Abstract
Purpose
To examine the effects of caffeine ingestion on physiological and perceptual responses in mentally fatigued individuals.
Methods
Eight male physically active subjects completed four cycling constant-workload tests in four experimental conditions at 80 % of maximal power output: control (C), mental fatigue (MF), mental fatigue plus caffeine ingestion (5 mg/kg) (MF-CAF), and mental fatigue plus placebo (MF-PLA). The mental fatigue was induced by a continuous performance task A-X version (AX-CPT). Before and after the AX-CPT, the profile of mood state (POMS) and blood samples for lactate measurement were collected. Oxygen consumption (\( \dot{V}{\text{O}}_{2} \)), rating of perceived exertion (RPE), and electromyography (EMG) activity were measured during the cycling test.
Results
The time to exhaustion in C, MF, MF-PLA, and MF-CAF were 251 ± 30, 222 ± 23, 248 ± 28, and 285 ± 42 s, respectively. Delta values (corrected by C condition) were higher in MF-CAF than MF (P = 0.031). MF-CAF reported higher Vigor scores when compared with C (P = 0.046) and MF (P = 0.020). RPE at the first minute was significantly higher in MF-PLA than in C (P = 0.050); at the second minute, RPE was higher in MF-PLA than in C (P = 0.049) and MF-CAF (P = 0.048). EMG activity was not different between the conditions.
Conclusions
Caffeine ingestion increased approximately 14 % endurance performance after the induction of mental fatigue. This effect was accompanied by a tendency to improvement in mood state (i.e., vigor). Therefore, caffeine ingestion can promote a beneficial effect on endurance performance in mentally fatigued individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical and mental fatigue have been defined as a decline in the ability to perform physical and mental activities, respectively, which are often accompanied by the sense of discomfort, desire to rest, and reduced motivation (Ishii et al. 2014; Tanaka et al. 2013). Physical fatigue is related to a number of physiological factors, including neuromuscular (Paavolainen et al. 1999), cardiorespiratory (Howley et al. 1995), and muscle-energetic-related mechanisms (Billat et al. 1996). Recent studies have demonstrated that endurance performance is reduced as a result of prolonged periods undergoing cognitive tasks designed to induce mental fatigue (e.g., AX-CPT computer task) (Brownsberger et al. 2013; Marcora et al. 2009; Pageaux et al. 2015). It is interesting to observe that this impairment of the endurance performance has not been accompanied by alterations in some traditional physiological variables related to endurance capacity, such as cardiac output, minute ventilation, and oxygen uptake (Marcora et al. 2009). On the other hand, the rating of perceived exertion (RPE) during the exercise task has been shown to increase with mental fatigue when compared with control. These findings have led some authors to suggest that endurance capacity at 80 % of maximal power output could be limited by central nervous system (CNS) via perceptive-based decision (Marcora et al. 2009; Pageaux et al. 2015). The mechanisms by which mental fatigue increase perceived exertion and impair the endurance performance remain to be fully elucidated. However, it has been argued that cognitive tasks could increase the accumulation of adenosine in the cortex (Pageaux et al. 2015; Rozand et al. 2014) and diminish the excitatory activity in CNS (Davis et al. 2003), which could result in an increased subjective feeling of tiredness and lack of energy. This could induce the individuals to reach their maximum level of perceived exertion earlier, leading them to give up from exercising (Marcora et al. 2009; Pageaux et al. 2015; Rozand et al. 2014).
Caffeine is a natural xanthine alkaloid able to alter positively the CNS function (Glade 2010; Lorist et al. 1994; Smith et al. 2005). It has been suggested that caffeine (3–5 mg kg−1 body weight) can easily cross the blood barrier and blockade the adenosine receptors in the CNS (Glade 2010). The adenosine inhibits the release of brain excitatory neurotransmitters (e.g., dopamine) and reduces arousal and spontaneous behavioral activity (Davis et al. 2003). It has also been reported that the consumption of caffeine is accompanied by an improvement in mood state as well as reduced subjective feelings of tiredness, lack of energy, and RPE during the exercise (Astorino et al. 2012; Spriet 2014). Considering its neural action, it is not surprising that the impact of caffeine on CNS would be more pronounced during mental fatigue state (Smith et al. 2005). Indeed, Lorist et al. (1994) found that caffeine produced lower peak latency during a target letter task in mental fatigued and in well-rested subjects. Moreover, the CNS function in mental fatigued and well-rested was similar after caffeine ingestion, suggesting that improvements in CNS function after caffeine ingestion could be dependent on the mental fatigue state of the subject (Lorist et al. 1994). Smith et al. (2005) also demonstrated positive effects of caffeine on cognitive performance and mood state in volunteers who had been mentally fatigued by a prolonged cognitive task. Although these findings suggest the beneficial effects on CNS of the caffeine in mentally fatigued individuals, it is still unknown whether caffeine ingestion could counteract the deleterious effects of mental fatigue on endurance performance.
In addition to act in CNS, caffeine also stimulates neuromuscular function and some muscle-energetic-related mechanisms, such as spare muscle glycogen and enhances fat oxidation. In a meta-analysis (Warren et al. 2010), it was demonstrated that caffeine enhanced maximal voluntary contraction (MVC) by 4 % (ES = 0.19, P = 0.0003). Furthermore, the previous findings have shown an increase of 29 % in electromyography activity (EMG) on time limit of endurance of knee extensors after the ingestion of caffeine (Plaskett and Cafarelli 2001). An increased EMG with caffeine has been attributed to an enhanced motor unit recruitment and synchronization (Bazzucchi et al. 2011; Plaskett and Cafarelli 2001). Other studies have also detected an increase of 6–10 % in the anaerobic energy supply after caffeine ingestion (Doherty 1998; Santos et al. 2013; Simmonds et al. 2010). By stimulating catecholamine production, it has been proposed that caffeine would increase the activity of the enzyme phosphofructokinase, thereby increasing anaerobic glycolysis (Bridge and Jones 2006). In addition, it has been suggested that caffeine ingestion also increases the release of calcium in the skeletal muscle, facilitating the conversion of the enzyme phosphorylase b to it more active form a, which would lead to an acceleration of glycogenolysis (Bridge and Jones 2006). Although this evidence suggests an improved endurance performance after caffeine ingestion, mainly mediated by an increased neuromuscular excitability (Park et al. 2008) and anaerobic contribution (Doherty 1998; Simmonds et al. 2010), it is important to observe that these studies did not involve mentally fatigued individuals. Therefore, whether caffeine can attenuate the deleterious effect of mental fatigue on physical capacity remains unknown.
Thus, the aim of the present study was to examine the effects of caffeine ingestion on endurance performance, perceptual responses, neuromuscular function, and anaerobic metabolism in mentally fatigued subjects. We hypothesized that the ingestion of caffeine would reduce the deleterious effects of mental fatigue on endurance performance, possible due to improvements in psychological (i.e., mood state and RPE) as well as muscular and metabolic (i.e., EMG and anaerobic contribution) variables.
Materials and methods
Participants
Eight male subjects (age 24 ± 2 years; height 176.3 ± 8.3 cm; body weight 73.8 ± 9.7 kg, and maximal oxygen uptake 45.2 ± 3 ml kg min−1) familiarized with exhaustive exercise participated in this study. Participants reported to be engaged in recreational sports (i.e., jogging, soccer, and cycling) 3–4 times per week for at least 1 year. Exclusion criteria included: low or high daily caffeine intake (<0.5 or >8 mg/kg); hypersensitivity to caffeine (Park et al. 2008), which was measured by a daily alimentation questionnaire repeated in three separated days in the same week. The subjects also were asked to complete a physical activity readiness questionnaire (PAR-Q, Canadian Society for Exercise Physiology) for any neuromuscular, cardiovascular dysfunction, and use of any medications. Participants received an explanation of the possible benefits, risks, and discomfort associated with the study and signed a written informed consent before participation. The study was approved by the local Ethics Committee for Human Studies (process 107358/2014).
Experimental design
Using a randomized, placebo-controlled, crossover, counterbalanced, double-blind study, each participant visited the laboratory on seven separate occasions over a 4-week period. Before data collection, the CAF and PLA capsules were coded, so that neither the investigators nor the participants were aware of the contents until completion of the analyses. The capsules were provided by a staff member of our research team who did not have any participation in the data acquisition, analyses, or interpretation. For the session order, each participant had their order randomized for the four experimental procedures using a free software, available at http://www.randomized.org. Each experimental session was performed, at least 48 h apart, in the same time of the day to avoid any circadian interference. In the first visit, they were submitted to a maximal cycling incremental test to measure the maximal oxygen uptake (\( \dot{V} \)O2max) and maximal power output (Wmax). A familiarization with cycling constant-workload test (80 % of Wmax) was performed in the second and third visits. Thereafter, the participants completed four cycling workload constant tests at 80 % Wmax until exercise tolerance after four different conditions: control (C); mental fatigue (MF); mental fatigue plus caffeine ingestion (MF-CAF); and mental fatigue plus placebo (MF-PLA). Participants were asked to replicate the same diet during the 24 h proceeding the experimental sessions. They were also asked to refrain from consuming caffeine-containing substances (i.e., coffee, chocolate, and soft drinks) or performing heavy training for 24 h before each experimental session. A list of caffeine-containing substances was given to participants. In the morning of the experimental session, the participants arrived at the laboratory at 0830 h after consuming a standardized breakfast (60 % carbohydrate, 25 % lipids, and 15 % protein) between 0700 and 0720 h. Participants were blinded to the objectives and hypotheses of the study. For this purpose, they were told that the objective of the study was to investigate different types of previous exercise acclimatization and interventions, a documentary or a computer test with placebo or caffeine ingestion. Only at the end of the study, the objectives and hypotheses were revealed. All tests were performed in a controlled laboratory environment (22.9 ± 1.3 °C and 56.1 ± 7.8 % relative humidity).
Maximal cycling incremental test
The maximal incremental exercise test was carried out on a mechanically braked cycle ergometer (Biotec 2100, Cefise, Nova Odessa, Brazil). The seat height was individually adjusted for the subject’s comfort, with the legs being near fully extended during each pedal revolution. The seat position was recorded and replicated during all subsequent experimental sessions. After a 5-min warm-up at 25 W, the test increased by 30 W min−1 until participants reached exercise tolerance. Participants exercised at a pedal cadence of between 60 and 50 rpm, and exercise tolerance was defined as the incapacity to maintain a minimum pedal cadence of 50 rpm during 5 s despite verbal encouragement. Participants received strong verbal encouragement to continue as long as possible. Gas exchange was measured breath-by-breath using a gas analyzer (Cortex Metalyzer 3B, Cortex Biophysik, Leipzig, Germany) and was subsequently averaged over 20 s intervals throughout the test. Before each test, the gas analyzer was calibrated according to the recommendations of the manufacturer. The maximal oxygen uptake (\( \dot{V} \)O2max) was considered as the oxygen uptake measured during the last 20 s. The maximal heart rate was defined, as the highest values obtained at the end of the test, and power output (Wmax) was defined by the given equation:
where W c is the load of the last completed stage, t is the time completed in the stage (s), 60 is the stage duration, and 30 is the power increment.
Cycling workload constant tests
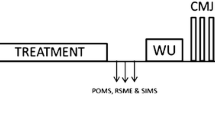
The cycle ergometer and all procedures used in the maximal incremental exercise test were adopted in the cycling constant-workload tests. Figure 1 shows an overview of other procedures adopted during the experimental sessions. Participants rested for 10 min after arriving in the laboratory. After this procedure, three maximal voluntary contractions (MVC) of the quadriceps muscles were made. During the MVC, the EMG was recorded to normalize the EMG signal measured during the cycling tests. Following the MVC, participants were asked to fill in the Profile of Mood State Questionnaire (POMS) to determine fatigue and vigor parameters before and after the treatments. Before executing the documentary or AX-CPT, blood samples were collected for glucose measurement. In capsule ingestion sessions, the capsules were ingested immediately before the documentary or AX-CPT intervention. As soon as the intervention was concluded, the subjects filled out the POMS questionnaire and blood samples were collected again. Finally, the subjects proceeded to the cycling workload constant test.
Before starting the cycling workload constant tests, the participants were asked to rest quietly in a standing position for 5 min to determine the oxygen uptake at rest (\( \dot{V} \)O2rest). Following, the participants performed a standardized warm-up exercise, which consisted of a 3-min cycling at 40 % of Wmax at a pedal cadence of 60 rpm. Immediately after the warm-up, the power output corresponding to 80 % Wmax (212 ± 27 W) was adjusted and the subjects were instructed to maintain a pedal cadence of 60 rpm. Exercise tolerance was defined as the incapacity to maintain pedal cadence of 60 rpm for more than 5 s despite verbal encouragement. The time to exercise tolerance was measured by a manual stopwatch. Throughout the test, the heart rate was continuously recorded (Polar Electro Oy, Kempele, Finland), and \( \dot{V} \)O2 was measured breath-by-breath (Cortex Metalyzer 3B, Cortex Biophysik, Leipzig, Germany). The peak oxygen uptake (\( \dot{V} \)O2peak) was established as the mean of the last 20 s of oxygen uptake, whereas the peak heart rate (HRpeak) was defined as the highest value measured at the end of the exercise. Blood samples (25 µl) were collected from the ear lobe before, immediately after exercise, during the 3rd and 5th minutes of recovery to determine the peak plasma lactate concentration using enzymatic calorimetric reactions in a spectrophotometer, as previously described (Silva-Cavalcante et al. 2013). The RPE was recorded every 1 min using the Borg 15-point category scale (Borg 1982). The instructions for RPE scale were standardized, in accordance with the procedures previously described (Pageaux et al. 2015). Briefly, subjects were asked to rate the conscious sensation of how hard, heavy, and strenuous the physical task was. For example, nine scores corresponded to a “very light” exercise. For a normal, healthy person, it is like walking slowly at his or her own pace for some minutes. Seventeen score corresponded to a “very hard” and strenuous exercise. A healthy person can still go on, but he or she really has to push him or herself. It feels very heavy, and the person is very tired. To include all tests in the statistical analyses, a 4-min isotime was adopted.
Mental fatigue
Mental fatigue was induced by a computerized cognitive task especially designed for this purpose (i.e., AX-CPT test), as previously described (Marcora et al. 2009). Using electroencephalogram technique, Brownsberger et al. (2013) demonstrated an increase in β-band electro cortical activity after AX-CPT test, confirming that AX-CPT elicits greater attention, information processing, and cognitive engagement. In addition, the previous findings have demonstrated that AX-CPT produces a significant reduction in the proportion of correct responses during the task without changes in blood glucose, reinforcing that the mental fatigue induced by this test is arising from cognitive function (Brownsberger et al. 2013; Marcora et al. 2009). During condition C, participants watched two episodes of a documentary series named “Earth Planet (BBC)” in a continuous 90-min sequence under identical conditions adopted in the MF. These procedures were conducted in a silent, temperature- and humidity-controlled room (22.9 ± 1.3 °C and 56.1 ± 7.8 % relative humidity).
Mood state assessment
The mood state was assessed before and after experimental treatments using the Profile of Mood State Questionnaire (POMS), as previously described (Morgan 1994). POMS consisted of a 65-item questionnaire related to the mood state, with a score ranging from zero (not at all) to four (extremely) for each item. The participants answered the questionnaire after receiving a detailed description of the POMS. To reduce the possibility of false responses, the confidentiality of the data was ensured, as recommended (Terry et al. 2003). The POMS was first applied at the first visit to familiarize participants with the questionnaire, and the participants were required to respond the following question: “How have you been feeling during the past week, including today?” (Terry et al. 2003). At the other visits, the POMS questionnaire was filled out before and after treatments based on the question “How are you feeling now?”. This procedure was adopted to avoid fluctuations in mood states, thus increasing the predictive capabilities of the method (Marcora et al. 2009). The results generated six factors related to mood state: tension/anxiety; depression; anger/hostility; vigor; fatigue; and mental confusion (39). For the purpose of the current study, only vigor and fatigue were considered, as in a previous publication (Marcora et al. 2009).
Caffeine and placebo ingestion
Immediately before the AX-CPT, approximately 90 min before the cycling workload constant tests, the participants ingested an opaque capsule containing either 5 mg kg−1 body weight of caffeine (MF-CAF) or microcrystalline cellulose (MF-PLA) with 150 mL of water. Previous findings have suggested that plasma caffeine levels rise to ~15–40 μmol l−1, peaking at 80 min after ingestion when caffeine doses are administrated in capsules containing 3–6 mg∙kg−1 body weight (36). Furthermore, dosage between 3 and 7.5 mg/kg has been suggested to counteract the mental fatigue effects (Ruijter et al. 1999) and improve endurance performance (Magkos and Kavouras 2004).
Acquisition and analysis of the electromyographic signal
The electromyographic signal was individually normalized using electromyographic signal measured during MVC. The MVC was performed in a chair with the trunk-thigh angle at 90° and the knee at 90° from full leg extension (0°). Before the MVC, participants performed a standardized warm-up consisting of four 5-s contractions of the right knee extensors, interspersed by 30-s rest periods, at intensities corresponding to 50, 60, 70, and 80 % of the maximum subjective force (Santos et al. 2013). Thereafter, participants performed three 5-s MVC (right leg), each separated by 60-s intervals. The peak force produced by the quadriceps muscles was recorded using a load cell (Cefise biotechnology, Nova Odessa, Brazil). The athletes were verbally encouraged during all MVCs to achieve their maximal force. During the MVC and time trials, EMG signals of the vastus lateralis (VL) muscle of the right leg were recorded via bipolar Ag–AgCl surface electrode (Hal, São Paulo, Brazil) at an interelectrode distance of 20 mm. We chose the VL muscle, because it has been reported as the most appropriate to monitor EMG activity in the lower limb during cycling (Hettinga et al. 2006). The reference electrode was placed over the anterior surface of the tibia. The skin preparation, placement, and location of the electrodes were in accordance with the recommendations of SENIAM (Hermens et al. 2000). To prevent movement artifact, the electrode wires were taped to the skin using adhesive tape (MicroporeTM 3 M, São Paulo, Brazil). Five seconds of raw EMG signal was recorded each minute with a sample rate of 2000 Hz in a four channel MyoTraceTM 400 (Noraxon, Scottdale, AZ, USA). Raw EMG signals were full-wave rectified and filtered with the second-order Butterworth bandpass filters with cut-off frequencies set at 10 and 400 Hz to remove external interference noise and movement artifacts. An envelope representing the muscle activation was determined using a moving RMS filter with a window of 50 ms. The period of activation during a burst was determined as the period, where the signal was above a threshold of 15 % of the maximum activity during the cycling test for at least 100 ms. These parameters were selected based on the signal–noise relationship of the EMG data and were visually verified to correctly identify periods of muscle activation (Damasceno et al. 2014; Santos et al. 2013). For each burst of EMG activation, we calculated the iEMG, defined as the area under the EMG versus time curve divided by the period of activation. Mean iEMG was calculated for last 5-s intervals at each minute throughout the cycling test, which was normalized by dividing the iEMG calculated at the point coinciding with peak torque of the highest MVC (De Andrade Nemezio et al. 2015). The procedures were performed using the Noraxon_s Myoresearch software (version 1.08).
Energy system contributions
The trapezoidal method was used to calculate the \( \dot{V} \)O2 area over time during cycling workload constant tests. Thus, the exercise aerobic energy contribution was calculated by subtracting \( \dot{V} \)O2rest from the \( \dot{V} \)O2 area integrated over time. The contribution of anaerobic metabolism was estimated by the oxygen deficit method. Initially, we fitted the breath-to-breath \( \dot{V} \)O2 on-transient response using a biexponential model (Eq. 2) (Origin 6.0, Microcal, MA, USA), as previously suggested (Ozyener et al. 2001). Thus, the oxygen deficit was obtained by Eq. (3). A caloric equivalent of 20.9 kJ l O −12 was considered for the two energy systems (di Prampero and Ferretti 1999). Total energy expenditure (W total) was calculated as the sum of the two energy systems:
where \( \dot{V} \)O2(t) is the oxygen uptake at time t, O2 baseline is the oxygen uptake at baseline, A is the amplitude, δ is the time delay, t = is the time constant, and 1 and 2 denote the fast and slow components, respectively.
Statistical analysis
Calculation of sample size was based on the assessment of a main effect for difference in cycling capacity after acute ingestion of caffeine described in previous studies (Bell et al. 1998). The sample size required was estimated from the equation n = 8e 2/d 2, as suggested by Hopkins (2000), where n, e, and d denote predicted sample size, coefficient of variation, and the magnitude of the treatment effect, respectively. Coefficient of variation was assumed to be 15.1 % (Laursen et al. 2007). Expecting a magnitude of effect for the treatment from 14.3 % (Bell et al. 1998), the detection of a very conservative 1 % difference as statistically significant would require at least eight participants. To account for midtrial dropouts, the sample size was increased to ten participants. Two participants dropped out because of personal reasons.
Data normality was assessed using the Shapiro–Wilk test. All variables were normally distributed. The results of the descriptive statistics were reported as mean ± SD. To mitigate the impact of inter-individual data variability, cycling capacity and POMS were also expressed as delta values from control condition and pre-treatment (post–pre), respectively. One-way analysis of variance with repeated measures was used to compare the changes in dependent variables (e.g., POMS, endurance capacity, physiological variables, EMG, and RPE) between the treatments. LSD post hoc analyses were used to localize the differences when necessary. Significance level was set at P ≤ 0.05. All statistical analyses were performed using a statistical software package (SPSS, version 13.0, Chicago, IL, USA).
Endurance capacity was also assessed for practical significance using magnitude-based inferences (Hopkins et al. 2009). This analysis was performed using a modified statistical spreadsheet (Paton and Hopkins 2006). The smallest worthwhile change/difference in all variables was calculated as 0.2 multiplied by the between-subject standard deviation, based on Cohen’s effect size principle. Threshold probabilities for a substantial effect based on the 90 % confidence limits were: 1 %, almost certainly not; 1–5 %, very unlikely; 5–25 %, unlikely; 25–75 %, possible; 75–95 %, likely; 95–99 %, very likely; and 0.99 %, almost certain. If chance of benefit and harm both >5 %, true effect was assessed as unclear (could be beneficial or harmful).
Results
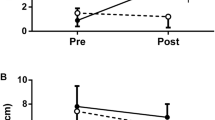
The mean values of the cycling capacity during C, MF, MF-PLA, and MF-CAF were 251 ± 30, 222 ± 23, 248 ± 28, and 285 ± 42 s, respectively. Endurance performance tended to be improved during MF-CAF when compared with MF (P = 0.070). Six out of eight subjects showed reduced exercise tolerance in the MF when compared with MF-CAF. Table 1 shows the practical significance of the influence of the caffeine consumption on endurance performance. MF-CAF resulted in “possible benefit”, “likely benefit”, and “possible benefit” when compared with C, MF, and MF-PLA, respectively. C and MF-PLA resulted in “possible benefit” compared with MF. When endurance capacity was expressed as delta changes (vs. control condition), it was found an improved endurance capacity in MF-CAF when compared with MF (P = 0.031) (Fig. 2A). Delta values of endurance capacity tended to be lower in MF than MF-PLA (P = 0.070) and showed no statistical difference between MF-PLA and MF-CAF (P = 0.212).
Delta values of the endurance capacity (A) and rating of perceived exertion (B) during the different experimental conditions. Values are presented as mean ± SD. C control, MF mental fatigue, MF-PLA mental fatigue + placebo, MF-CAF mental fatigue + caffeine. a Significantly different (P = 0.031). b Mental fatigue + placebo condition significantly higher than control condition (P = 0.050). c Mental fatigue + placebo condition significantly higher than caffeine condition (P = 0.048)
RPE measured during first minute in MF-PLA was significantly higher than C (P = 0.050), while the RPE measured during the second minute in MF-PLA was significantly higher than both C (P = 0.049) and MF-CAF (P = 0.048) (Fig. 2B). The analysis of variance revealed that there were not significant differences for vigor (13 ± 7, 14 ± 5, 9 ± 6, and 12 ± 5 scores for C, MF, MF-PLA, and MF-CAF, respectively) and fatigue (7 ± 4, 8 ± 6, 10 ± 3, 7 ± 5 scores for C, MF, MF-PLA, and MF-CAF, respectively) feelings measured by POMS (P = 0.491) after the respective protocol interventions. However, when vigor feeling was expressed as delta values from pre-experimental condition to mitigate inter-individual variations, it was found a lower value in C (P = 0.046) and MF (P = 0.020) when compared with MF-CAF (Fig. 3A). Higher delta values of the fatigue feeling were observed and approached statistical significance in MF (P = 0.066) and MF-PLA (P = 0.068) compared with MF-CAF (Fig. 3B).
Table 2 shows the physiological, EMG, energy system contributions, and total energy expenditure measured during cycling workload constant tests. It was found that MF-CAF resulted in a higher W total than C (P = 0.050). None of the other variables measured during the cycling workload constant tests were modified by treatments (P > 0.05). Furthermore, MVC values at the begging of experimental session were not different (C: 618 ± 136, MF: 638 ± 138, MF-PLA: 613 ± 107, and MF-CAF: 586 ± 120 N, P > 0.05).
Discussion
Previous findings have demonstrated that mental fatigue, which has been induced by sustained cognitive demand, can impair CNS function and endurance capacity in both self-paced (Brownsberger et al. 2013) and constant-workload tests (Marcora et al. 2009). There is also a growing number of evidence suggesting that caffeine can improve CNS function during mental fatigue (Glade 2010; Spriet 2014). However, it is still unknown whether the ingestion of caffeine is able to attenuate the deleterious effects of mental fatigue on endurance performance. Therefore, to the best of our knowledge, this is the first study testing the influence of caffeine ingestion on endurance performance in mentally fatigued men. Consistent with our hypothesis, caffeine ingestion promoted an increase of ~14 % in endurance performance after mental fatigue. This was accompanied by a tendency of improvements in mood state.
Endurance performance
The AX-CPT, which requires sustained vigilance, working memory, and response inhibition, has been effectively used to induce the mental fatigue (Marcora et al. 2009). In the current study, the AX-CPT might had induced mental fatigue, as evidenced by lower and higher delta values of vigor and fatigue scores, respectively, in MF condition. This is in accordance with previous findings showing that mental fatigue increased the feelings of general fatigue after an cognitive test, which was measured by perceptive scales and questionnaires, such as VAS and POMS, respectively (Brownsberger et al. 2013; Marcora et al. 2009). Mental fatigue also produced a reduction of ~6 % on endurance performance in comparison with C condition. Furthermore, we detected a “possible harm” effect on endurance performance in MF condition when compared with C condition. This negative effect on endurance performance is quite similar to that reported in previous study, in which reduction of 7–16 % in time to exhaustion during cycling tests at fixed power output relative to 11 and 15 RPE was observed (Brownsberger et al. 2013). Some authors have suggested that during exhaustive exercise, the recruited muscles become fatigued, resulting in an increase of motor output from primary motor cortex to maintain the performance (Ishii et al. 2014). However, it is interesting to note that the impaired endurance capacity in the MF condition was not accompanied by changes in EMG. Alternatively, it has been proposed that mental fatigue may alter mood state resulting in increased subjective feeling of “tiredness” and “lack of energy” before exercise due to the accumulation of adenosine in CNS (Pageaux et al. 2015). This would produce a higher than expected perception of effort and induce the individuals to disengage from the exercise (Marcora et al. 2009). Thus, it is possible that endurance performance was impaired in MF condition due to an increased inhibitory input produced by prolonged period of cognitive task before the exercise. These findings reinforce the previous suggestion that mental fatigue might impair endurance performance mainly due to a conscious decision of disengage from exercise (Marcora et al. 2009; Pageaux et al. 2015).
Caffeine has become one of the most ergogenic supplements used by athletes to improve athletic performance (Chester and Wojek 2008). This is probably due to a wide number of evidence showing that caffeine promotes positive effects in endurance-type activities. Indeed, Santos et al. (2013) found an increased mean power output during a 4000-m cycling time trial, whereas Denadai and Denadai (1998) reported an enhanced endurance capacity during cycling workload constant test at intensity 10 % below the anaerobic threshold, both after the ingestion of 5 mg kg−1 body weight of caffeine. It is interesting to observe that in the current study, the MF-CAF tended to produce a significant higher (~36 %, P = 0.070) time to exercise tolerance when compared with MF. This improvement on endurance performance was substantially higher than the average effect observed in endurance athletes, which is approximately ~21 % (Denadai and Denadai 1998). In addition, we observed a “likely beneficial” effect in caffeine when compared with placebo (i.e., MF-CAF vs. MF). This suggests that the effects of caffeine on endurance performance were more pronounced when individuals experienced mental fatigue state. This is in agreement with a previous suggestion that the effects of caffeine in the CNS function are more pronounced in individuals’ mentally fatigued (Lorist et al. 1994). Importantly, we showed that caffeine can reduce the negative impact of mental fatigue on endurance performance. These results have clear implications for many practical situations, because they indicate that caffeine might be used to attenuate the deleterious effects of the mental fatigue on mood state and endurance performance in recreational exercisers.
Psychological and mood state variables
In the current study, the improvement on endurance performance in MF-CAF was accompanied by maintenance in vigor feeling (Fig. 3A) and trend to reduction in fatigue feeling (Fig. 3B). The maintenance of mood state in MF-CAF condition is in accordance of previous results, where caffeine ingestion had positive effects on POMS values of vigor and fatigue in situations of mental strain (Penetar et al. 1993). In contrast, except for total energy expenditure, no significant differences were found in cardiorespiratory, metabolic, and neuromuscular variables between the experimental conditions. These findings suggest that the effects of caffeine on endurance performance may have been mainly mediated through a modulation in CNS function, in accordance with previous observations (Davis et al. 2003; Spriet 2014). It has been speculated that blocked adenosine receptors in the CNS may produce positive changes on CNS function, such as improvement in mood state (Smith et al. 2005; Spriet 2014). Our data allow us speculating that the ability of caffeine to counteract the negative effects of mental fatigue on endurance performance might be mediated by the blockade of the adenosine receptors in the CNS.
It is well known that RPE is generated as a result of the both internal (e.g., physiological responses) and external (e.g., environment) cues (Morgan 1994). Previous studies have demonstrated that mental fatigue could act as external cues increasing RPE and reducing overall athletic performance (Brownsberger et al. 2013; Marcora et al. 2009). In contrast, caffeine has been shown to elicit improvements in endurance performance while attenuating RPE (Cornish et al. 2015). In this study, the participants were able to produce a greater amount of work during MF-CAF condition, as evidenced by increased Wtotal and time to exercise tolerance, but with RPE values equivalent to the other conditions, in particular in the last minutes. It has been proposed that caffeine acts on the CNS by improving attentional focus and arousal feelings (Lorist and Tops 2003), which in part might be related to lower RPE values during the physical activity (Boksem and Tops 2008). These findings reinforce the idea that caffeine could affect CNS function, reducing RPE, thereby enabling individuals to perform a greater amount of work (Cornish et al. 2015; Stadheim et al. 2013).
This study had some limitations for considerations and conclusions. First, the participants were recreationally trained subjects; therefore, these data cannot be generalized to athletes. It is well known that athletes involved in endurance-type sports often consume caffeine supplements to improve performance. Since there is evidence suggesting that heavy consumers appear to be less sensitive to ergogenic effects of caffeine than individuals who do not consume caffeine frequently (Warren et al. 2010), one could speculate that athletes could experience a less pronounced effect with caffeine supplementation. Although the subjects were strongly advised to keep the same diet throughout experimental conditions, we were unable to measure dietary caffeine intake in this study, precluding any inference in relation to an influence of habitual caffeine intake on the effects of caffeine supplementation. Finally, it is important to recognize that we did not perform direct measurements of CNS function (e.g., electroencephalogram). Therefore, further studies are necessary to confirm the hypothesis that caffeine may improve endurance performance in mentally fatigued subjects by directly modulating CNS.
Conclusion
In conclusion, the findings of the present study suggest that the beneficial effects of the caffeine in endurance performance might be more pronounced during mental fatigue state. Caffeine ingestion increased approximately 14 % endurance performance after the induction of mental fatigue. This effect was accompanied by a tendency to improvement in mood state (i.e., vigor). From practical standpoint, these findings suggest that caffeine can be considered as a promising ergogenic resource to minimize the negative effects of the mental fatigue produces on endurance performance.
Abbreviations
- AX-CPT:
-
AX continuous performance task
- C:
-
Control group
- CNS:
-
Central nervous system
- EMG:
-
Eletromiography
- iEMG:
-
Integrated eletromiography
- MVC:
-
Maximal voluntary contraction
- MF:
-
Mental fatigue group
- MF-CAF:
-
Mental fatigue plus caffeine ingestion group
- MF-PLA:
-
Mental fatigue plus placebo ingestion group
- RMS:
-
Root mean square
- RPE:
-
Rating of perceptive exertion
- POMS:
-
Profile of mood state questionnaire
- VL:
-
Lateral vastus
- VO2max :
-
Maximal oxygen uptake
- W max :
-
Maximal power output
References
Astorino TA, Cottrell T, Talhami Lozano A, Aburto-Pratt K, Duhon J (2012) Effect of caffeine on RPE and perceptions of pain, arousal, and pleasure/displeasure during a cycling time trial in endurance trained and active men. Physiol Behav 106(2):211–217
Bazzucchi I, Felici F, Montini M, Figura F, Sacchetti M (2011) Caffeine improves neuromuscular function during maximal dynamics exercise. Muscle Nerve 43(6):839–844
Bell DG, Jacobs I, Zamecnik J (1998) Effects of caffeine, ephedrine and their combination on time to exhaustion during high-intensity exercise. Eur J Appl Physiol Occup Physiol 77(5):427–433
Billat V, Beillot J, Jan J, Rochcongar P, Carre F (1996) Gender effect on the relationship of time limit at 100 % VO2max with other bioenergetic characteristics. Med Sci Sports Exerc 28(8):1049–1055
Boksem MA, Tops M (2008) Mental fatigue: costs and benefits. Brain Res Rev 59(1):125–139
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Bridge CA, Jones MA (2006) The effect of caffeine ingestion on 8 km run performance in a field setting. J Sports Sci 24(4):433–439
Brownsberger J, Edwards A, Crowther R, Cottrell D (2013) Impact of mental fatigue on self-paced exercise. Int J Sports Med 34(12):1029–1036
Chester N, Wojek N (2008) Caffeine consumption amongst British athletes following changes to the 2004 WADA prohibited list. Int J Sports Med 29(6):524–528
Cornish RS, Bolam KA, Skinner TL (2015) Effect of caffeine on endurance performance and function in prostate cancer survivors. Med Sci Sports Exerc 47(3):468–475
Damasceno MV, Duarte M, Pasqua LA, Lima-silva AE, MacIntosh BR, Bertuzzi R (2014) Static stretching alters neuromuscular function and pacing strategy but not performance during a 3-km running time-trial. PLoS One 9(6):e99238
Davis JM, Zhao Z, Stock HS, Mehl KA, Bugy J, Hand GA (2003) Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Regul Integr Comp Physiol 284(2):r399–r404
De Andrade Nemezio KM, Bertuzzi R, Correira-Oliveira CR, Gualano B, Bishop DJ, Lima-Silva AE (2015) Effects of creatine loading on oxygen uptake during a 1-km cycling time trial. Med Sci Sports Exerc 47(12):2660–2668
Denadai BS, Denadai ML (1998) Effects of caffeine on time to exhaustion in exercise performed below and above the anaerobic threshold. Braz J Med Biol Res 31(4):581–585
di Prampero PE, Ferretti G (1999) The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respir Physiol 118(2–3):103–115
Doherty M (1998) The effects of caffeine on the maximal accumulated oxygen deficit and short-term running performance. Int J Sport Nutr 8(2):95–104
Glade MJ (2010) Caffeine—not just a stimulant. Nutrition 26(10):932–938
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374
Hettinga FJ, De Koning JJ, Broersen FT, Van Geffen P, Foster C (2006) Pacing strategy and the occurrence of fatigue in 4000-m cycling time trials. Med Sci Sports Exerc 38(8):1484–1491
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports Med 30(1):1–15
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41(1):3–13
Howley ET, Basset DRJR, Welch HG (1995) Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc 27:1292–1301
Ishii A, Tanaka M, Watanabe Y (2014) Neural mechanisms of mental fatigue. Rev Neurosci 25(4):469–479
Laursen PB, Francis GT, Abbiss CR, Newton MJ, Nosaka K (2007) Reliability of time-to-exhaustion versus time-trial running tests in runners. Med Sci Sports Exerc 39(8):1374–1379
Lorist MM, Tops M (2003) Caffeine, fatigue and cognition. Brain Cogn 53(1):82–94
Lorist MM, Snel J, Kok A, Mulder G (1994) Influence of caffeine on selective attention in well-rested and fatigued subjects. Psychophysiology 31(6):525–534
Magkos F, Kavouras SA (2004) Caffeine and ephedrine: physiological, metabolic and performance-enhancing effects. Sports Med 34(13):871–889
Marcora SM, Staiano W, Manning V (2009) Mental fatigue impairs physical performance in humans. J Appl Physiol 106(3):857–864
Morgan WP (1994) Psychological components of effort sense. Med Sci Sports Exerc 26(9):1071–1077
Ozyener F, Rossiter HB, Ward SA, Whipp BJ (2001) Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 533:891–902
Paavolainen L, Häkkinen K, Hämäläinen I, Nummela A, Rusko H (1999) Explosive-strength training improves 5-km running time by improving running economy and muscle power. J Appl Physiol 86(5):1527–1533
Pageaux B, Marcora SM, Rozand V, Lepers R (2015) Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front Hum Neurosci 9:67
Park ND, Maresca RD, McKibans KI, Morgan DR, Allen TS, Warren GL (2008) Caffeine’s beneficial effect on maximal voluntary strength and activation in uninjured but not injured muscle. Int J Sport Nutr Exerc Metab 18(6):639–652
Paton CD, Hopkins WG (2006) Ergometer error and biological variation in power output in a performance test with three cycle ergometers. J Sports Med 27(6):444–447
Penetar D, McCann U, Thorne D, Kamimori G, Galinski C, Sing H, Thomas M, Belenky G (1993) Caffeine reversal of sleep deprivation effects on alertness and mood. Psychopharmacology 112(2–3):359–365
Plaskett CJ, Cafarelli E (2001) Caffeine increases endurance and attenuates force sensation during submaximal isometric contractions. J Appl Physiol 91(4):1535–1544
Rozand V, Pegeaux B, Marcora SM, Papaxanthis C, Lepers R (2014) Does mental exertion alter maximal muslce activation? Front Hum Neurosci 8:755
Ruijter J, Lorist MM, Snel J (1999) The influence of different doses of caffeine on visual task performance. J Psychophysiol 13:37–48
Santos RdA, Kiss MAPDM, Silva-Cavalcante MD, Correia-Oliveira CR, Bertuzzi R, Bishop DJ, Lima-Silva AE (2013) Caffeine alters anaerobic distribution and pacing during a 4000-m cycling time trial. PLoS ONE 8(9):e75399. doi:10.1371/journal.pone.0075399
Silva-Cavalcante MD, Correia-Oliveira CR, Santos RA et al (2013) Caffeine increases anaerobic work and restores cycling performance following a protocol designed to lower endogenous carbohydrate availability. PLoS One 8(8):e72025
Simmonds MJ, Minahan CL, Sabapathy S (2010) Caffeine improves supramaximal cycling but not the rate of anaerobic energy release. Eur J Appl Physiol 109(2):287–295
Smith A, Sutherland D, Christopher G (2005) Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J Psychopharmacol 19(6):620–626
Spriet LL (2014) Exercise and sport performance with low doses of caffeine. Sports Med 44(2):S175–S184
Stadheim HK, Kvamme B, Olsen R, Drevon CA, Ivy JL, Jensen J (2013) Caffeine increases performance in cross-country double-poling time trial exercise. Med Sci Sports Exerc 45(11):2175–2183
Tanaka M, Ishii A, Watanabe Y (2013) Neural effect of mental fatigue on physical fatigue: a magnetoencephalography study. Brain Res 1542:49–55
Terry PC, Lane AM, Fogarty GJ (2003) Construct validity of the profile of mood states adolescents for use with adults. Psych Sport Exerc 4(2):125–139
Warren GL, Park ND, Maresca RD, Mckibans KI, Millard-Stafford ML (2010) Effect of caffeine ingestion on muscular strength and endurance: a meta-analysis. Med Sci Sports Exerc 42(7):1375–1387
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Azevedo, R., Silva-Cavalcante, M.D., Gualano, B. et al. Effects of caffeine ingestion on endurance performance in mentally fatigued individuals. Eur J Appl Physiol 116, 2293–2303 (2016). https://doi.org/10.1007/s00421-016-3483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3483-y