Abstract
It is now well established that reactive oxygen species (ROS) play a dual role as both deleterious and beneficial species. In fact, ROS act as secondary messengers in intracellular signalling cascades; however, they can also induce cellular senescence and apoptosis. Aging is an intricate phenomenon characterized by a progressive decline in physiological functions and an increase in mortality, which is often accompanied by many pathological diseases. ROS are involved in age-associated damage to macromolecules, and this may cause derangement in ROS-mediated cell signalling, resulting in stress and diseases. Moreover, the role of oxidative stress in age-related sarcopenia provides strong evidence for the important contribution of physical activity to limit this process. Regular physical activity is considered a preventive measure against oxidative stress–related diseases. The aim of this review is to summarize the currently available studies investigating the effects of chronic and/or acute physical exercise on the oxidative stress process in healthy elderly subjects. Although studies on oxidative stress and physical activity are limited, the available information shows that acute exercise increases ROS production and oxidative stress damage in older adults, whereas chronic exercise could protect elderly subjects from oxidative stress damage and reinforce their antioxidant defences. The available studies reveal that to promote beneficial effects of physical activity on oxidative stress, elderly subjects require moderate-intensity training rather than high-intensity exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aging is associated with increased free-radical generation, which induces cell damage. |
Acute exercise increases free-radical generation and oxidative injury in the elderly. |
Regular physical activity could prevent oxidative stress–related diseases in the elderly. |
1 Introduction
Despite prolongation of the lifespan in developed countries in the last several decades, a proportion of the elderly population are frail and vulnerable to physical and mental disorders that impair the quality of their later lives. Numerous anti-aging measures have been proposed to ameliorate and/or retard age-associated declines in physiological functions and/or the onset of diseases [1]. The free-radical theory of aging [2] has been the basis of these major anti-aging strategies, although this theory has been criticized as being not definitely proven [3]. In fact, among the various theories that attempt to explain the aging process, disruption of the whole signalling network involving reactive oxygen species (ROS) has received increasing recognition over the past two decades [4–6]. ROS can be generated by a variety of enzymes and metabolic pathways, including mitochondrial complexes I–III in the electron transport chain (ETC) [7], dihydrolipoamide dehydrogenase in α-keto acid dehydrogenase complexes, peroxisomes, cytochrome P450, xanthine oxidase and nicotinamide dinucleotide phosphate (NADPH) oxidase, although the quantitative significance of each of the pathways in generative ROS and causing cells to age is not clear [8]. All of these systems may induce oxidative stress under appropriate conditions, even if there are differences in the rate of ROS production and susceptibility to oxidative damage among various organs and tissues [9]. Basal levels of ROS are indispensable for redox signalling and cell survival. However, high levels of ROS would be detrimental to cells and have been thought to contribute to the development of age-related diseases and to promote the aging process [10]. Oxidative stress is a situation whereby cellular concentrations of ROS overwhelm cellular antioxidant capacities, leading to extensive modifications or damage to macromolecules, including DNA, proteins and lipids, and playing an important role in many physiological and pathophysiological conditions [11, 12]. However, it has recently been shown that protein or lipid oxidation can be beneficial for cell survival, which is termed ‘positive oxidative stress’, as suggested by Yan [13]. In fact, these oxidation products are usually caused by a moderate level of oxidative stress and can induce or are part of an adaptive response, which protects cells against subsequent severe challenges that otherwise would trigger widespread oxidative damage and cell death. Thus, even if the imbalance between ROS production and antioxidant defences in elderly subjects can modulate the expression of many transcription factors responsible for shifting protein synthesis to protein degradation and leading to muscle sarcopenia [14], lipid peroxidation by-products and protein oxidation adducts can have very large prophylactic effects on aging-related diseases, including redox signalling and activation of transcriptional factors [15].
Aging has also been shown to predispose skeletal muscle to increased levels of oxidative stress [16], suggesting that oxidative stress has a role in mediating disuse-induced and sarcopenia-associated muscle loss. In fact, provoked by continuous antigenic and oxidative stress, a phenomenon appears in the elderly, denoted by the term ‘inflammaging’, which is used to elucidate a low-grade chronic inflammatory state in the aged population [17]. This chronic low-grade inflammation induced by oxidative stress has been shown to be detrimental to skeletal muscle in humans [18, 19]. In fact, under normal conditions, there is a balanced and continuous degradation and resynthesis of skeletal muscle proteins. However, during the aging process and the resulting increased oxidative stress, this balance is disrupted [20], particularly because of blunted anabolic signalling (e.g. growth factor [IGF]-1) and increased catabolic signalling (e.g. interleukin [IL]-6 and tumour necrosis factor [TNF]-α).
In daily life, numerous physical activities, such as walking, climbing and cleaning, require aerobic metabolism and therefore increased ROS production. In this area, numerous studies have demonstrated changes in both ROS production and antioxidant defence activities in response to acute exercise [21, 22]. These changes would be more pronounced in elderly people, in view of the deficiency in the mitochondrial respiratory chain and the decrease in antioxidant efficiency, which are characteristics of the aging process. Nevertheless, it has been shown that regular exercise has a significant effect on the prevention of these age-associated losses [23]. Moreover, regular exercise could be considered as a preventive measure against oxidative stress–related diseases by improving the ability to scavenge ROS. This paradoxical effect is due to the ability of the exercise itself to increase the formation of ROS to a level that may induce significant yet tolerable damage, which can, in turn, induce beneficial adaptations [24]. However, the balance between beneficial and deleterious effects of exercise on oxidative stress in elderly people remains unclear, even though it has been reported that physical activity—in particular, aerobic exercise training—decreases the level of oxidative stress markers and increases enzymatic and non-enzymatic antioxidant capacity in middle-aged and elderly individuals [23–26]. However, to our knowledge, few studies have investigated the relationship between different types (chronic or acute) or modalities of exercise (resistance or aerobic) on oxidative stress markers in these populations.
Given the fact that the data on oxidative stress and aging are a subject of debate, the purpose of this review is to examine this topic in relation to physical activity in order to improve our knowledge and highlight some of the mechanisms involved. To that end, we searched the electronic databases PubMed, ISI Web of Knowledge, SPORTDiscus and Google Scholar (up to February 2015). Key terms that were included and combined were ‘elderly’, ‘oxidative stress’, ‘reactive oxygen species’, ‘antioxidants’, ‘exercise’ and ‘training’. Only scientific research using accepted methods that provided relevant information about physical activity and oxidative stress in the elderly were included in the current review.
2 Reactive Oxygen Species
Aerobic respiration generates energy in the mitochondria of eukaryotic cells. As a result of this oxidative metabolism, several compounds are produced. These compounds are highly reactive molecules, which consist of a number of diverse chemical species, including superoxide anion (O2 ⋅−), hydroxyl radical (OH⋅) and hydrogen peroxide (H2O2) [27]. H2O2 is relatively stable and membrane permeable. It can be diffused within the cell and can be removed by cytosolic antioxidant systems, such as catalase (CAT), glutathione peroxidase (GPX) and thioredoxin peroxidase [28]. Because of their potential to cause oxidative deterioration of DNA, proteins and lipids, ROS have been implicated as one of the causative factors of aging [29]. However, it is now accepted that a moderate degree of ROS production might be an important mechanism in the regulation of signal transduction, gene expression or enzyme regulation [30, 31], even if the line that separates the biological effects of ROS from oxidative damage is not clear [32]. Indeed, by activating proteins such as tyrosine kinases, mitogen-activated protein kinases (MAPK) or Ras proteins, ROS are important mediators of signal transduction pathways. Depending on cell types, ROS have been found to function as signalling molecules in cell proliferation, cellular senescence or cell death.
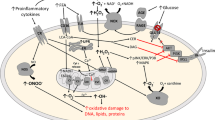
ROS represent the most important class of radical species generated in living systems [33]. The addition of one electron to dioxygen forms the superoxide anion radical (O2 ⋅−), considered to be the ‘primary’ ROS. It can further interact with other molecules to generate ‘secondary’ ROS, which are more aggressive, either directly or prevalently through enzyme or meta-catalysed processes. Various ROS formation pathways are outlined in Fig. 1. Their ‘steady state’ concentrations are determined by the balance between their rates of production and their rates of removal by various antioxidants.
Pathways of reactive oxygen species formation. Reaction 1: a superoxide anion radical (O2 ⋅−) is formed by the process of reduction of molecular oxygen, mediated by several pathways, such as those involving nicotinamide dinucleotide phosphate (NADPH) oxidases and xanthine oxidase. Reaction 2: the superoxide radical is dismuted by superoxide dismutase (SOD) to hydrogen peroxide (H2O2). Reaction 3: Hydrogen peroxide is most efficiently scavenged by the enzyme glutathione peroxidase (GPX), which requires glutathione (GSH) as the electron donor. Reaction 4: the oxidized glutathione (GSSG) is reduced back to GSH by the enzyme glutathione reductase (Gred), which uses NADPH as the electron donor. Reaction 5: some transition metals (e.g. Fe2+) can break down hydrogen peroxide to a reactive hydroxyl radical (OH⋅; Fenton reaction). Reaction 6: the superoxide anion radical can combine with nitric oxide (NO) to produce peroxynitrite (ONOO−). Reprinted with modifications from [51], with permission from Elsevier
Sources of internal oxidative stress also include peroxisomes and enzymes—in particular, detoxifying enzymes from the P450 complex, xanthine oxidase and NADPH oxidase complexes, which include the Nox family. Cyclooxygenase, aldehyde oxidase, dihydroorotate dehydrogenase, tryptophan dioxygenase, nitric oxide (NO) synthase and xanthine oxidase also contribute to ROS production [33]. In addition to being generated during cellular metabolism in mitochondria, ROS can be produced in response to different environmental stimuli, such as growth factors, inflammatory cytokines, ionizing radiation, UV, chemical oxidants, chemotherapeutics, hyperoxia, toxins and transition metals [34].
Once they are produced, ROS react with lipids, proteins and nucleic acids, causing oxidative damage to these macromolecules. In fact, when lipids react with ROS, they can undergo a highly damaging chain reaction of lipid peroxidation. The interaction of ROS and lipids consists of three different steps: initiation, propagation and termination. During these steps, a large number of toxic by-products are formed, which can have effects at a site away from the area of generation, behaving as secondary messengers [35]. The damage caused by lipid peroxidation can be detrimental to the functioning of the cell. Indeed, lipid peroxidation has been shown to perturb the bilayer structure and modify membrane properties, such as membrane fluidity, permeability to different substances and bilayer thickness. Consequently, membrane permeability caused by lipid peroxidation can disrupt ion gradients, therefore altering metabolic processes [36]. The process of lipid peroxidation gives rise to many products of toxicological interest, including malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and F2-isoprostanes [37]. MDA and HNE have the potential to modify DNA and proteins. They are also capable of inducing apoptosis or necrosis in various cells. Thiobarbituric acid (TBA) reacts with numerous chemical species (including nucleic acids, amino acids, proteins, phospholipids and aldehydes) when heated under acidic conditions [38]. Nevertheless, it appears that lipid peroxidation products could have beneficial effects for cell survival [13].
Oxidation of proteins by ROS can generate a range of stable as well as reactive products, such as protein hydroperoxides, which can generate additional radicals, particularly upon interaction with transition metal ions [39]. Although most oxidized proteins that are functionally inactive are rapidly removed, some can gradually accumulate over time and thereby contribute to the damage associated with aging, as well as various diseases. Depending on the ROS present, the resulting damage to the protein may take the form of nitration or oxidation of various amino acid residues [40]. ROS may also result in the formation of advanced glycation end products (AGE) or advanced oxidation protein products (AOPP), both of which are stable markers of oxidative stress [41].
ROS readily attack DNA and generate a variety of DNA lesions, such as oxidized DNA bases, abasic sites and DNA strand breaks, which ultimately lead to genomic instability [42]. 7,8-Dihydro-8-oxo-deoxyguanosine (8-oxo-dG) is one of the most abundant and well-characterized DNA lesions caused by ROS [43, 44]. It is a highly mutagenic lesion, which results in G:C to T:A transversions. Continuous oxidative damage to DNA may lead to alterations in signalling cascades or gene expression, may induce or arrest transcription, and may cause replication errors [38].
To limit the cellular damage caused by ROS, cells have evolved a number of sophisticated defence mechanisms. ROS-generated DNA lesions are repaired mainly by base excision repair, as well as other DNA repair pathways, including nucleotide excision repair, double-strand break repair and mismatch repair [45]. In addition, the damaging effects of ROS can be neutralized via elevated antioxidant defences, which include superoxide dismutase (SOD), CAT and GPX to scavenge ROS to nontoxic forms [46].
Exposure to free radicals has led organisms to develop a series of defence mechanisms involving prevention mechanisms, repair mechanisms, physical defences and antioxidant defences. Enzymatic antioxidant defences include SOD, which is characterized by three isoforms, CAT and GPX. Two of the SOD isoforms are located within cells, whereas the third SOD isoform is found in the extracellular space [47]. SOD1 requires copper–zinc as a cofactor and is primarily located in the cytosol and the mitochondrial intermembrane space. SOD2 uses manganese as a cofactor and is located in the mitochondrial matrix. Finally, SOD3 requires copper–zinc as a cofactor and is located in the extracellular space. The main role of this enzyme is to catalyse the dismutation of superoxide to hydrogen peroxide and oxygen [48]. CAT is an iron-containing enzyme primary found in peroxisomes. It is predominantly concentrated in the liver, and it detoxifies hydrogen peroxide by catalysing a reaction between two peroxide molecules, resulting in production of water and oxygen. GPX consists of several components, including the enzymes GPX and glutathione reductase (Gred), and the cofactors glutathione (GSH) and NADPH. Together, these components efficiently remove H2O2. This reaction uses GSH and transforms it into oxidized glutathione (GSSG); GPX and CAT have the same action upon H2O2, but GPX seems more efficient with a high ROS concentration, whereas CAT may have an important action with a lower H2O2 concentration [33]. These defences can be modified by exercise, training, nutrition and aging [49].
Non-enzymatic antioxidants include a variety of free-radical quenchers, such as ascorbic acid, alpha-tocopherol, carotenoids, flavonoids, thiols (which include GSH), ubiquinone Q10, uric acid, bilirubin, ferritin, albumin, transferrin, lactoferrin and micronutrients, which act as enzymatic cofactors. Essential micronutrients include cobalt, copper, chromium, fluorine, iron, iodine, manganese, selenium and zinc. Under normal conditions, there is a balance between both the activities and the intracellular levels of antioxidants—this balance being essential for the survival of organisms and their health. Thus, each cell is characterized by a concentration of electrons (a redox state) stored in many cellular constituents, and the redox state of a cell and its oscillation determine cellular functioning—the term ‘redox state’ also describing the redox environment of a cell.
At the molecular level, cellular stress response pathways are controlled by four categories of molecules and transcriptional regulators: insulin/insulin-like IGF-1 signalling, sirtuins, target of rapamycin and adenosine monophosphate–activated protein kinase (AMPK)-dependent pathways. All of these pathways have one molecular target in common, named forkhead box protein O1 (FoxO1) [50]. In mammals, there are four FoxO molecules, which are encoded by different genes, and they all affect the differentiation, proliferation and survival of a variety of cells, including adipocytes, hepatocytes and myoblasts. FoxO activity is regulated by a variety of external stimuli, including insulin, growth factors, hormones, cytokines and oxidative stress, through a plethora of post-translational modification of FoxOs, including phosphorylation, ubiquitination, acetylation and methylation. The antioxidant defence provided by FoxOs is ultimately overwhelmed by high levels of oxidative stress or oxidative stress–activated pathways that interfere with the activity of FoxOs, or both. ROS are widely recognized for their dual roles as being both deleterious and beneficial, since they can be either harmful or beneficial to living systems, particularly by playing a physiological role in intracellular signalling and regulation as secondary messengers. In fact, depending on the type of antioxidants, the intensity and the time of redox imbalance, as well as the type of cells, oxidative stress can play a role in the regulation of important processes through modulation of signal pathways, influencing synthesis of antioxidant enzymes, repair processes, inflammation, apoptosis and cell proliferation [51]. The magnitude and duration of the stress, as well as the cell type involved, are important factors in determining which pathways are activated, and the particular outcome reflects the balance between these pathways. The initiation and proper functioning of several signal transduction pathways rely on the action of ROS as signalling molecules, which may act on different levels in the signal transduction cascade by causing reversible oxidation of proteins at cysteine residues. Such reversible amino acid oxidations regulate extracellular signal–regulated kinases (ERKs), jun N-terminal kinases, p38, MAPK and the Wnt/β-catenin signalling pathway, as well as the activity of transcription factors, including nuclear factor (NF)-kB and activator protein (AP)-1. ROS can thus play a very important physiological role as secondary messengers. However, signalling via ROS is harmful, as overproduction of reactive signal molecules may be destructive.
3 Aging and Oxidative Stress
The process of aging may be defined as a progressive decline in the physiological functions of an organism. The first free-radical theory of aging proposed by Harman [29] postulated that aging results from accumulation of deleterious effects caused by free radicals, and the ability of an organism to cope with cellular damage induced by ROS plays an important role in determining organismal lifespan. In agreement with this theory, increased ROS production by mitochondria and increased 8-oxo-dG content in mitochondrial DNA (mtDNA) are frequently detected in aged tissues [45], suggesting that progressive accumulation of oxidative DNA damage is a contributory factor to the aging process.
Martins Chaves et al. [52] and Bailey et al. [53] noted higher rates of free-radical generation in elderly compared with young subjects in various compartments of measurement (blood or muscle). The authors explained this increased production of ROS by a decline in mitochondrial respiratory function with aging. In fact, respiratory control, the efficiency of oxidative phosphorylation, the rates of resting (state 4) and ADP-stimulated (state 3) respiration, and the activities of respiratory enzyme complexes have all been observed to decline with age in various human tissues [54, 55]. These findings have led to the proposal that impairment of mitochondrial respiratory function is associated with or is responsible for higher free-radical levels in older subjects. It has been shown that aging induces modifications in factors that affect mitochondrial free-radical production [56]. Among those, three factors have been extensively studied: mitochondrial membrane potential (Δψ), intracellular Ca2+ and NO. Figure 2 illustrates the factors that contribute to the rise in mitochondrial ROS production with aging. First, generation of mitochondrial ROS is dependent on Δψ. High Δψ seems to favour the production of ROS, particularly at complex III. This is thought to be due to slowed electron transport and prolongation of ubisemiquinone radical occupancy in the complex [57]. However, most studies with animal models have reported a decrease in Δψ with aging [58, 59]. Therefore the Δψ factor cannot explain the rise in ROS production in older subjects. Second, Ca2+ increases mitochondrial ROS production under conditions of partial mitochondrial membrane depolarization or in the presence of some degree of mitochondrial electron transport inhibition. Several mechanisms have been suggested: (1) Ca2+ stimulates the tricarboxylic acid (TCA) cycle and enhances electron flow into the respiratory chain; (2) Ca2+ stimulates NO production from NO synthase, resulting in inhibition of complex IV; and (3) Ca2+ dissociates cytochrome c from the inner mitochondrial membrane and at higher concentrations induces release of cytochrome c across the outer membrane [60]. Cytochrome c is a potent antioxidant, and its loss can result in increased ROS liberation from the mitochondria. To our knowledge, there have been no studies investigating aging effects on mitochondrial Ca2+ in humans. Only a few studies have focused on this topic in animal models, and they indicated an increase in mitochondrial Ca2+ with aging in the liver [61] and heart tissues [62], which could partly explain the higher ROS production in mitochondria in older tissues. Third, the mitochondrial ROS production in endothelial cells is also under regulation by NO. The best-understood interaction between the mitochondria and NO occurs at complex IV. In fact, low concentrations of NO reversibly bind and inhibit complex IV, thereby modulating mitochondrial respiration and oxygen consumption, and facilitating the release of mitochondrial ROS [57]. Martins Chaves et al. [52] reported higher levels of both NO and ROS in older rats compared with younger rats, supporting the link between NO levels and ROS release with aging. Finally, the increase in free-radical leakage could also be due to lower mitochondrial capacity to scavenge free radicals with aging [63, 64]. In a general manner, most of the studies have shown a decrease in antioxidant levels with age [65–67]. This decrease could be explained by an alteration in cell signalling pathways with age, including NF-kB and AP-1 [68]. Conversely, some studies have shown that aging is accompanied by an increase in the activity of antioxidants. Specifically, studies by Pansarasa et al. [69] and Inal et al. [70] found higher levels of some enzymatic antioxidants in older subjects compared with a younger group.
It should also be noted that other potential sources of ROS generation, including endothelial cells (via xanthine oxidase and/or NADPH oxidase) and activated leukocytes (via NADPH oxidase), could also contribute to increased production of ROS with aging [71]. The activation of neurohumoral factors commonly seen in older adults, including catecholamines and NO, can in fact contribute to the generation of ROS [72].
It also appears that an age-related imbalance between ROS and antioxidant defences may be a primary causal factor in producing chronic inflammatory reactions [73]. Besides impairing the ability to remove ROS, this redox imbalance could activate redox-sensitive transcription factors and generate numerous proinflammatory mediators, such as cytokines, chemokines and inducible NO synthase. These molecules can produce products that include both reactive oxygen and reactive nitrogen species [74], and it is postulated that accumulation of these reactive species contributes to the pathogenesis of age-related diseases [75]. According to Kregel and Zhang [76], a positive feedback loop is also activated with generation of these reactive species, which serves to further augment the cascade of inflammatory processes and exacerbate inflammation-induced cellular and tissue damage.
Despite a large body of evidence supporting the role of ROS in aging, the free-radical theory of aging faces some challenges [77]. In heterozygous mice, for example, the reduction in MnSOD activity with aging results in increased DNA damage but does not accelerate the aging process [78]. In this sense, overexpression of antioxidant enzymes in mice, such as SOD1 or CAT, does not extend the lifespan [79]. Studies of long-lived rodents also have not found a convincing correlation between levels of oxidative damage and aging [80]. Moreover, generally speaking, pharmacological intervention with antioxidants in humans and mice has little effect in prolonging the lifespan. Vina et al. [81] suggested that free radicals can not only cause molecular damage to cells but also serve as signals, leading to the proposal that they act as modulators of physiological processes. Ristow and Zarse [82] reported that oxidative stress may increase longevity and improve metabolic health, and these authors put forward the concept of mitochondrial hormesis or mitohormesis. They emphasized that by promoting an increase in ROS and an increase in mild oxidative damage, one can foster stress defences in mitochondria and increase metabolic health and lifespan. In summary, it seems that ROS cause aging as they alter—sometimes irreversibly—the signalling network of the cell. If the cell can cope with the stress caused by relatively mild action of ROS, then adaptation takes place and damage does not occur. If, however, the cell is overwhelmed by the action of radicals, subcellular damage and aging will take place. Indeed, radicals serve as signals, and interaction between them is tightly balanced [81].
Thus, given that ROS are short-lived molecules and that their molecular signatures imprinted on lipids and proteins can execute the function of positive oxidative stress, including redox signalling and activation of transcriptional factors [13], further studies are needed to identify more targets and their mechanisms, especially their prophylactic roles in aging.
4 Oxidative Stress and Exercise in Elderly Subjects
Both inactivity and aging are known to increase basal ROS concentrations in skeletal muscle. New data and literature reviews show that chronic ROS overproduction induced by physical inactivity may exacerbate the activation of some redox-sensitive signalling pathways involved in age-related sarcopenia [68–84]. In contrast, regular physical activity is an important determinant in maintaining an optimal state of health and preventing chronic diseases. Conversely, acute exercise increases oxygen consumption, which induces an increase in the leakage of ROS, such as superoxide and hydroxyl radicals, as well as products of oxidative phosphorylation of the ETC, and subsequently promotes an imbalance between oxidants and antioxidants [85].
4.1 Exercise and Hormesis: Effects on Oxidative Stress in Elderly Subjects
The thesis of the hormesis theory is that biological systems respond to exposure to chemicals, toxins and radiation with a bell-shaped curve. In toxicology, hormesis is a dose–response phenomenon characterized by a low dose of stimulation and a high dose of inhibition response [86–88]. Recently, Radak et al. [89] extended the hormesis theory to free-radical species, which appear to plateau when modulated by aging or physical exercise. Therefore, they proposed that exercise modulates oxidative stress and the effects can be described by the hormesis curve.
It has been clearly shown that a single bout of exercise exceeding a certain intensity or duration results in increased production of ROS and causes oxidative damage to lipids, proteins and DNA [90, 91]. On the other hand, it is also well established that regular exercise is a preventive measure against oxidative stress–related diseases, including cardiovascular diseases, stroke and certain cancers [92–94]. Radak et al. [89] proposed that this paradoxical effect is due to the ability of exercise to increase the formation of ROS to a level that may induce significant but tolerable damage, which can, in turn, induce beneficial adaptations, in keeping with the theory of hormesis. The available data regarding the effects of exercise on oxidative stress in elderly subjects suggest that regular physical activity improves antioxidant defences and reduces free-radical damage [95, 96]. However, these adaptations are often related to the exercise intensity. In general, exercise at high intensity or for a long duration can enhance free-radical production, induce damage in various tissues [97] and decrease circulating antioxidants [98]. However, exercise at moderate intensity and duration can generally be regarded as an up-regulator of antioxidant defences and a down-regulator of free-radical production under basal conditions and during exercise [99]. A recent study by Bouzid et al. [100] compared the effects of regular physical activity at high and moderate intensity on oxidative stress in older adults. The authors concluded that both low and high physical exercise levels help to maintain better antioxidant defences in older adults. However, greater physical fitness could increase lipid peroxidation damage more than a low physical fitness level. According to Radak et al. [89], this differential oxidative stress training adaptation between high and moderate intensity comes from the fact that older tissues need a lower level of ROS to prevent diseases associated with oxidative stress and to retard the aging process. Therefore, the molecular basis of the preventive effect of regular exercise is due, in part, to intermittent, brief increases in the formation of ROS, thereby altering signalling pathways and/or causing molecular damage that can induce adaptive responses that protect against a subsequent stronger stress. In other words, exercise-induced increases in the production of ROS may protect against ROS-associated diseases. Moreover, regular physical exercise mediates health and longevity promotion involving sirtuin 1 (SIRT1)-regulated pathways. The anti-aging activity of SIRT1 is achieved, at least in part, by means of fine-tuning of the AMPK pathway through prevention of the transition of an originally pro-survival programme into a pro-aging mechanism. Additionally, SIRT1 promotes mitochondrial function and reduces the production of ROS through regulation of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α, the master controller of mitochondrial biogenesis [101].
Figure 3 presents the relationship between training intensity and oxidative stress adaptation in older adults.
4.2 Oxidative Stress and Acute Exercise in Elderly Subjects
There are few studies that have evaluated the effect of acute exercise on ROS production in healthy elderly subjects. Table 1 summarizes the data available in the literature.
In a general manner, the generation of ROS in skeletal muscle increases during physical exercise or isolated contractions [102]. Contraction-induced changes in ROS modulate some of the adaptive responses that occur in skeletal muscle following contractile activity. Thus, muscle ROS generation during contractions increases the expression of genes involved in antioxidant defence (e.g. heat shock protein [HSP] 60, HSP70, NF-kB, AP-1) and also in mitochondrial biogenesis (e.g. PGC-1α) [103], and thus has a positive effect.
Koechlin et al. [104] reported increased production (+29 %) of superoxide radicals (O2 ⋅−) after a dynamic quadriceps endurance test at 40 % of maximal strength among elderly subjects. Bailey et al. [53] also noted that ubisemiquinone radical (UQ−) production was higher after maximal knee extensor exercise in elderly subjects compared with young subjects. The reason for the increased ROS production in aged subjects is not entirely clear. Age-related defects in the mitochondrial ETC are considered a major mechanism. In fact, mitochondria from aged animals demonstrate much lower cytochrome c oxidase (complex IV) activity than those from young animals, whereas enzyme activities in complexes I and II show smaller changes [105]. This alteration in the ETC favours greater electron ‘leakage’ and formation of superoxide anions (O2 ⋅−) found in the senescent organism. Moreover, although older adults achieve only lower maximal exercise intensities and may have defects in ROS-induced cellular processes, such as impaired ETC in mitochondria, they also demonstrate much lower antioxidant capacity than young subjects, which could also enhance ROS production during acute exercise.
A post-exercise inflammatory reaction could also accentuate exercise-induced free-radical production in elderly subjects [106]. In addition, strenuous exercise triggers release of TNF-α, IL-1 and IL-6 from immune cells and/or damaged muscle tissue [107]. During the early phase of muscle injury, these cytokines play an important role in inflammatory responses by promoting adhesion molecule expression and NO synthase induction in the endothelial cells [108]. The resulting increase in vasodilatation due to NO production facilitates the movement of polymorphoneutrophils (PMN) and circulating cytokines to the affected area. In addition to stimulating PMN infiltration, and hence ROS production, some cytokines can bind with membrane receptors and activate specific ROS-generating enzymes, such as lipooxygenase, NADPH oxidase and xanthine oxidase. In a general manner, acute exercise increases muscle oxygen flux and induces intracellular events that can lead to increased oxidative injury, and the paradox arises as to whether exercise is advisable for the aged population, who are prone to muscle injury and sensitive to inflammatory responses [68]. On the other hand, studies have noted that ROS modulate some of the adaptive responses that occur in skeletal muscle following contractile activity [109, 110].
Figure 4 shows the main mechanisms responsible for ROS production in response to acute exercise.
The overall cellular oxidative stress during aging or inactivity is determined not only by ROS production but also by the defence capacity of antioxidant systems [106]. As previously described, most of the studies have shown a decrease in antioxidant levels with age [65–67]. However, some studies have reported that aging increases the activity of MnSOD in both human and rodent skeletal muscles [111, 112]. The same findings have been reported concerning the mitochondrial activity of GPX [69]—these increases being a defence mechanism that fights against mitochondrial ROS overproduction in old skeletal muscle.
Regarding the effect of aerobic exercise on the activity of antioxidant enzymes, most studies have shown an increase in antioxidant activity in the blood and muscle after aerobic exercise. This response appears immediately after exercise in the blood and up to 48 h post-exercise in muscle cells [25].
In summary, acute exercise induces increased production of free radicals in elderly subjects. In response, the antioxidant defences increase the rate of enzymatic and non-enzymatic antioxidants. However, given the decline in antioxidant defences with age, it seems that the antioxidant defences would not be able to neutralize all of the free radicals produced during exercise, thus setting up a status of oxidative stress. Currently, there are only sparse data concerning exercise and oxidative stress in the elderly population, in comparison with the volume of data in young subjects. Future studies should focus on the relationship between exercise intensity and oxidative stress in the elderly, and the relationship between different types of exercise (aerobic, anaerobic or mixed) and oxidative stress in this population.
4.3 Oxidative Stress and Endurance Training in Elderly People
Table 2 summarizes the investigations in this area. A number of studies have examined the effects of endurance training on markers of oxidative stress and antioxidant capacity [25, 26, 113]. These studies found that endurance training attenuated resting and exercise-induced oxidative stress, and increased protection against oxidative stress by increasing the efficiency of enzymatic and non-enzymatic antioxidant defence systems, suggesting training-induced adaptations of oxidative stress.
For example, Fatouros et al. [25] reported a 16 % decrease in the MDA level following a period of endurance training at 50–80 % of the maximal heart rate (HRmax). These results are in agreement with those reported by Karolkiewicz et al. [114], who demonstrated a 20 % decrease in plasma TBA reactive substances (TBARS) after endurance training at 70–80 % of maximal oxygen consumption (VO2max). Ghosh et al. [115] showed a decrease in the basal production of H2O2 in muscle tissue after a training period of 16 weeks at 65 % of VO2max in older adults (mean age 72.6 ± 1.6 years). This decrease may have been due to greater mitochondrial capacity to scavenge free radicals [116–118]. Training may also induce modifications in factors that affect mitochondrial free-radical production. ROS production, during basal conditions, seems to be controlled in part by the mitochondrial membrane potential (ΔΨ m). According to Daussin et al. [119], the induction of uncoupling protein (UCP) by regular physical activity may activate mitochondrial pathways, which decreases ΔΨ m and increases basal respiration. Such effects could prevent ROS release in mitochondria.
Moreover, it appears that regular endurance training is able to stimulate the oxidative damage repair system [120–122]. In fact, it has been reported that regular exercise increases the activity of the proteasome complex, which is believed to be responsible for degradation of oxidatively modified proteins [123]. The proteasome complex has a very important role—namely, reduction of oxidatively modified proteins—leading to better and more efficient cell function by more rapid turnover of proteins [124]. Although the proteasome complex remains operational and functional in elderly human cells [125, 126], some studies have reported that the rate of increase of protein oxidation with aging may exceed the proteasome complex capacity to degrade the majority of oxidized proteins [127, 128]. Keller et al. [129] reported that the proteasome is able to degrade a vast array of proteins, including oxidized proteins. The ability of the proteasome in young tissue to efficiently degrade these proteins is due to its ability to interchange proteasome subunits, as well as the binding of specialized 11S and 19S proteasome caps. In elderly subjects, the plasticity of the proteasome probably becomes inefficient, resulting in impairment of the ability of the proteasome to respond to oxidative stress damage [130], which ultimately causes deleterious accumulation of a diverse array of proteins. It is therefore possible that proteasome inhibition contributes to the aging process through such deleterious accumulations in intracellular proteins [131]. Nevertheless, as we mentioned earlier, these changes in proteasome complex efficiency in elderly subjects could be restored by exercise training.
In addition to an increase in damaged protein-degrading systems, the activities of DNA and lipid peroxidation damage-repairing enzymes are also up-regulated by regular exercise [132, 133]. However the repair process of DNA damage and lipid peroxidation induced by regular exercise in humans remains unclear and little studied; only a few studies have focused on this topic, all of which involved animal models [120–123, 134].
Concerning the antioxidant systems, most of the studies did not identify any changes in the activity of SOD (in blood), MnSOD or Cu-ZnSOD (in muscle tissue) following endurance training at rest [115–135]. These findings are consistent with those reported by Fatouros et al. [25], who demonstrated no effect of endurance training at 50–80 % of HRmax in GPX activity, but they noted an increase in GPX activity and total antioxidant status in response to acute exercise. This adaptation may be due to regular exercise activating signal transduction pathways, resulting in enhancement of endogenous antioxidant systems. According to Lambertucci et al. [136], ROS may modulate antioxidant enzyme activities by regulating the messenger RNA (mRNA) level through activation of signalling pathways. Franco et al. [137] demonstrated that induction of antioxidant enzyme mRNA levels coincides with increases in oxidative protein damage, supporting the postulated relationship between oxidative stress and antioxidant enzyme mRNA expression. In fact, ROS play a very important role in regulating cell functions by acting as secondary messengers and activating specific redox-sensitive transcription factors, such as AP-1 and NF-kB [103]. AP-1 and NF-kB response elements are present in the promoter regions of genes encoding CAT, GPX, Mn-SOD and Cu-ZnSOD [138]. Combinations of AP-1 and NF-kB with other redox-sensitive transcription factors may determine how an antioxidant enzyme is induced and to what extent. Therefore, by activating these signalling pathways, endurance training can lead to an improvement in antioxidant activity that would appear during major oxidative stress such as after acute exercise among elderly subjects.
In summary, endurance training may induce a decrease in the production of free radicals in the basal condition in elderly people. These results may relate to both improved antioxidant defences and better control of mitochondrial ROS production. In view of the findings in the literature, optimal aerobic training for oxidative/antioxidant balance effects can be achieved with intensities between the two ventilatory thresholds (50–80 % of VO2max) and with a frequency of 2–3 sessions per week.
4.4 Oxidative Stress and Resistance Training in Elderly People
Differences in methodological procedures and the numerous selected markers of oxidative stress from one study to another make it difficult to draw a conclusion about the effect of resistance training on oxidative stress damage markers. Table 3 summarizes the most important data on this topic. Nevertheless, Bloomer et al. [139] reported a decrease (16 %) in the basal production of H2O2 radicals after 8 weeks of resistance training in seniors with Parkinson’s disease. The decrease in free-radical production after resistance training may be attributable to regulation of the mitochondrial respiratory chain. In fact, Parise et al. [140] noted an increase in the complex IV to complex I + III ratio of the mitochondrial respiratory chain following resistance training in older adults. An increase in the complex IV to complex I + III ratio may result in a greater driving force down the ETC, thus reducing the amount of electron leakage and resulting in a decrease in ROS production.
The response of markers of free-radical damage following resistance training depends on the measurement compartment. Rall et al. [141], who implemented resistance training at 80 % of one-repetition maximum (1RM), did not detect any change in urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels following resistance training. However the study by Parise et al. [140] showed a decrease in this marker in muscle tissue after resistance training at 50–80 % of 1RM. On the other hand, other markers such as TBARS have been shown to decrease after a period of resistance training in both serum [142] and plasma [143] compartments. Other studies have not found an effect of resistance training on other markers of free-radical damage. For example, a study by Bobeuf et al. [144] did not detect any differences in plasma MDA and urinary F2-isoprostane levels between before and after resistance training in older adults. The differences in these studies’ results may be related to the choice of markers of free-radical damage, as well as the measuring compartments (blood, muscle, plasma). Differences in methodological procedures and the numerous selected markers between one study and another make it difficult to draw a conclusion about the effect of resistance training on oxidative stress damage markers.
To our knowledge, only one study in elderly subjects has shown a significant improvement in the activity of antioxidant enzymes after resistance training [145]. This improvement can be attributed to an adaptation in the response to free radicals produced during training sessions—this adaptation being related to the training intensity. In fact, if the exercise intensity during training resistance is too low and thus insufficient, the majority of free radicals produced during the training sessions will be eliminated by the antioxidant defences, and consequently there will be no change in the antioxidant system [146].
Moreover, a lack of change in any of the antioxidant enzyme protein content may not necessarily mean that these systems do not respond to resistance exercise. For example, Oh-Ishi et al. [147] reported significantly higher Cu-ZnSOD enzyme activity in rat muscle following exercise training, despite there being no increase in Cu-ZnSOD protein content, suggesting that these adaptations resulted from post-translational modifications to existing proteins. Further analysis is necessary to determine whether antioxidant enzyme activity is increased following resistance exercise training.
On the other hand, aging is accompanied by an ‘anabolic resistance’ phenomenon, which could impair the response of muscles to resistance training in elderly subjects. Oxidative stress and chronic inflammation with aging are the major factors that explain this phenomenon. In fact, it is well known that increased oxidative stress and inflammation co-exist in many skeletal muscle–associated diseases and dysfunctions such as the anabolic resistance phenomenon [16]. An age-related disruption in the intracellular redox balance appears to be a primary causal factor in producing a chronic state of low-grade inflammation through activation of redox-sensitive transcription factor NF-kB. In fact, some reports have demonstrated that age-related up-regulation of proinflammatory cytokines, such as IL-6 and TNF-α, is mediated by NF-kB [148]. In this sense, Reid et al. [149] showed that ROS appear to function as secondary messengers for TNF-α in skeletal muscle by activating NF-kB transcription factor. TNF-α is one of the primary signals that induce cellular apoptosis in muscle. Apoptosis and inflammation closely interact with oxidative damage, and they are involved in age-related reductions in muscle mass and strength [149]. Roubenoff [150] reported that chronic inflammation may negatively influence skeletal muscle through direct catabolic effects or through indirect mechanisms, such as decreases in growth hormone and IGF-1 levels. Such a reduction in the IGF-1 level is associated with sarcopenia and resistance to normally robust anabolic stimuli, such as resistance exercise—the anabolic resistance phenomenon.
In view of the literature, it may be assumed that resistance training could modify the balance between oxidants/antioxidants in elderly subjects by improving antioxidant defences. It would appear that these improvements are related to the intensity of training. To optimize the results of resistance training, training protocols must contain sufficient volume for each muscle group (3–5 sets, 10 repetitions) and with intensities between 50 and 80 % of 1RM.
5 Conclusion
Aging is a complex process involving a multitude of factors. Many studies have demonstrated that oxidative stress and mitochondrial dysfunction are two important factors contributing to the aging process. On the other hand, recent studies demonstrate that ROS can have positive effects on aging disease—ROS being signalling molecules, which activate oxidative stress–sensitive signal transduction pathways in mammalian tissues.
Moreover, the role of oxidative stress in age-related sarcopenia provides strong evidence for the important contribution of physical activity in limiting this process. Conversely, exercise is associated with increased ROS generation, which may aggravate oxidative damage to the aged cell. At the same time, however, muscle ROS generation during contractions increases the expression of genes involved in antioxidant defence, but also in mitochondrial biogenesis, and thus has a positive effect. In the context of chronic exercise, repeated exposure to oxidative stress during exercise sessions could induce adaptation in the elderly. However, as previously mentioned, it remains unclear whether exercise-induced oxidative modifications induce harmful oxidative damage or are an integral part of redox regulation.
References
Goto S, Radák Z. Hormetic effects of reactive oxygen species by exercise: a view from animal studies for successful aging in human. Dose Response. 2009;8:68–72.
Harman D. Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009. Biogerontology. 2009;10:773–81.
Muller FL, Lustgarten MS, Jang Y, et al. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503.
Buffenstein R, Edrey YH, Yang T, et al. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordrecht). 2008;30:99–109.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47.
Vina J, Borras C, Gomez-Cabrera MC. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19:779–87.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Bejma J, Ramires P, Ji LL. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–51.
Sawada M, Sester U, Carlson JC. Superoxide radical formation and associated biochemical alterations in the plasma membrane of brain, heart and liver during the lifetime of the rat. J Cell Biochem. 1992;48:296–304.
Malinin NL, West XZ, Byzova TV. Oxidation as “the stress of life”. Aging (Albany N. Y.). 2011;3:906–10.
Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57.
Patel RS, Al Mheid I, Morris AA, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–5.
Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2C:165–9.
Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–29.
Milisav I, Poljsak B, Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13:10771–806.
Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–26.
Wu J, Xia S, Kalionis B, et al. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int. 2014;2014:615312.
Howard C, Ferrucci L, Sun K, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol. 2007;103:17620.
Woods JA, Wilund KR, Martin SA, et al. Exercise, inflammation and aging. Aging Dis. 2012;3:130–40.
Koopman R, van Loon LJ. Aging, exercise and muscle protein metabolism. J Appl Physiol. 2009;106:2040–8.
McBride JM, Kraemer WJ, Triplett-McBride T, et al. Effect of resistance exercise on free radical production. Med Sci Sports Exerc. 1998;30:67–72.
El Abed K, Rebai H, Bloomer RJ, et al. Antioxidant status and oxidative stress at rest and in response to acute exercise in judokas and sedentary men. J Strength Cond Res. 2011;25:2400–9.
Laforest S, St-Pierre DM, Cyr J, et al. Effect of age and regular exercise on muscle strength and endurance. Eur J Appl Physiol Occup Physiol. 1990;60:104–11.
Polidori MC, Mecocci P, Cherubini A, et al. Physical activity and oxidative stress during aging. Int J Sports Med. 2000;21:154–7.
Fatouros IG, Jamurtas AZ, Villiotou V, et al. Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc. 2004;36:2065–72.
Takahashi M, Miyashita M, Kawanishi N, et al. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur J Appl Physiol. 2013;113:1117–26.
Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44.
Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–312.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–24.
Zheng M, Storz G. Redox sensing by prokaryotic transcription factors. Biochem Pharmacol. 2000;59:1–6.
Sardina JL, Lopez-Ruana G, Sanchez-Sanchez B, et al. Reactive oxygen species: are they important for haematopoiesis? Crit Rev Oncol Hematol. 2012;81:257–74.
Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36:327–58.
Wink DA, Hanbauer I, Grisham MB, et al. Chemical biology of nitric oxide: regulation and protective and toxic mechanisms. Curr Top Cell Regul. 1996;34:159–87.
Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–8.
Wong-Ekkabut J, Xu Z, Triampo W, et al. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. 2007;93:4225–36.
Erejuwa OO, Sulaiman SA, Ab Wahab MS. Evidence in support of potential applications of lipid peroxidation products in cancer treatment. Oxid Med Cell Longev. 2013;2013:931251.
Crohns M. Antioxidants, cytokines and markers of oxidative stress in lung cancer: associations with adverse events, response and survival. 1st ed. Saar-Brücken: Lambert Academic Publishing; 2010.
Dean RT, Fu S, Stocker R, et al. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18.
Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–6.
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13.
Krokan HE, Standal R, Slupphaug G. DNA glycosylases in the base excision repair of DNA. Biochem J. 1997;325:1–16.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39.
Subash P, Gurumurthy P, Sarasabharathi A, et al. Urinary 8-OHdG: a marker of oxidative stress to DNA and total antioxidant status in essential hypertension with South Indian population. Indian J Clin Biochem. 2010;25:127–32.
Maynard S, Schurman SH, Harboe C, et al. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10.
Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354.
Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76.
Mates M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104.
Franzke B, Halper B, Hofmann M, et al. The impact of six months strength training, nutritional supplementation or cognitive training on DNA damage in institutionalised elderly. Mutagenesis. 2015;30:147–53.
Kousteni S. FoXOs: unifying links between oxidative stress and skeletal homeostatis. Curr Osteoporos Rep. 2011;9:60–6.
Filaire E, Dupuis C, Galvaing G, et al. Lung cancer: what are the links with oxidative stress, physical activity and nutrition. Lung Cancer. 2013;82:383–9.
Martins Chaves M, Rocha-Vieira E, Pereira dos Reis A, et al. Increase of reactive oxygen (ROS) and nitrogen (RNS) species generated by phagocyting granulocytes related to age. Mech Ageing Dev. 2000;119:1–8.
Bailey DM, McEneny J, Mathieu-Costello O, et al. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109:449–56.
Kovalenko SA, Kopsidas G, Kelso JM, et al. Deltoid human muscle mtDNA is extensively rearranged in old age subjects. Biochem Biophys Res Commun. 1997;232:147–52.
Melov S, Hinerfeld D, Esposito L, et al. Multi-organ characterization of mitochondrial genomic rearrangements in ad libitum and caloric restricted mice show striking somatic mitochondrial DNA rearrangements with age. Nucleic Acids Res. 1997;25:974–82.
Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013;19:1420–45.
Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–31.
Kokoszka JE, Coskun P, Esposito LA, et al. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–83.
Duicu OM, Mirica SN, Gheorgheosu DE, et al. Ageing-induced decrease in cardiac mitochondrial function in healthy rats. Can J Physiol Pharmacol. 2013;91:593–600.
Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33.
Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–8.
Petrosillo G, Moro N, Paradies V, et al. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340–6.
Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med. 1998;24:1477–84.
Lustgarten MS, Jang YC, Liu Y, et al. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505.
Guemouri L, Artur Y, Herbeth B, et al. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991;37:1932–7.
Ceballos-Picot I, Trivier JM, Nicole A, et al. Age-correlated modifications of copper–zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes. Clin Chem. 1992;38:66–70.
Andersen HR, Nielsen JB, Nielsen F, et al. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–8.
Bar-Shai M, Carmeli E, Ljubuncic P, et al. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med. 2008;44:202–14.
Pansarasa O, Bertorelli L, Vecchiet J, et al. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–22.
Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305:75–80.
Oliveira BF, Nogueira-Machado JA, Chaves MM. The role of oxidative stress in the aging process. Sci World J. 2010;10:1121–8.
Canton M, Menazza S, Di Lisa F. Oxidative stress in muscular dystrophy: from generic evidence to specific sources and targets. J Muscle Res Cell Motil. 2014;35:23–36.
Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80.
Mariani E, Polidori MC, Cherubini A, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75.
Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23.
Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36.
Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8.
Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37.
Chen X, Liang H, Van Remmen H, et al. Catalase transgenic mice: characterization and sensitivity to oxidative stress. Arch Biochem Biophys. 2004;422:197–210.
Andziak B, O’Connor TP, Qi W, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–71.
Vina J, Borras C, Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–54.
Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45:410–8.
Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79.
Derbre F, Gratas-Delamarche A, Gomez-Cabrera MC, et al. Inactivity-induced oxidative stress: a central role in age-related sarcopenia? Eur J Sport Sci. 2014;14:S98–108.
Radak Z, Zhao Z, Koltai E, et al. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–46.
Calabrese EJ, Baldwin LA. U-shaped dose–responses in biology, toxicology, and public health. Annu Rev Public Health. 2001;22:15–33.
Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21:91–7.
Cook RR, Calabrese EJ. Hormesis is biology, not religion. Environ Health Perspect. 2006;114:A688.
Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–5.
Davies KJ, Quintanilha AT, Brooks GA, et al. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–205.
Radak Z, Pucsok J, Mecseki S, et al. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med. 1999;26:1059–63.
Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;351:1603–8.
Hamilton ML, Van RH, Drake JA, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–74.
Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33:877–88.
Rowiński R, Kozakiewicz M, Kędziora-Kornatowska K, et al. Markers of oxidative stress and erythrocyte antioxidant enzyme activity in older men and women with differing physical activity. Exp Gerontol. 2013;48:1141–6.
Traustadóttir T, Davies SS, Su Y, et al. Oxidative stress in older adults: effects of physical fitness. Age (Dordrecht). 2012;34:969–82.
Cooper CE, Vollaard NB, Choueiri T, et al. Exercise, free radicals and oxidative stress. Biochem Soc Trans. 2002;30:280–5.
Bergholm R, Mäkimattila S, Valkonen M, et al. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999;145:341–9.
Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–31.
Bouzid MA, Hammouda O, Matran R, et al. Influence of physical fitness on antioxidants activities and malondialdehyde level in healthy older adults. Appl Physiol Nutr Metab. 2015;4:1–8.
Bayod S, Guzmán-Brambila C, Sanchez-Roige S, et al. Voluntary exercise promotes beneficial anti-aging mechanisms in SAMP8 female brain. J Mol Neurosci. 2015;55:525–32.
Gomez-Cabrera MC, Borras C, Pallardo FV, et al. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–20.
Kang C, O’Moore KM, Dickman JR, et al. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–400.
Koechlin C, Couillard A, Simar D, et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2004;169:1022–7.
Tatarková Z, Kuka S, Račay P, et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–9.
Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–47.
Gökbel H, Okudan N, Gül I, et al. Effects of repeated bouts of supramaximal exercise on plasma adiponectin, interleukin-6, and tumor necrosis factor-α levels in sedentary men. J Strength Cond Res. 2012;26:1675–9.
Flohé L, Brigelius-Flohé R, Saliou C, et al. Redox regulation of NF-kappa-B activation. Free Radical Biol Med. 1997;22:1115–26.
Jackson MJ, Papa S, Bolaños J, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–85.
Khassaf M, Child RB, McArdle A, et al. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–5.
Gianni P, Jan KJ, Douglas MJ, et al. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–400.
Marzani B, Felzani G, Bellomo RG, et al. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40:959–65.
Johnson ML, Irving BA, Lanza IR, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol A Biol Sci Med Sci. 2014 (in press).
Karolkiewicz J, Michalak E, Pospieszna B, et al. Response of oxidative stress markers and antioxidant parameters to an 8-week aerobic physical activity program in healthy, postmenopausal women. Arch Gerontol Geriatr. 2009;49:67–71.
Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–60.
Higuchi M, Cartier LJ, Chen M, et al. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–6.
Chandwaney R, Leichtweis S, Leeuwenburgh C, et al. Oxidative stress and mitochondrial function in skeletal muscle: effects of aging and exercise training. Age (Omaha). 1998;21:109–17.
Bori Z, Zhao Z, Koltai E, et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47:417–24.
Daussin FN, Rasseneur L, Bouitbir J, et al. Different timing of changes in mitochondrial functions following endurance training. Med Sci Sports Exerc. 2012;44:217–24.
Radak Z, Kaneko T, Tahara S, et al. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27:69–74.
Radak Z, Kaneko T, Tahara S, et al. Regular exercise improves cognitive function and decrease oxidative damage in rat brain. Neurochem Int. 2001;38:17–23.
Sato Y, Nanri H, Ohta M, et al. Increase of human MTH1 and decrease of 8-hydroxydeoxyguanosine in leukocyte DNA by acute and chronic exercise in healthy male subjects. Biochem Biophys Res Commun. 2003;305:333–8.
Radak Z, Naito H, Kaneko T, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–8.
Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–5.
Agarwal S, Sohal RS. Aging and proteolysis of oxidized proteins. Arch Biochem Biophys. 1994;309:24–8.
Carrard G, Dieu M, Raes M, et al. Impact of ageing on proteasome structure and function in human lymphocytes. Int J Biochem Cell Biol. 2003;35:728–39.
Lilienbaum A. Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol. 2013;4:1–26.
Pickering AM, Davies KJ. Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci. 2012;109:227–48.
Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing Res Rev. 2002;1:279–93.
Shringarpure R, Davies KJ. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med. 2002;32:1084–9.
Ding Q, Dimayuga E, Markesbery WR, et al. Proteasome inhibition induces reversible impairments in protein synthesis. FASEB J. 2006;20:1055–63.
Radak Z, Apor P, Pucsok J, et al. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–33.
Wittwer M, Billeter R, Hoppeler H, et al. Regulatory gene expression in skeletal muscle of highly endurance trained humans. Acta Physiol Scand. 2004;180:217–27.
Nakamoto H, Kaneko T, Tahara S, et al. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol. 2007;42:287–95.
Zago AS, Park JY, Fenty-Stewart N, et al. Effects of aerobic exercise on the blood pressure, oxidative stress and eNOS gene polymorphism in pre-hypertensive older people. Eur J Appl Physiol. 2010;110:825–32.
Lambertucci RH, Levada-Pires AC, Rossoni LV, et al. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev. 2007;128:267–75.
Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–32.
Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–16.
Bloomer RJ, Schilling BK, Karlage RE, et al. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40:1385–9.
Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40:173–80.
Rall LC, Roubenoff R, Meydani SN, et al. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J Nutr Biochem. 2000;11:581–4.
Vincent KR, Vincent HK, Braith RW, et al. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur J Appl Physiol. 2002;87:416–23.
Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring). 2006;14:1921–30.
Bobeuf F, Labonte M, Dionne IJ, et al. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging. 2011;15:883–9.
Parise G, Phillips SM, Kaczor JJ, et al. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med. 2005;39:289–95.
Lovlin R, Cottle W, Pyke I, et al. Are indices of free radical damage related to exercise intensity? Eur J Appl Physiol. 1987;56:313–6.
Oh-ishi S, Kizaki T, Nagasawa J, et al. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin Exp Pharmacol Physiol. 1997;24:326–32.
Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30.
Carter CS, Hofer T, Seo AY, et al. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954–66.
Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295–9.
Meydani M, Evans WJ, Handelman G, et al. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am J Physiol. 1993;264:992–8.
Di Massimo C, Taglieri G, Penco M, et al. Influence of aging and exercise-induced stress on human platelet function. Clin Hemorheol Microcirc. 1999;20:105–10.
Meijer EP, Coolen SA, Bast A, et al. Exercise training and oxidative stress in the elderly as measured by antipyrine hydroxylation products. Free Radic Res. 2001;35:435–43.
Sacheck JM, Milbury PE, Cannon JG, et al. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med. 2003;34:1575–88.
Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1664–9.
Radak Z, Bori Z, Koltai E, et al. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic Biol Med. 2011;51:417–23.
Bouzid MA, Hammouda O, Matran R, et al. Low intensity aerobic exercise and oxidative stress markers in older adults. J Aging Phys Act. 2014;22:536–42.
Jessup JV, Horne C, Yarandi H, et al. The effects of endurance exercise and vitamin E on oxidative stress in the elderly. Biol Res Nurs. 2003;5:47–55.
Acknowledgments
No sources of funding were used in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review. The authors express thanks to Mr. Henrick Grenu for his assistance in improving the English in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouzid, M.A., Filaire, E., McCall, A. et al. Radical Oxygen Species, Exercise and Aging: An Update. Sports Med 45, 1245–1261 (2015). https://doi.org/10.1007/s40279-015-0348-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-015-0348-1