ten studies investigating physiological MF countermeasures, half of which provided positive results [12, 14, 39, 42, 48] (see Table 4). Details regarding the specific MF markers that were employed can be found in Table 4.
3.4.3 Psychological
Three included studies used a psychological countermeasure against MF (see the Sect. 2 for a definition) [26, 37, 46]. Axelsen et al. [26] and Loch et al. [46] analysed the differences between a psychological and behavioural countermeasure (see Sect. 3.4.1). In the study of Axelsen et al. [26], the difference between binaural beats and mindfulness (where the authors made the additional distinction between novice and experienced practitioners) and their effects on MF was explored. Furthermore, Loch and colleagues [46] investigated the effect of systematic breathing with and without mental imagery in comparison with a powernap as recovery from MF. Lastly, one study conducted an experiment in which the researchers attempted to enhance motivation through a time-based reward manipulation during the MF-inducing task [37].
Table 4 presents the outcome measures of these studies. All three studies that evaluated a psychological countermeasure used performance on cognitive tasks as behavioural markers to determine whether the employed countermeasures were successful in counteracting MF. As such, Axelsen et al. [26] and Hopstaken et al. [37] observed significant results (see Table 6). The study of Hopstaken and colleagues [37] was the only one of the included studies that searched for and also found a significant difference in pupil diameter, which they used as a physiological marker of MF. In their study, Hopstaken et al. [37] evaluated the MF-counteracting properties of a reward manipulation (i.e., the length of the cognitive task depended on how well they performed). They observed a significant decrease in pupil diameter due to MF and an increase in it after introducing the reward manipulation. Three other studies [20, 27, 39] assessed electrooculography but failed to observe significant changes due to the countermeasure used (see Table 4). Subjective outcomes were utilised by two [37, 46] of these three studies, but the psychological manipulations only improved subjective markers of MF in one of these [37] (see Table 4 for more information on this matter).
3.5 Timing of the Countermeasure
3.5.1 Up to a Week Before the Mental Fatigue-Inducing Activity
A total of five studies investigated the implementation of a physiological countermeasure over a period of 5–7 days before the moment of MF [13, 14, 48, 56, 58]. Two of these protracted strategies [13, 14] were required due to the typical loading phase of creatine utilised [63]. While, on the other hand, the commercially available chicken essence used by Nagai et al. [48] and Yamano et al. [58] is traditionally used over a period of 7 days or longer due to its potential as remedy for physical and mental fatigue. A 7-day loading strategy was also utilised in a study of Ataka et al. [56], wherein the MF-counteracting properties of caffeine and D-ribose were compared. Ataka et al. [56] suggested that both interventions could possibly counteract MF via their potential to increase the adenine nucleotide availability (see Table 4 for details and Table 5 for an overview).
3.5.2 On the Same Day as the Mental Fatigue-Inducing Activity
Nine studies explored the effect of physiological countermeasures minutes to hours before the MF task [11, 44, 50, 51, 53, 57, 60,61,62]; full details on the moment of application can be found in Tables 4 and 5. Six of the studies used countermeasures, such as caffeine and cocoa flavanols, that have short-term impacts and were administered within an hour of the MF protocol [11, 44, 50, 51, 60, 61]. In the three other studies, countermeasures were given 90 min before the MF task [53, 62] or, in the case of Kennedy et al. [57], the MF task was employed 1, 3 and 6 h after the intake of the countermeasure.
3.5.3 During the Mental Fatigue-Inducing Activity
Ten studies investigated the effect of countermeasures during the MF task [12, 20, 27, 37,38,39, 41, 42, 49, 52] (see Table 5 for an overview). Six studies included countermeasures which did not directly interfere with the MF-inducing task [20, 38, 39, 41, 49, 52]. Two studies implemented an odour during the MF task [39, 52], three used a non-invasive brain stimulation intervention [38, 41, 49] and Guo et al. [20] implemented music during the MF task. The four other investigations [12, 27, 37, 42] were incorporated in the MF task, with two studies using mouth rinses at predetermined moments during the MF task [12, 42]. Hopstaken et al. [37] implemented a time-based reward in the last block of the MF task, and Hayashi and colleagues [27] investigated the difference between a 20-min nap or rest break in the middle of the task. The duration of each countermeasure can be found in Table 4.
3.5.4 After the Mental Fatigue-Inducing Activity
Nine studies assessed the restorative effect of countermeasures after the MF task [26, 40, 43, 45,46,47, 54, 55, 59], of which three studies inspected a countermeasure with a stress-relieving background [47, 54, 55] and one study used a carbohydrate mouth rinse after the MF task. Axelsen et al. [26] investigated the differences between binaural beats (i.e., an auditory intervention suspected to have an impact on cognitive functioning [64]) and mindfulness, and applied both interventions immediately after the MF task for a duration of 12 min. Loch et al. [46] used 20-min sessions of a powernap and systematic breathing with and without mental imagery as recovery strategies and applied these countermeasures 5 min after the mentally fatiguing task. Lim and colleagues [45] studied the combination of binaural beats and mechanical massage for 20 min as strategies to counteract MF 5 min after the MF task. Full details on the moment and duration of application can be found in Table 4.
4 Discussion
The purpose of this review was to provide an overview of countermeasures against the negative consequences of MF. A total of 33 studies were included in the present systematic review. Of these, eight studies investigated a behavioural countermeasure, 22 a physiological one, one study a psychological countermeasure and two studies investigated both a behavioural and psychological countermeasure. The vast majority of the included studies reported that, based on an observed positive impact on behavioural (e.g., task or sport performance) and/or subjective markers of MF (e.g., visual analogue scale for MF or alertness), the investigated countermeasure successfully counteracted MF. However, regarding the (neuro)physiological markers of MF, it is difficult to draw a strong conclusion as only half of the included studies evaluated the effect on these markers in addition to evaluating the effect on behavioural and/or subjective markers. Nonetheless, first indications show that the used countermeasures had a positive impact on MF-related brain activity changes (e.g., elevated P3-amplitudes and alpha activity in favour of the used countermeasure [20, 39, 40]). See Table 6 for a general overview.
4.1 Countermeasures and Their Mechanism(s)
4.1.1 Physiological Countermeasures
To counter and/or mitigate the effects of MF, 22 studies have evaluated interventions that are proposed to act directly through physiological pathways. Fourteen of them used a form of neuroenhancer, that is, a substance with the purpose to augment learning and memory, attention and vigilance, mood and behaviour [65]. Among the substances used to combat MF, the most tested is caffeine. Azevedo et al. [11] found that 5 mg.kg−1 caffeine resulted in a 14% increase in endurance performance. This was confirmed by Franco-Alvarenga et al. [61], who reported a reduced (1.7%) time to complete a 20-km cycling session and an increase (3%) in mean power. Based on these two studies, it appears that caffeine is able to reduce MF-related decrements in endurance performance and that a 5-mg.kg−1 dosage is sufficient to do so. Furthermore, caffeine also successfully countered the negative effect of MF on cognitive performance [44, 56]. Ataka et al. [56] found that after a 7-day intake period of 200 mg of caffeine per day, task performance on an advanced trail-making test in a mentally fatigued state was improved in comparison with the placebo. Further, Kennedy and Scholey [44] reported an improved cognitive performance following the combined intake of caffeine and carbohydrates, but only with the combination of 46 mg of caffeine with 68 g of glucose. Other combinations (38 mg of caffeine with 68 g of glucose and 33 mg caffeine with 60 g of glucose) were less successful in mediating the effects of MF. This would suggest that there is a certain minimal dose of caffeine required to counteract MF, which emphasizes the importance of using a relative dosage of caffeine that is based on body weight. Furthermore, Van Cutsem et al. [12] used a combination of caffeine and maltodextrin during the MF task, but as a mouth rinse. A beneficial effect of this solution was observed on the subjective feeling of MF and also improved task performance on the Stroop and flanker task (more specifically the accuracy) in a mentally fatigued state. This may be due to the activation of both the orbitofrontal and dorsolateral prefrontal cortex, which improved cognitive performance [66]. The use of solely a carbohydrate mouth rinse during the MF task also appears to be useful. The latter finding was demonstrated by Bailey et al. [42], who found improvements of both subjective and neurophysiological markers of MF. Additionally, Brietzke et al. [43] found a positive effect of mouth rinsing with carbohydrates on a maximal incremental test, but they applied it after the MF task. The above shows that nutritional MF countermeasures, such as caffeine, are currently known to work in two ways. The first follows ingestion and thus involves the mechanism of absorption via the gastrointestinal system. Caffeine ultimately crosses the blood–brain barrier and exerts its effect on brain neurotransmission. In other words, by blocking the adenosine receptors it counteracts most of the inhibitory effects of adenosine on neuroactivity, dopamine release and arousal (certainly in the prefrontal cortex and hippocampus, brain areas that could be linked to MF [16, 17]). Secondly, caffeine may act through the activation of specific taste receptors (specifically in the study by Van Cutsem et al. [12, 67], bitter taste receptors were suggested to play a role) that trigger a transduction cascade to the brain and thus may result in the activation of brain regions that play an important role in MF, such as the anterior cingulate cortex. Also, for carbohydrate mouth rinses a transduction cascade can occur. This was supported by research in which the limbic system as well as the primary sensorimotor cortex and neural networks involved in sensory perception were activated after the intervention [68].
The possible importance of receptors that are present in the mouth and nose to counteract MF, and as such the subsequent brain activation [69], is further substantiated by two studies that investigated the effect of odour on MF [39, 52]. Both studies found significant improvements in the odour conditions. Kato et al. [39] found a significantly reduced increase in reaction time in the MF group compared with the control group, while Saito et al. [52] found a better response rate when using a newly developed odour. This newly developed odour (called MCMP, a honey-like floral odour) was created to work on the Hex-Hex Mix receptors. Activating these receptors has been shown to attenuate fatigue and, as such, to effectively counteract MF [52]. However, more general and well-known odours, as used by Kato et al. [39], are also apparently able to counter MF. The suggested explanation for these findings currently is the connection of the olfactory bulb with higher brain centres like the amygdala-hippocampal complex [70]. The amygdala plays an important role in the process of the value of rewards associated with cognitive effort [71, 72], which is then projected to the anterior cingulate cortex for decision making, based on the evaluation of rewards and potential costs. Based on their results, Kato et al. [39] suggested that intermittent exposure to several odours (i.e., citral, green and menthol) of neutral pleasantness leads to positive feelings and as such mitigates MF-related effects on cognitive performance. With this suggestion they emphasized the importance of positive feelings and affective valence as a mediating mechanism of MF-counteracting properties of odours.
Besides caffeine, multiple other nutritional interventions were evaluated. Kennedy et al. [60] used a nutritional supplement with added guarana (Berocca Boost®) and found an improvement in speed (~ 30 ms in favour of the intervention) and accuracy (approximately 11% more accurate) on performing the Rapid Visual Information Processing task, while attenuating the subjective feelings of MF. While it might seem that guarana could be a good countermeasure against MF, it should be noted that the used supplement also contained 40 mg of caffeine. Given the mix of guarana and caffeine, and the high risk of bias within this study, no conclusion on the use of guarana alone against MF could be drawn and subsequently these results could also be interpreted as additional evidence for the use of caffeine as a MF countermeasure. Two studies [13, 14] investigated the ability of creatine supplementation to counteract MF due to its cognitive function improvement properties [73]. They both found an increase in the performances of their cognitive tasks [13, 14], but Van Cutsem et al. [13] did not find an effect of creatine on the evaluated sport-specific visuomotor performance (i.e., badminton-related reaction time) in a mentally fatigued state. Van Cutsem et al. [13] argued that the occurrence of MF could be (partly) linked to a drop in the brain’s phosphocreatine concentration; as such, creatine supplementation might be able to increase brain energy resources and postpone the drop in phosphocreatine and subsequently also the emergence of MF. Further evidence is, however, necessary to substantiate this mechanism. Besides creatine supplementation, cocoa flavanols were, based on their positive impact on subjective and behavioural markers of MF [53], proven effective to counteract MF. This can presumably be explained by their capacity to improve the endothelial function with an increase in blood flow, possible glucose uptake, cerebral oxygenation and ultimately cognition [74]. However, the link between MF and oxygenation is an underexplored mechanism. Kennedy et al. [57] explored the use of orally administered capsules filled with a certain dose of essential oils (Mentha spicata and M. piperita) and their effects on brain function. They found an increase in performance in a visual sustained attention task at 1 h and 3 h after the highest dose, and an attenuated subjective feeling of fatigue with both doses. Two studies explored the use of chicken essence in countering MF [48, 58]. Chicken essence is rich in amino acids and the dipeptides carnosine and anserine [75, 76]. These have shown to be effective in elderly subjects and patients with mild cognitive impairment, with clinical efficacy against the cognitive decline [77]. Also, several amino acids (e.g., tyrosine, which is a precursor of dopamine) seem to improve cognitive performance [74, 78]. Both Nagai et al. [48] and Yamano et al. [58] showed that consuming chicken essence in the morning for a 7-day period is effective in reducing the feeling of fatigue when performing cognitive tasks and improves task performance. Therefore, it seems that chicken essence is a useful countermeasure for MF; however, it should be noted that both studies were supported by the same company that also provided the chicken essences (and which are commercially available). A non-sponsored study or one investigating the active components of chicken essence might therefore be of added value to confirm the usefulness of chicken essence in overcoming MF.
Interventions other than nutritional ones may also have a positive effect on MF through physiological mechanisms. The pharmacological intervention modafinil is perhaps a logical one to combat MF given its action as a modulator of dopaminergic and adrenergic pathways [65, 79] (see Colzato and Mourits [80] for more information on the mechanisms of action of modafinil). However, it should be noted that modafinil is a banned drug in competition, which limits its use outside a scientific setting but which could be useful in the military [81] and certain occupational settings. In specific situations (e.g., when sleep deprived), modafinil is known to increase attention and vigilance and reduce reaction time and general fatigue [65, 81], while possibly influencing the effort-related decision-making pathways [71, 82]. Additionally, it is also suggested that it has fewer side effects and a longer-lasting effect in comparison with caffeine [65]. However, despite the theoretical advantages of modafinil on MF, Rattray et al. [62] only found improved performance on a cognitive task evaluating executive functioning and not on cycling endurance performance following an MF-inducing task. Additionally, they also found higher heart rates and disrupted sleep patterns in the intervention group, which are important side effects of modafinil.
Interventions with non-invasive brain stimulation techniques were also evaluated to perhaps positively impact MF via a physiological mechanism, possibly by altering the threshold required for an action potential within the nervous system [83, 84]. Three studies used a magnetic-based intervention during the MF-inducing task [38, 41, 49]. Ishihara et al. [38] used a general magnetic field to combat MF, but failed to show differences in MF exposure after the task. Li and colleagues [41] used a more targeted magnetic stimulation on acupuncture points and did find a significant difference in reaction time between the control and study group post-task in favour of the study group. This observation was confirmed by Yang et al. [85] and as such it is assumed that magnitopuncture is an adequate countermeasure against MF. However, it is important to note that the study of Yang et al. [85] did not include a control group, which lowers the quality of the study and puts their conclusions into question. Also, it remains unclear whether participants were blinded for the intervention in the study of Li et al. [41]; therefore, the conclusions should be read with caution. Penna et al. [49] used tDCS in order to counter the decrements due to MF in swimming performance. By stimulating the left temporal lobe, the excitability of the insular cortex would increase and therefore influence the awareness of subjective feelings (e.g., subjective feeling of physical exertion) playing a role during exercise. Unfortunately, they were unable to prove that MF negatively affected swimming performance, which makes it difficult to give a conclusive answer regarding the effectiveness of tDCS and MF-related decrements in physical performance. Despite the absence of an effect of MF on swimming performance, the effectiveness of tDCS to counteract MF could still be evaluated. Penna et al. [49] observed a greater fatigue perception and a drop in cognitive performance due to prolonged performance on the mentally fatiguing task (i.e., a Stroop task). However, no difference in perceived fatigue or cognitive performance was found between the brain stimulation and sham condition. The inability of tDCS to counteract MF while performing a prolonged cognitive task could be due to the stimulated area not being related to cognitive performance. It would therefore be interesting to see if the stimulation of other regions known to impact cognitive function would be more successful in order to make a firm conclusion regarding the use of tDCS as a MF countermeasure [12, 86].
4.1.2 Behavioural and Psychological Countermeasures
Eleven studies used a behavioural and/or psychological countermeasure against MF. One of the most-used behavioural countermeasures was the application of auditory stimuli. Guo et al. [20] used instrumental folk music, while Jacquet et al. [40] let the participants themselves select the songs. The choice to use music as a countermeasure is a reasonable one as music has the potential to improve cognitive and physical performance [21, 22, 87], most plausibly via its beneficial effects on affective valence and motivation [87]. Motivation and mental fatigue are two psychobiological states that can be dissociated from each other [88] but that have clearly been shown to impact one another [37, 89,90,91]. Indeed, in the study of Guo et al. [20], they found that the intervention group maintained their performance through the MF task while listening to music. Also, the subjective feeling of MF was significantly lower in comparison with the control groups [20]. Two other studies [26, 45] used binaural beats as auditory stimuli because of the positive effect of binaural beats on mind wandering and cognitive flexibility [64, 92], two actions which play a role in MF. Therefore, influencing these two processes might combat the decrements in performance due to MF. Indeed, both studies found a positive effect of binaural beats in reducing the effect of MF. Axelsen et al. [26] even observed a slight increase of 3.8% on cognitive performance after using binaural beats to counter the negative effects of MF, possibly mediated by less mind wandering. However, it should be noted that in the study of Lim et al. [45], this was combined with a mechanical massage chair. The combination of binaural beats and mechanical massage could have reinforced each other. Also the mechanical massage alone was found to positively impact the recovery from MF, a finding that is also confirmed by the study of Mizuno et al. [47] where they implemented a massage provided by a mild stream function while bathing. They suggested that this could be related to the stress-alleviating effect of mild-stream bathing, which could also underly the positive effect of listening to music [93, 94], sitting next to a pellet stove [54] and exposure to a natural environment [55, 95] on MF. Therefore, potentially, similar brain mechanisms (e.g., increase in dopamine) might play a role in both creatine/caffeine and non-nutritional countermeasures (such as music and massage).
Axelsen et al. [26] also evaluated the potential benefits of on-the-spot mindfulness in the recovery from MF. Mindfulness is a behavioural therapy that provides training in self-regulation in a systematic way [92], which might be appropriate for countering MF [96]. Self-regulation is the ability to exert control over one’s own behaviour. It is defined as the ability to overcome urges and habitual reactions, such as emotions and impulses. Importantly, self-regulation is regarded as a capability based on a limited capacity of internal resources. By engaging in activities that require self-regulation, such as cognitive tasks used to induce MF, this internal reserve of resources is being drained (i.e., being mentally fatigued). By training to improve this self-regulation capacity, it is suggested that future self-regulation activities will cost fewer internal resources [96,97,98]. This was the case in the study of Axelsen et al. [26], but only for the experienced mindfulness group, the novice mindfulness group performed even worse on the cognitive task compared with the control group. Since mindfulness was new to the novice mindfulness group, and thus requiring a certain level of self-regulatory resources, this impaired performance might be explained by the cognitive task (i.e., trying mindfulness for the first time) requiring extra self-regulatory resources and even aggravating the emergence of MF. Therefore, it can be concluded that mindfulness could be a helpful recovery strategy for MF, but only after a certain period of training [99]. Another long-term countermeasure against MF could be brain endurance training [25, 100]. The possible mechanism behind this is that the ability to tolerate a certain amount of mental exertion is trainable and as such could prevent/postpone the occurrence of MF. Although this is still a relatively unknown field, the first studies seem rather promising [25, 99, 100].
Loch and colleagues [46] evaluated the recovery effect of a 20-min powernap on MF but failed to find a significant effect. This led them to the conclusion that a period of rest with mental recovery strategies might be enough to recover from MF. This is exactly what Hayashi et al. [27] did, but in contrast to Loch et al. [46], they introduced this 20-min rest period during the MF task and compared it with a 20-min nap. They found that a short nap was more successful in maintaining subjective alertness and kept performance at a higher level and subjective feeling of MF at a lower level than a moment of rest. This could indicate that a nap is more useful as a countermeasure during the MF task compared with after the MF task. However, given the heterogeneity in duration and the type of MF task and MF markers, it is difficult to formulate a final conclusion on this matter. The use of a mid-task break also seems to be a countermeasure worth looking further into, as previously published research already provided promising results by making use of tasks shorter than 30 min [101, 102]. The choice of activity during this break of course seems to be of importance. Gao et al. [103] stated that implementing a mid-task 15-min cycling exercise led to an improved reaction time and response accuracy. However, it should be noted that they compared this to a group without a break. On the other hand, given the benefits of physical activity regarding executive functioning [104], Jacquet et al. [40] and Oberste et al. [59] investigated the effect of light physical aerobic exercise after the MF task to counter the negative consequences due to MF. They both found a positive effect or no negative effect of their intervention, meaning that a physical activity to recover from a mentally fatiguing task is useful. An explanation for the effectivity of this countermeasure can be found in the dopamine-releasing effect of physical activity, which also strengthens the theory that adenosine and dopamine may play an important mechanistic role in the MF-associated decrease in performance [17, 104, 105].
A last non-physiological countermeasure is the use of motivation against MF. In the study of Hopstaken et al. [37], it was found that by adding a time-based reward towards the end of the task, participants could restore task engagement and performance despite staying subjectively mentally fatigued. This is in accordance with the results of Herlambang et al. [90] and Brown and Bray [106], who used monetary incentives to overcome MF, but it should be taken into account that both studies might be biased due to their study design. However, it is clear that motivation may be an effective countermeasure for MF if introduced in a proper way [88, 91, 107, 108].
4.2 Practical Application of Countermeasures
Given the wide variety in all discussed countermeasures, it may be difficult to decide which countermeasure could be useful, effective and easy to use within a setting where MF could emerge and where it is undesired. For example, during a long game or ride with no possibility to take a break, an easy-to-consume nutritional countermeasure could be the preferred option to counteract MF. On the other hand, when competing in a tournament with multiple rounds (e.g., judo, badminton), behavioural interventions such as listening for 20 min to music could specifically be of added value to deal with MF [26, 45]. If an individual knows there will be a cognitively demanding period within days, countermeasures such as creatine [13, 14] or chicken essence [48, 58] seem to be good candidates, but are of course more time consuming and require some planning. If MF is likely to occur within hours, the most appropriate countermeasure would be a 5 mg.kg−1 of body mass dose of caffeine [11, 44, 61], a caffeine-maltodextrin mix [12], cocoa flavanols [53] or one of the other countermeasures used by the research groups of Kennedy, Reay and Scholey [50, 51, 53, 57, 60]. These are all nutritional-based countermeasures that are easy and fast to use. However, one should be aware of the possible side effects of the mentioned countermeasures. For example, using higher doses of caffeine might lead to gastrointestinal distress [74], which might be unwanted in multiple situations (e.g., endurance performance), but also cause tremors [65], which could be a disadvantage for sport- and work-specific psychomotor performance. Regarding the other countermeasures, further research is needed to exclude undesirable side effects and to pinpoint the most advantageous dosage.
If there is no time to anticipate MF, countermeasures during the mentally demanding activity could be of use. Depending on the situation one might choose to use odours in overcoming the negative effects of MF [39, 52]. However, given the volatility of odours, their use is only possible in situations where one stays in the same place (e.g., table tennis [5], driving a car [109] or esports [110, 111]) or where the odour can be sprayed on clothing or the body [112]. In addition, the use of non-invasive brain stimulation techniques in counteracting MF [38, 41, 49] is also a possibility. This kind of countermeasure might, however, be impractical due to the required equipment. One of the most practical interventions in overcoming MF is the use of motivational stimuli [37]. The difficulty there is finding the adequate motivational determinants as this is very individually dependent [108, 113].
To recover from MF, different countermeasures have also been identified. The use of 20 min of binaural beats [26, 45] and long-term mindfulness [26] are promising countermeasures for athletes as these require no extra material or supplements and are easy to implement in a training regimen. Besides that, activities enhancing dopamine release [45, 47, 54, 55] can be easy to conduct after a MF task. However, as with motivation, it is a question of finding the adequate countermeasure for each individual.
4.3 Limitations and Future Perspectives for Research
The field of MF research in sport science lacks homogeneity in the methodological inducement of MF. There is no consensus on the gold standard method to induce MF; discussions on the optimal length and intensity of the cognitive challenge and on the most valid behavioural, subjective and (neuro)physiological markers of MF are still ongoing. Without a doubt, these discussions are of major importance to further unravel MF and the mechanism(s) behind it. The degree of MF induced by a certain task depends on the skill of the performer/participant, on the way it cognitively engages the performer/participant, the motivational level, etc. Moreover, the most valid marker of MF, be it a behavioural, subjective and/or (neuro)physiological one, will differ in each specific situation. As such, this challenging field of research will, most probably, keep on struggling with standardization issues. This does not need to be a major issue if study results are interpreted while taking into account these possible methodological differences and their impact on MF and on the observed efficacy of the evaluated MF countermeasure. Throughout the present review, however, it was clear that methodological differences exist, which complicated the comparison of the efficacy of each of the evaluated MF countermeasures. Specifically, the heterogeneity in MF inducement methods and in the employed MF markers resulted in the inability to execute any type of meta-analyses that would have been of added value.
Based on the present systematic review, two important research avenues can be distinguished to advance this field of research; a practical and a mechanistic line. In terms of the practical research avenue, as stated by a recent systematic review [114], it would be interesting to gather more insight on the optimal dosage and timing of each of the evaluated MF countermeasures. Regarding caffeine, it is clear that a dosage of 5 mg.kg−1 of body mass has been proven to be effective to counter MF. While for creatine, because the two included studies used a different dose (i.e., 20 g/day [13] and 8 g/day [14]) and different periods of supplementation (i.e., 7 days [13] and 5 days [14]), additional research to find the optimal design for application is needed. This would be important not only for creatine, but also for the other included MF countermeasures in the present systematic review; dosage and timing studies to evaluate the minimal dosage and timing that is needed to effectively counteract MF are necessary. In addition, before proceeding to dosage and timing studies, the usefulness of multiple countermeasures needs to be confirmed in replication studies [43, 50, 53].
From a mechanistic perspective, it would be worthwhile to see the mechanistic pathways that are suggested to be in place for some MF countermeasures confirmed. This could be attained via studies that incorporate outcome measures that are able to give insight into these mechanistic pathways (e.g., electroencephalography, near infrared spectroscopy) or via studies that evaluate the effectiveness of other interventions that we know counteract MF via the same mechanistic pathway, to eventually confirm the presence of that particular mechanistic pathway. For example, modafinil is thought to counteract MF via its impact on dopaminergic activity; the importance of this impact on dopaminergic activity to counteract MF could be further substantiated by evaluating the ability of methylphenidate (i.e., a dopamine reuptake inhibitor) to counteract MF [115].
To end, no countermeasure totally resolved MF, and therefore it would be interesting to see if the combination of different types of countermeasures (e.g., caffeine and music) would lead to better results in countering MF. The combination of the mechanistic pathways through which different countermeasures are suggested to positively impact MF could work synergistically in the sense that the positive impact of these different countermeasures could enhance each other, or could even reinforce one another.
5 Conclusion
Mental fatigue is known to impair physical and/or cognitive performance. The purpose of this review was therefore to create an overview of possible MF countermeasures and to discuss the underlying mechanisms and the practical application of these MF countermeasures, to eventually be able to use them across multiple contexts (e.g., sporting, occupational or military contexts). A total of 33 studies investigated a specific type of countermeasure for MF and its negative effects on sport performance or on cognitive performance. In general, a vast majority of the included countermeasures had a positive effect on behavioural and subjective markers of MF. Unfortunately, only 19 of the included studies used (neuro)physiological outcome measures, which hindered drawing a definitive conclusion on the effect of the employed countermeasures on (neuro)physiological markers of MF. The most investigated countermeasures with the most reliable evidence seem to be the use of caffeine before and/or during the occurrence of MF and the use of a noticeable and pleasant odour during the MF task. In addition, the use of strategies (e.g., rewards) to increase motivation, seems to be a promising psychological method to counteract MF. To provide in-detail practical guidelines for the real-life application of MF countermeasures, more research has to be performed on the underlying mechanisms and the optimal dosage and timing of application/intake.
References
Rozand V, Lebon F, Papaxanthis C, Lepers R. Effect of mental fatigue on speed-accuracy trade-off. Neuroscience. 2015;297:219–30. https://doi.org/10.1016/j.neuroscience.2015.03.066.
Van Cutsem J, Marcora S, De Pauw K, et al. The effects of mental fatigue on physical performance: a systematic review. Sports Med. 2017;47(8):1569–88. https://doi.org/10.1007/s40279-016-0672-0.
Brown DMY, Graham JD, Innes KI, et al. Effects of prior cognitive exertion on physical performance: a systematic review and meta-analysis. Sport Med. 2020. https://doi.org/10.1007/s40279-019-01204-8.
McMorris T, Barwood M, Hale BJ, et al. Cognitive fatigue effects on physical performance: a systematic review and meta-analysis. Physiol Behav. 2018;188:103–7. https://doi.org/10.1016/j.physbeh.2018.01.029.
Habay J, Van Cutsem J, Verschueren J, et al. Mental fatigue and sport-specific psychomotor performance: a systematic review. Sports Med. 2021;51(7):1527–48. https://doi.org/10.1007/s40279-021-01429-6.
Smith MR, Thompson C, Marcora SM, et al. Mental fatigue and soccer: current knowledge and future directions. Sports Med. 2018;48(7):1525–32. https://doi.org/10.1007/s40279-018-0908-2.
Galy E, Mélan C. Effects of cognitive appraisal and mental workload factors on performance in an arithmetic task. Appl Psychophysiol Biofeedback. 2015;40(4):313–25. https://doi.org/10.1007/s10484-015-9302-0.
Borghini G, Vecchiato G, Toppi J et al. Assessment of mental fatigue during car driving by using high resolution EEG activity and neurophysiologic indices. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 70 (cm); 2012. pp. 6442–6445. https://doi.org/10.1109/EMBC.2012.6347469.
Verschueren J, Tassignon B, Pluym B, et al. Bringing context to balance: development of a reactive balance test within the injury prevention and return to sport domain. Arch Physiother. 2019;9(1):6. https://doi.org/10.1186/s40945-019-0057-4.
Sun Y, Lim J, Dai Z, et al. The effects of a mid-task break on the brain connectome in healthy participants: a resting-state functional MRI study. Neuroimage. 2017;152:19–30. https://doi.org/10.1016/j.neuroimage.2017.02.084.
Azevedo R, Silva-Cavalcante MD, Gualano B, et al. Effects of caffeine ingestion on endurance performance in mentally fatigued individuals. Eur J Appl Physiol. 2016;116(11–12):2293–303. https://doi.org/10.1007/s00421-016-3483-y.
Van Cutsem J, De Pauw K, Marcora S, et al. A caffeine-maltodextrin mouth rinse counters mental fatigue. Psychopharmacology. 2018;235(4):947–58. https://doi.org/10.1007/s00213-017-4809-0.
Van Cutsem J, Roelands B, Pluym B, et al. Can creatine combat the mental fatigue-associated decrease in visuomotor skills? Med Sci Sports Exerc. 2020;52(1):120–30. https://doi.org/10.1249/MSS.0000000000002122.
Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42(4):279–85. https://doi.org/10.1016/S0168-0102(02)00007-X.
Glade MJ. Caffeine-not just a stimulant. Nutrition. 2010;26(10):932–8. https://doi.org/10.1016/j.nut.2010.08.004.
Cappelletti S, Daria P, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2014;13(1):71–88. https://doi.org/10.2174/1570159x13666141210215655.
Martin K, Meeusen R, Thompson KG, et al. Mental fatigue impairs endurance performance: a physiological explanation. Sports Med. 2018;48(9):2041–51. https://doi.org/10.1007/s40279-018-0946-9.
Meeusen R, Van Cutsem J, Roelands B. Endurance exercise-induced and mental fatigue and the brain. Exp Physiol. 2020. https://doi.org/10.1113/EP088186.
Davis JM, Zhao Z, Stock HS, et al. Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Regul Integr Comp Physiol. 2003;284(253–2):399–404. https://doi.org/10.1152/ajpregu.00386.2002.
Guo W, Ren J, Wang B, Zhu Q. Effects of relaxing music on mental fatigue induced by a continuous performance task: behavioral and ERPs evidence. PLoS One. 2015;10(8):1–12. https://doi.org/10.1371/journal.pone.0136446.
Franco-Alvarenga PE, Brieztke C, Canestri R, Pires FO. Psychophysiological responses of music on physcial performance: a critical review. Revista Brasileira de Ciência e Movimento. 2019;27(2):218. https://doi.org/10.31501/rbcm.v27i2.9908.
Angel LA, Polzella DJ, Elvers GC. Background music and cognitive performance. Percept Mot Skills. 2010;110(3C):1059–64. https://doi.org/10.2466/04.11.22.pms.110.c.1059-1064.
Russell S, Jenkins D, Smith M, et al. The application of mental fatigue research to elite team sport performance: new perspectives. J Sci Med Sport. 2019;22(6):723–8. https://doi.org/10.1016/j.jsams.2018.12.008.
Roelands B, Kelly V, Russell S, Habay J. The physiological nature of mental fatigue: current knowledge and future avenues for sport science. Int J Sports Physiol Perform. 2022;17(2):149–50. https://doi.org/10.1123/ijspp.2021-0524.
Dallaway N, Lucas SJE, Ring C. Concurrent brain endurance training improves endurance exercise performance. J Sci Med Sport. 2021;24:405–11. https://doi.org/10.1016/j.jsams.2020.10.008.
Axelsen JL, Kirk U, Staiano W. On-the-spot binaural beats and mindfulness reduces the effect of mental fatigue. J Cogn Enhanc. 2020;4(1):31–9. https://doi.org/10.1007/s41465-019-00162-3.
Hayashi M, Chikazawa Y, Hori T. Short nap versus short rest: recuperative effects during VDT work. Ergonomics. 2004;47(14):1549–60. https://doi.org/10.1080/00140130412331293346.
Boksem MAS, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006;72(2):123–32. https://doi.org/10.1016/j.biopsycho.2005.08.007.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71.
Giboin LS, Wolff W. The effect of ego depletion or mental fatigue on subsequent physical endurance performance: a meta-analysis. Perform Enhanc Health. 2019;7(1–2): 100150. https://doi.org/10.1016/j.peh.2019.100150.
Fortes LS, Lima-Junior D, Nascimento-Júnior JRA, et al. Effect of exposure time to smartphone apps on passing decision-making in male soccer athletes. Psychol Sport Exerc. 2019;44:35–41. https://doi.org/10.1016/j.psychsport.2019.05.001.
Gantois P, Caputo Ferreira ME, de Lima-Junior D, et al. Effects of mental fatigue on passing decision-making performance in professional soccer athletes. Eur J Sport Sci. 2020;20(4):534–43. https://doi.org/10.1080/17461391.2019.1656781.
Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science. 1983;220(4594):327–9. https://doi.org/10.1126/science.6836276.
Smith MR, Coutts AJ, Merlini M, et al. Mental fatigue impairs soccer-specific physical and technical performance. Med Sci Sports Exerc. 2016;48(2):267–76. https://doi.org/10.1249/MSS.0000000000000762.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. https://doi.org/10.1186/s13643-016-0384-4.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. https://doi.org/10.1136/bmj.l4898.
Hopstaken JF, Van Der LD, Bakker AB. Shifts in attention during mental fatigue: evidence from subjective, behavioral, physiological, and eye-tracking data [Journal of Experimental Psychology: Human Perception and Performance, 42, 6, (2016), (878-889)], doi: 10.1037/xhp0000189. J Exp Psychol Hum Percept Perform. 2016;42(9):1442. https://doi.org/10.1037/xhp0000300.
Ishihara I, Ikushima M, Horikawa J, et al. A very low level of magnetic field exposure does not affect a participant’s mental fatigue and stress as much as VDT work. J UOEH. 2005;27(1):25–40. https://doi.org/10.7888/juoeh.27.25.
Kato Y, Endo H, Kobayakawa T, et al. Effects of intermittent odours on cognitive-motor performance and brain functioning during mental fatigue. Ergonomics. 2012;55(1):1–11. https://doi.org/10.1080/00140139.2011.633175.
Jacquet T, Poulin-Charronnat B, Bard P, et al. Physical activity and music to counteract mental fatigue. Neuroscience. 2021;478:75–88. https://doi.org/10.1016/j.neuroscience.2021.09.019.
Li Z, Jiao K, Chen M, Wang C. Reducing the effects of driving fatigue with magnitopuncture stimulation. Accid Anal Prev. 2004;36(4):501–5. https://doi.org/10.1016/S0001-4575(03)00044-7.
Bailey SP, Harris GK, Lewis K, et al. Impact of a carbohydrate mouth rinse on corticomotor excitability after mental fatigue in healthy college-aged subjects. Brain Sci. 2021;11(8):972. https://doi.org/10.3390/brainsci11080972.
Brietzke C, Franco-Alvarenga PE, Canestri R, et al. Carbohydrate mouth rinse mitigates mental fatigue effects on maximal incremental test performance, but not in cortical alterations. Brain Sci. 2020;10(8):1–15. https://doi.org/10.3390/brainsci10080493.
Kennedy DO, Scholey AB. A glucose-caffeine “energy drink” ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite. 2004;42(3):331–3. https://doi.org/10.1016/j.appet.2004.03.001.
Lim JH, Kim H, Jeon C, Cho S. The effects on mental fatigue and the cognitive function of mechanical massage and binaural beats (brain massage) provided by massage chairs. Complement Ther Clin Pract. 2018;32(April):32–8. https://doi.org/10.1016/j.ctcp.2018.04.008.
Loch F, Hofzum BA, Ferrauti A, et al. Acute effects of mental recovery strategies after a mentally fatiguing task. Front Psychol. 2020;11(December):1–13. https://doi.org/10.3389/fpsyg.2020.558856.
Mizuno K, Tanaka M, Tajima K, et al. Effects of mild-stream bathing on recovery from mental fatigue. Med Sci Monit. 2009;16(1):CR8–14.
Nagai H, Harada M, Nakagawa M, et al. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl Hum Sci J Physiol Anthropol. 1996;15(6):281–6. https://doi.org/10.2114/jpa.15.281.
Penna EM, Filho E, Campos BT, et al. No effects of mental fatigue and cerebral stimulation on physical performance of master swimmers. Front Psychol. 2021. https://doi.org/10.3389/fpsyg.2021.656499.
Reay JL, Kennedy DO, Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19(4):357–65. https://doi.org/10.1177/0269881105053286.
Reay JL, Kennedy DO, Scholey AB. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained “mentally demanding” tasks. J Psychopharmacol. 2006;20(6):771–81. https://doi.org/10.1177/0269881106061516.
Saito N, Yamano E, Ishii A, et al. Involvement of the olfactory system in the induction of anti-fatigue effects by odorants. PLoS One. 2018;13(3):1–18. https://doi.org/10.1371/journal.pone.0195263.
Scholey AB, French SJ, Morris PJ, et al. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24(10):1505–14. https://doi.org/10.1177/0269881109106923.
Tanaka M, Yamada H, Nakamura T, Watanabe Y. Effects of pellet stove on recovery from mental fatigue. Med Sci Monit. 2012;18(3):1–4. https://doi.org/10.12659/MSM.882519.
Tanaka M, Yamada H, Nakamura T, et al. Fatigue-recovering effect of a house designed with open space. Explore J Sci Heal. 2013;9(2):82–6. https://doi.org/10.1016/j.explore.2012.12.006.
Ataka S, Tanaka M, Nozaki S, et al. Effects of oral administration of caffeine and D-ribose on mental fatigue. Nutrition. 2008;24(3):233–8. https://doi.org/10.1016/j.nut.2007.12.002.
Kennedy D, Okello E, Chazot P, et al. Volatile terpenes and brain function: Investigation of the cognitive and mood effects of mentha × piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients. 2018. https://doi.org/10.3390/nu10081029.
Yamano E, Tanaka M, Ishii A, et al. Effects of chicken essence on recovery from mental fatigue in healthy males. Med Sci Monit. 2013;19(1):540–7. https://doi.org/10.12659/MSM.883971.
Oberste M, de Waal P, Joisten N, et al. Acute aerobic exercise to recover from mental exhaustion—a randomized controlled trial. Physiol Behav. 2021;241(September): 113588. https://doi.org/10.1016/j.physbeh.2021.113588.
Kennedy DO, Haskell CF, Robertson B, et al. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added guaraná (Paullinia cupana). Appetite. 2008;50(2–3):506–13. https://doi.org/10.1016/j.appet.2007.10.007.
Franco-Alvarenga PE, Brietzke C, Canestri R, et al. Caffeine improved cycling trial performance in mentally fatigued cyclists, regardless of alterations in prefrontal cortex activation. Physiol Behav. 2019;204:41–8. https://doi.org/10.1016/j.physbeh.2019.02.009.
Rattray B, Martin K, Hewitt A, et al. Effect of acute modafinil ingestion on cognitive and physical performance following mental exertion. Hum Psychopharmacol. 2019. https://doi.org/10.1002/hup.2700.
Burke LM. Practical issues in evidence-based use of performance supplements: supplement interactions, repeated use and individual responses. Sports Med. 2017;47(S1):79–100. https://doi.org/10.1007/s40279-017-0687-1.
Hommel B, Sellaro R, Fischer R, et al. High-frequency binaural beats increase cognitive flexibility: evidence from dual-task crosstalk. Front Psychol. 2016;7(AUG):1–7. https://doi.org/10.3389/fpsyg.2016.01287.
Daubner J, Arshaad MI, Henseler C, et al. Pharmacological neuroenhancement: current aspects of categorization, epidemiology, pharmacology, drug development, ethics, and future perspectives. Neural Plast. 2021;2021:8823383. https://doi.org/10.1155/2021/8823383.
De Pauw K, Roelands B, Knaepen K, et al. Effects of caffeine and maltodextrin mouth rinsing on P300, brain imaging, and cognitive performance. J Appl Physiol. 2015;118(6):776–82. https://doi.org/10.1152/japplphysiol.01050.2014.
Van Cutsem J, Marcora S. The effects of mental fatigue on sport performance—an update. Motiv Self Regul Sport Exerc. 2021. https://doi.org/10.4324/9781003176695.
Turner CE, Byblow WD, Stinear CM, Gant N. Carbohydrate in the mouth enhances activation of brain circuitry involved in motor performance and sensory perception. Appetite. 2014;80:212–9. https://doi.org/10.1016/j.appet.2014.05.020.
De Pauw K, Roelands B, Van Cutsem J, et al. Electro-physiological changes in the brain induced by caffeine or glucose nasal spray. Psychopharmacology. 2017;234(1):53–62. https://doi.org/10.1007/s00213-016-4435-2.
Broughan C. Odours, emotions, and cognition—how affect cognitive performance. Int J Aromather. 2002;12(2):92–8. https://doi.org/10.1016/S0962-4562(02)00033-4.
Chong TT-J, Apps M, Giehl K, et al. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 2017;15(2): e1002598. https://doi.org/10.1371/journal.pbio.1002598.
Masuo Y, Satou T, Takemoto H, Koike K. Smell and stress response in the brain: review of the connection between chemistry and neuropharmacology. Molecules. 2021;26(9):2571. https://doi.org/10.3390/molecules26092571.
Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D. Effects of creatine supplementation on cognitive function of healthy individuals: a systematic review of randomized controlled trials. Exp Gerontol. 2018;108:166–73. https://doi.org/10.1016/j.exger.2018.04.013.
Meeusen R, Decroix L. Nutritional supplements and the brain. Int J Sport Nutr Exerc Metab. 2018;28(2):200–11. https://doi.org/10.1123/ijsnem.2017-0314.
Suttiwan P, Yuktanandana P, Ngamake S. Effectiveness of essence of chicken on cognitive function improvement: a randomized controlled clinical trial. Nutrients. 2018;10(7):845. https://doi.org/10.3390/nu10070845.
Teoh S, Sudfangsai S, Lumbiganon P, et al. Chicken essence for cognitive function improvement: a systematic review and meta-analysis. Nutrients. 2016;8(1):57. https://doi.org/10.3390/nu8010057.
Caruso G, Godos J, Castellano S, et al. The therapeutic potential of carnosine/anserine supplementation against cognitive decline: a systematic review with meta-analysis. Biomedicines. 2021;9(3):253. https://doi.org/10.3390/biomedicines9030253.
Charernboon T, Jaisin K, Pattanaseri K. Chicken essence and cognitive function: a systematic review and meta-analysis. J Med Assoc Thai. 2016;99(July):S93–101.
Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–502. https://doi.org/10.1038/sj.npp.1301534.
Colzato LS, Mourits R. Modafinil. In: Theory-driven approaches to Cogn. Enhanc. Cham, Springer International Publishing; 2017. pp. 83–93
Van Puyvelde M, Van Cutsem J, Lacroix E, Pattyn N. A state-of-the-art review on the use of modafinil as a performance-enhancing drug in the context of military operationality. Mil Med. 2021. https://doi.org/10.1093/milmed/usab398.
Webber HE, Lopez-Gamundi P, Stamatovich SN, et al. Using pharmacological manipulations to study the role of dopamine in human reward functioning: a review of studies in healthy adults. Neurosci Biobehav Rev. 2021;120:123–58. https://doi.org/10.1016/j.neubiorev.2020.11.004.
Ahmed Z, Wieraszko A. Pulsed magnetic stimulation modifies amplitude of action potentials in vitro via ionic channels-dependent mechanism. Bioelectromagnetics. 2015;36(5):386–97. https://doi.org/10.1002/bem.21917.
Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J ECT. 2018;34(3):144–52. https://doi.org/10.1097/YCT.0000000000000510.
Yang S, Qiao Y, Wang L, Hao P. Magnetic stimulation at acupoints relieves mental fatigue: an event related potential (P300) study. Technol Health Care. 2017;25(S1):157–65. https://doi.org/10.3233/THC-171318.
Mcmorris T. Cognitive fatigue effects on physical performance: the role of interoception. Sports Med. 2020;50:1703–8. https://doi.org/10.1007/s40279-020-01320-w.
Terry PC, Karageorghis CI, Curran ML, et al. Effects of music in exercise and sport: a meta-analytic review. Psychol Bull. 2020;146(2):91–117. https://doi.org/10.1037/bul0000216.
Gergelyfi M, Jacob B, Olivier E, Zénon A. Dissociation between mental fatigue and motivational state during prolonged mental activity. Front Behav Neurosci. 2015. https://doi.org/10.3389/fnbeh.2015.00176.
Herlambang MB, Cnossen F, Taatgen NA. The effects of intrinsic motivation on mental fatigue. PLoS One. 2021;16(1):1–22. https://doi.org/10.1371/journal.pone.0243754.
Herlambang MB, Taatgen NA, Cnossen F. The role of motivation as a factor in mental fatigue. Hum Factors. 2019;61(7):1171–85. https://doi.org/10.1177/0018720819828569.
Schiphof-Godart L, Roelands B, Hettinga FJ. Drive in sports: how mental fatigue affects endurance performance. Front Psychol. 2018. https://doi.org/10.3389/fpsyg.2018.01383.
Kirk U, Wieghorst A, Nielsen CM, Staiano W. On-the-spot binaural beats and mindfulness reduces behavioral markers of mind wandering. J Cogn Enhanc. 2019;3(2):186–92. https://doi.org/10.1007/s41465-018-0114-z.
Ferreri L, Mas-Herrero E, Zatorre RJ, et al. Dopamine modulates the reward experiences elicited by music. Proc Natl Acad Sci. 2019;116(9):3793–8. https://doi.org/10.1073/pnas.1811878116.
Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–84. https://doi.org/10.1016/j.neuroimage.2005.05.053.
Kimura T, Yamada T, Hirokawa Y, Shinohara K. Brief and indirect exposure to natural environment restores the directed attention for the task. Front Psychol. 2021. https://doi.org/10.3389/fpsyg.2021.619347.
Englert C, Taylor IM. Motivation and self-regulation in sport and exercise. 1st ed. 2021. https://doi.org/10.4324/9781003176695.
Martin K, Thompson KG, Keegan R, Rattray B. Are individuals who engage in more frequent self-regulation less susceptible to mental fatigue? J Sport Exerc Psychol. 2019;41(5):289–97. https://doi.org/10.1123/jsep.2018-0222.
Leyland A, Rowse G, Emerson L-M. Experimental effects of mindfulness inductions on self-regulation: systematic review and meta-analysis. Emotion. 2019;19(1):108–22. https://doi.org/10.1037/emo0000425.
Coimbra DR, Bevilacqua GG, Pereira FS, Andrade A. Effect of mindfulness training on fatigue and recovery in elite volleyball athletes: a randomized controlled follow-up study. J Sports Sci Med. 2021. https://doi.org/10.52082/jssm.2021.1.
Filipas L, Martin K, Northey JM, et al. A 4-week endurance training program improves tolerance to mental exertion in untrained individuals. J Sci Med Sport. 2020;23(12):1215–9. https://doi.org/10.1016/j.jsams.2020.04.020.
Qi P, Gao L, Meng J, et al. Effects of rest-break on mental fatigue recovery determined by a novel temporal brain network analysis of dynamic functional connectivity. IEEE Trans Neural Syst Rehabil Eng. 2020;28(1):62–71. https://doi.org/10.1109/TNSRE.2019.2953315.
Sun Y, Lim J, Dai Z, et al. The effects of a mid-task break on the brain connectome in healthy participants: a resting-state functional MRI study. Neuroimage. 2017;152(February):19–30. https://doi.org/10.1016/j.neuroimage.2017.02.084.
Gao L, Zhu L, Hu L, et al. Mid-task physical exercise keeps your mind vigilant: evidences from behavioral performance and EEG functional connectivity. IEEE Trans Neural Syst Rehabil Eng. 2021;29:31–40. https://doi.org/10.1109/TNSRE.2020.3030106.
Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev. 2013;20(1):73–86. https://doi.org/10.3758/s13423-012-0345-4.
Marques A, Marconcin P, Werneck AO, et al. Bidirectional association between physical activity and dopamine across adulthood—a systematic review. Brain Sci. 2021;11(7):829. https://doi.org/10.3390/brainsci11070829.
Brown DMY, Bray SR. Effects of mental fatigue on physical endurance performance and muscle activation are attenuated by monetary incentives. J Sport Exerc Psychol. 2017;39(6):385–96. https://doi.org/10.1123/jsep.2017-0187.
Perry J, Ross M, Weinstock J, Gfeller J. Examining the interrelationships between motivation, conscientiousness, and individual endurance sport performance. J Sport Sci. 2017. https://doi.org/10.17265/2332-7839/2017.03.002.
Collins D. Motivation in sport and exercise. Sport Psychol. 2016. https://doi.org/10.1123/tsp.7.3.331.
Sikander G, Anwar S. Driver fatigue detection systems: a review. IEEE Trans Intell Transp Syst. 2019;20(6):2339–52. https://doi.org/10.1109/TITS.2018.2868499.
Nicholson M, Poulus D, McNulty C. Letter—more physiological research is needed in esports. Int J Esports. 2020;1(1):1–6.
Koshy A, Koshy GM (2020) The potential of physiological monitoring technologies in esports. Int J Esports. 1(1):1–11.
Stevens CJ, Best R. Menthol: a fresh ergogenic aid for athletic performance. Sports Med. 2017;47(6):1035–42. https://doi.org/10.1007/s40279-016-0652-4.
Geller K, Renneke K, Custer S, Tigue G. Intrinsic and extrinsic motives support adults’ regular physical activity maintenance. Sports Med Int Open. 2018;02(03):E62–6. https://doi.org/10.1055/a-0620-9137.
Oliver LS, Sullivan JP, Russell S, et al. Effects of nutritional interventions on accuracy and reaction time with relevance to mental fatigue in sporting, military, and aerospace populations: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;19(1):307. https://doi.org/10.3390/ijerph19010307.
Moeller SJ, Tomasi D, Honorio J, et al. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2(10):e176–e176. https://doi.org/10.1038/tp.2012.110.
Acknowledgements
We would like to thank the Luxemburg Institute of Research in Orthopedics, Sport Medicine and Science (LIROMS) and the Strategic Research Program Exercise and the Brain in Health & Disease: The Added Value of Human-Centered Robotics (SRP17) for their valuable contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest
Matthias Proost, Jelle Habay, Jonas De Wachter, Kevin De Pauw, Ben Rattray, Romain Meeusen, Bart Roelands and Jeroen Van Cutsem have no conflicts of interest relevant to the content of this review.
Compliance with ethical standards
Matthias Proost, Jelle Habay, Jonas De Wachter, Kevin De Pauw, Ben Rattray, Romain Meeusen, Bart Roelands and Jeroen Van Cutsem declare that the systematic review complies with all ethical standards.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors declare that all data supporting the findings of this study are available within the manuscript.
Code availability
Not applicable.
Authorship contribution
The design of the search strategy was performed by MP, and subsequently revised by JH, JDW, BaRo and JVC. Screening on title and abstract was done by MP and JH, while full-text screening was conducted by four authors, i.e., MP, JH, BaRo and JVC. Data analysis was first conducted by MP and JH and was later revised and updated by MP. RoB assessment was performed by MP who also designed the RoB figures. MP wrote the first draft of the manuscript which was later altered by JH, JDW, KDP, BeRa, RM, BaRo and JVC. All authors read, revised and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Proost, M., Habay, J., De Wachter, J. et al. How to Tackle Mental Fatigue: A Systematic Review of Potential Countermeasures and Their Underlying Mechanisms. Sports Med 52, 2129–2158 (2022). https://doi.org/10.1007/s40279-022-01678-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-022-01678-z