Abstract
Introduction and objective
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system. MS is considered incurable; however, disease treatment has advanced significantly over the past several decades with the introduction of disease-modifying therapies (DMTs). The current study reviewed the cost-effectiveness analyses of DMTs in relapsing–remitting MS (RRMS) patients.
Methods
A systematic literature search of bibliographic databases was conducted to identify economic evaluations published after 2007. The relevant population, intervention, comparators, outcomes, and study design (PICOS) were considered. The outcomes of interest were incremental cost-effectiveness ratios (ICERs), net monetary benefits, incremental benefits, and incremental costs. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement was used to assess the reporting quality of published studies.
Results
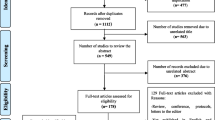
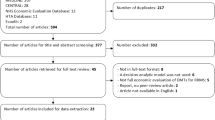
A total of 1370 potentially relevant citations were identified, of which 33 published articles and four Health Technology Assessment (HTA) reports prepared for the UK were included in the final analysis. Almost all studies were based on a health economic model and considered RRMS as the phase of disease at study entry. The studies were conducted in 10 different countries, with approximately 50% based in the US. Study outcomes were rarely comparable due to the different settings, input data, and assumptions. Even within the same country, the discrepancy between study criteria was considerable. The compliance with reporting standards of the CHEERS statement was generally high.
Conclusions
Internationally, a large number of health economic assessments of DMTs in RRMS were available, yielding difficult to compare, and at times conflicting, results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The level of compliance in the identified cost-effectiveness papers with reporting standards of the CHEERS statement was generally high. |
The outcomes of the studies were difficult to compare due to the different settings, input data, and assumptions; a high level of heterogeneity and sometimes conflicting results were observed even between studies conducted in the same country. |
While few conclusions can be drawn due to the variance in study inputs, the following general trends were of interest: |
Pegylated interferon (pegIFN) and dimethyl fumarate (DMF) were mostly cost effective in all studies in which they were analyzed. |
With the exception of three US studies, oral treatments were cost effective when compared with injection treatments. |
The main drivers of the incremental cost-effectiveness ratios (ICERs) were treatment effectiveness and direct treatment costs. |
1 Introduction and Objective
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, in which the protective myelin layer covering the nerve cells in the brain and spinal cord are damaged, resulting in progressive disability [1, 2].
MS takes several forms, with new symptoms either occurring in isolated attacks (relapsing form) or building up over time (progressive form) [3]. Between attacks, symptoms may disappear completely; however, permanent neurological problems often remain, especially as the disease advances [3]. In 85% of patients with MS, the onset form is relapsing–remitting MS (RRMS) [4].
MS affects approximately 2.3 million people all over the world, with a ratio of about 2:1 women to men. The prevalence of the disease is estimated to be > 100 per 100,000 in North America and across most of Europe [4]. The disease onset occurs when patients are of working age, and therefore, the chronicity and progression of the disease heavily impacts on patients’ quality of life (QoL) and, more broadly, on societal costs. MS is currently considered incurable [5]; however, treatment has advanced significantly over the past several decades [6,7,8,9,10,11]. Disease-modifying therapies (DMTs) can reduce the frequency and severity of clinical relapses, reduce the development of new lesions within the brain and spinal cord, and delay the progression of disability [12]. As is the case in many different therapeutic areas, clinical progress in treating the disease has accompanied a rise in costs to purchase biologic products [13]. DMTs have experienced a swift rise in acquisition costs over the last 15 years, despite the ongoing approval of novel DMTs as well as the recent introduction of the first generic in the US. Ultimately, a consequence of high drug prices for patients with MS in the US is the potential for reduced access to DMTs, and costs to major payers (i.e., Medicare) although often discounted, must be considered in the context of value [13]. Value for cost is a concept not limited to the US. In 2002 the NHS in the UK initiated a risk access scheme for interferon-beta (IFNβ) and glatiramer acetate (GA) that closely monitored patient outcomes in an attempt to confirm the cost effectiveness of the DMTs [14]. In this light, economic evaluations are key elements for healthcare decision making [13, 15]. Recently, cost-effectiveness studies of DMTs have been the subject of systematic literature reviews. Yamamoto and Campbell [16] conducted a general review of the literature with the aim of providing a comprehensive understanding of the cost effectiveness of DMTs for the treatment of MS, including the evaluation of the quality of recent cost-effectiveness studies through the use of the 16-item Quality of Health Economic Studies (QHES) instrument [17]. Koeser and McCrone [18] conducted a limited cost-effectiveness analyses review of a single specific DMT (natalizumab [NTZ]). Hawton et al. [19] analyzed the methodological challenges and suggested practical recommendations for future research, providing a statement on the scope and characteristics of the cost-effectiveness literature. Similarly, Thompson et al. [20] focused on the methodological challenges of modeling the cost effectiveness of MS treatments. They identified the key parameters influencing the cost effectiveness of DMTs and described the approaches taken including the major areas of weakness and uncertainty. Guo et al. [21] limited the review of cost-effectiveness analyses to studies published over the last decade that had a long-term time horizon (≥ 10 years), and homogeneous contexts of analysis (i.e., similar study objectives, comparators, and target populations). A recent review by Allen et al. [22] focused on modeling methods and analyzed data sources, techniques, and assumptions of health economic models of DMTs for RRMS in the UK. Unlike the previous reviews discussed, Allen et al. included the website of the UK’s National Institute for Health and Care Excellence (NICE) and in doing so added to their analysis the health economic evidence available from the process of Health Technology Assessment (HTA) submissions. Overall, most of the recent systematic literature reviews of health economic evidence for DMTs in MS focused on aspects related to modeling techniques, while only Yamamoto and Campbell reviewed the quality of reporting in the analyzed studies [16]. Despite a lack of review of the quality of reporting in the field, several new biologic DMTs have been presented to the market in recent years, and the pharmacoeconomic value of these newer DMTs have been assessed in HTA submissions as well as in the general literature. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement has been developed and accepted by the scientific community and was produced by a dedicated task force on good reporting practice from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [23].
The objective of the present work was to conduct a systematic literature review of economic evidence from cost-effectiveness analyses of MS treatment to (i) summarize the evidence on cost effectiveness of the different treatments; (ii) provide an assessment of the quality of the studies (i.e., using the CHEERS checklist); and (iii) identify and discuss the main drivers of cost effectiveness.
2 Methods
2.1 Data Sources and Search Strategy
A systematic literature search of bibliographic biomedical and economic databases (MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials [CENTRAL], the Centre for Reviews and Dissemination [CRD] HTA database, the National Health System Economic Evaluations Database [NHS EED] and Econlit) was conducted on December 22, 2016. Results were limited to economic evaluations published after the year 2007, in order to limit the analysis to the most recent evidences developed in the past 10 years. The search strategy comprised both selected subject headings and keywords relating to MS and relevant treatments. In addition, a search for grey literature, including HTAs, was undertaken. For a full description of the search strategy and resources used please see the electronic supplementary materials. The search was conducted in accordance with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24].
2.2 Selection Criteria
To address the research question, criteria were developed to describe the relevant population, intervention, comparators, outcomes, and study design (PICOS). Publications identified in the search of the different databases were combined and duplicates were removed.
Population The population of interest was adults (aged ≥ 18 years) diagnosed with MS.
Interventions All the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) authorized DMTs, including IFNβ-1a, IFNβ-1b, pegylated interferon (pegIFN) β-1a, GA, mitoxantrone, teriflunomide, NTZ, dymethilfumarate (DMF), fingolimod hydrochloride (FGM), alemtuzumab, and daclizumab were included in the search.
Comparators No restriction by comparator was applied.
Outcomes of interest Comparative outcomes from economic analyses, such as incremental cost-effectiveness ratios (ICERs), net monetary benefits (NMBs), incremental benefits, and incremental costs were considered.
Study design of interest Fully published economic analyses that considered costs and benefits, while simultaneously comparing the results in more than two interventions, such as cost-effectiveness, cost-utility, and cost-benefit analyses were included.
The first step of the screening process involved study selection based on title/abstract, followed by full-text screening for articles that were not definitively categorized via title/abstract. Two independent reviewers (SI and CI) simultaneously screened all abstracts and articles for inclusion/exclusion. A third reviewer (LP) was available to resolve disagreements. No limitation by language was applied in the searches. However, studies in languages other than English were excluded during screening.
2.3 Data Extraction and Quality Assessment
A comprehensive data extraction form was created in Microsoft Excel and used to compile the data. The study characteristics included type of economic analysis, geographic region, evidence base (i.e., model or real-world evidence), type of model, time horizon, disease phase at study entry (i.e., RRMS, primary-progressive or secondary-progressive MS), treatment and doses, use of network meta-analysis (NMA) for effectiveness inputs, and base-case results. Data were extracted by one researcher (CI) and validated by a second researcher (SI). A third researcher (LP) performed a random control on 50% of the selected papers. For all studies not reporting values in US dollars ($US), both original and converted values (listed in square brackets if different from the original value) were reported in the text. The following conversion rates were used, as of May 2017: $US1 = $Can1.346 = €0.895 = £0.782 = Kr8.688.
The CHEERS statement was used to assess the reporting quality of published studies. Quality of reporting control was not applied to HTA reports. The CHEERS criteria were checked for reporting in each selected paper, and when positive, page numbers were noted.
3 Results
The search strategy provided a total of 1370 potentially relevant citations, of which 33 published articles [6, 7, 10, 11, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] and four HTA reports [8, 9, 54, 55] were included in the final analysis. A flow diagram of the search and selection strategy is shown in Fig. 1.
3.1 Modeling Approach
In the majority of cases (n = 33), the analysis was based on a health economic model. In three studies, the evidence base was mixed (i.e., a model and real-world data [30, 47, 51]), and in one case the study was completely based on observational data [45]. Among the model-based studies, ten analyses were simple decision trees that measured the economic value of DMTs in terms of cost per relapse avoided over a limited time horizon of between 2 and 4 years [25,26,27,28, 30, 32, 37, 48,49,50]. Twenty-four analyses employed a Markov technique, with a longer time horizon variable of between 5 years and lifetime, and two made use of more sophisticated dynamic modeling techniques [38, 47]. The ten studies based on a decision tree did not adjust effectiveness data to account for the heterogeneity of sources (naïve comparison), and as such sourced input data directly from published randomized clinical trials. The other 26 studies were based on more sophisticated modeling techniques (i.e., Markov or dynamic simulations). Of these, ten cases made use of published or unpublished mixed treatment comparisons (MTC) [6, 8,9,10, 31, 39, 40, 53,54,55], five cases were directly based on head-to-head trials [7, 33, 38, 51] or real-world efficacy data [47], and 11 cases used a naïve comparison [11, 29, 34,35,36, 41,42,43,44, 46, 52] (Table 1).
3.2 Economic Value of Disease-Modifying Therapies (DMTs)
Most of the studies were performed in North America (n = 19) or Europe (n = 15). The remaining three studies were performed in Iran [41, 45, 46]. The included studies used a cost-effectiveness or cost-utility analysis. Only one study added the cost-benefit technique to the standard cost effectiveness and thus reported results also in terms of net-monetary benefit [11]. About 62% of the studies (n = 23) performed the economic evaluation from a third-party payer cost perspective, while the remaining 14 also considered a societal perspective in their evaluation. All studies included in the review considered RRMS as the phase of the disease at study entry, with the only exception being the model developed by Noyes at al. [47], who considered both patients in RRMS and in secondary-progressive MS (SPMS). The cost-effective treatment was defined as the one with the lowest ICER, the dominant treatment, or the treatment with an ICER below a willingness-to-pay threshold cited by the authors. Given the lack of transferability of economic analysis results between different geographic locations [56], the results reported by the studies included in the review in the remainder of this section were grouped by country (Table 2).
3.2.1 Analysis in the Canadian Setting
Su et al. [10] performed a cost-utility analysis that compared DMF with GA and IFNβ-1a 44 μg from the Canadian healthcare system perspective, reporting an ICER of $Can44,118 [$US32,767]/quality-adjusted life-year(QALY) and of $Can10,672 [$US7926]/QALY, respectively. At a threshold range of $Can50,000–$Can60,000 [$US37,150–$US44,580], DMF was cost effective [10].
3.2.2 Analysis in the French Setting
Chevalier et al. [31] analyzed the cost effectiveness of DMF to other DMTs from both the perspective of the French healthcare system and of the broad society. From both perspectives, DMF and IFNβ-1a 44 μg were the two optimal treatments, as they dominated the other comparative treatments (IFNβ-1a 30 μg, IFNβ-1b 250 μg, teriflunomide, GA, and FGM) on the efficiency frontier. From the societal perspective, DMF versus IFNβ-1a 44 μg had an ICER of €13,110 [$US14,655]/QALY, while from the payer perspective the ICER was €29,047 [$US32,474]/QALY.
3.2.3 Analysis in the German Setting
Nuijten and Mittendorf compared DMTs in terms of cost per relapse avoided [48]. From the perspective of society, the ICER of each DMT compared with no active treatment was IFNβ-1a 30 μg €133,770 [$US149,583]/relapse avoided; GA €71,416 [$US79,858]/relapse avoided; IFNβ-1b 250 μg €54,475 [$US61,918]/relapse avoided; IFNβ-1a 44 μg €51,250 [$US57,280]/relapse avoided. From the perspective of the third-party payer, the ICER was IFNβ-1a 30 μg €140,728 [$US157,287]/relapse avoided; GA €73,385 [$US82,065]/relapse avoided; IFNβ-1b 250 μg €57,034 [$US63,791]/relapse avoided; IFNβ-1a 44 μg €54,244 [$US60,636]/relapse avoided. Under both perspectives, IFNβ-1a 44 μg was the cost-effective therapy [48].
3.2.4 Analyses in the Iranian Setting
Two studies compared DMTs with placebo (symptom management alone) in the perspectives of society [46] and of the third-party payer [41]. Nikfar et al. found that the ICER ranged from a minimum of $US904/QALY for IFNβ-1a 30 μg(biopharmaceutical copy) to a maximum of $US19,954/QALY for IFNβ-1a 30 μg, showing the convenience and cost effectiveness of biopharmaceutical copies [46]. However, Imani and Golestani reported an almost opposite result, with a minimum ICER of $US607,397/QALY for IFNβ-1a 30 μg and a maximum ICER of $US1,010,429/QALY for IFNβ-1a 30 μg(biopharmaceutical copy). In this study, IFNβ-1a 30 μg was found to be the cost-effective therapy [41]. A more recent Iranian analysis by Najafi et al. analyzed a head-to-head comparison between IFNβ-1a 30 μg and IFNβ-1a 30 μg(biopharmaceutical copy) and showed that, in the perspective of the third-party payer, the latter is dominant [45].
3.2.5 Analysis in the Scottish Setting
Hernandez et al. evaluated the cost effectiveness of pegIFNβ-1a when compared with IFNβ-1a, IFNβ-1b or GA in the perspective of the Scottish NHS [40]. According this analysis, pegIFNβ-1a was dominant over all the other IFNs, and had an ICER of £5773 [$US7382]/QALY versus GA [40].
3.2.6 Analysis in the Serbian Setting
Jankovic et al. compared the different IFNs and GA with placebo (symptom management) from the perspective of Serbian society [42]. The ICER for all DMTs considered exceeded a billion Serbian dinars (> $US90 million) per QALY gained, making each of the four immunomodulatory therapies not cost effective.
3.2.7 Analyses in the Spanish Setting
Two studies adopted the perspective of Spanish society and made a paired comparison of IFNs and GA [52] or compared them with best supportive care (symptomatic treatment and treatment of relapses) [34]. Sánchez-de la Rosa et al. found that none of the IFNs were cost effective when compared with GA as first-line therapy (ICERs ranging from €117,914 [$US131,827]/QALY of IFNβ-1a 30 μg vs GA to €618,146 [$US691,083]/QALY of IFNβ-1a 44 μg vs GA) [52]. In Dembek et al., none of the DMTs were shown to be cost effective; the best ICER (€168,629 [$US188,505]/QALY) was reported for IFNβ-1a 30 μg [34]. A third analysis by Darbà et al. considered the third-party payer perspective and based on the effectiveness data of the head-to-head CombiRX trial, showed that GA was dominant over IFNβ-1a 30 μg [33].
3.2.8 Analyses in the Swedish Setting
Two economic analyses studied DMTs for MS in the Swedish setting [43, 50]. The O’Day et al. analysis, based on a short-term decision tree, adopted the perspective of the Swedish healthcare system and showed that NTZ was cost effective when compared with FGM, with an ICER of Kr25,448 [$US2929]/relapse avoided. In the sub-population of patients with rapidly evolving, severe disease, NTZ dominated FGM [50]. The second analysis by Kobelt et al. was developed from the perspective of society and showed that NTZ was dominant over a ‘standard therapy’ defined as a mix of IFNs and GA [43].
3.2.9 Analyses in the UK Setting
Six studies from the UK setting were included in the systematic review, of which four were part of the NICE Technology Appraisal processes [8, 9, 54, 55] and two were journal publications [36, 44]. The four analyses that were part of a NICE process adopted the perspective of the UK NHS. The NICE analysis of NTZ was cost effective (i.e., with an ICER below the threshold of £30,000 [$US38,356]/QALY) versus IFNs and GA in the sub-group of patients with rapidly evolving severe disease, but not in the general population [54]. FGM was not cost effective and had an ICER of £55,634 [$US71,131]/QALY versus IFNβ-1a 30 μg [8]. DMF was not cost effective when compared with IFNs and GA (with ICERs above £100,000 [$US127,855]/QALY in all comparisons), but was dominant over NTZ [9]. Alemtuzumab strongly or extendedly dominated IFNs, NTZ, and FGM, and the ICER of alemtuzumab compared with GA was £8924 [$US11,408]/QALY [55]. Gani et al. adopted the perspective of UK society and showed that NTZ was cost effective, with ICERs of £2300 [$US2946]/QALY versus pooled IFN-β, £2000 [$US2562]/QALY versus GA, and £8200 [$US10,508]/QALY versus best supportive care [36]. Maruszczak et al. concluded that FGM was cost effective versus DMF in the perspective of the UK NHS and presented an ICER of £12,528 [$US16,014]/QALY [44].
3.2.10 Analysis in the US Setting
The majority of analyses were conducted in the US setting (18 studies). Among the studies that adopted the societal perspective (6 studies), a diverse set of conclusions were reported. Zhang et al. showed that DMF was the most cost-effective strategy among IFNβ-1a 30 μg, teriflunomide, and FGM [11]. Lee et al. found that FGM provided better outcomes at an increased cost when compared with IFNβ-1a 30 μg, with an ICER of $US73,975/QALY [7]. Pan et al. estimated the impact of early treatment with IFNβ-1b and concluded it was cost effective with an ICER of $US46,357/QALY vs placebo [51]. Earnshaw et al. found that GA and NTZ were dominant over symptom management alone [35]. Two analyses assessed IFNs and GA versus best supportive care with a different modeling approach and different input data, but came to the same conclusion that they were not cost effective (ICERs ranging from $US901,319/QALY to $US2,178,555/QALY in Noyes et al., [47] and above $US200,000/QALY in Bell et al. [29]).
The other 12 studies adopted the cost perspective of the third-party payer. Among these, two studies assessed the economic value of DMTs in terms of cost per QALY [39, 53]: Hernandez et al. concluded that pegIFNβ-1a is an efficient option, being dominant over IFNβ-1a 44 μg and GA [39], while Tappenden et al. concluded that the ICER of IFNs and GA as compared with best supportive care was expected to be in excess of $US100,000 per QALY gained [53].
The remaining ten studies estimated the cost-effectiveness ratio mainly as cost per relapse avoided, and again presented a diverse set of results. Agashivala and Kim showed that the cost per relapse avoided improved if FGM was initiated early rather than late ($US83,125 and $US103,624 per relapse avoided, respectively) [25]. Two studies (Brandes et al. [30] and Agashivala et al. [26]) compared FGM with IFNs and GA and concluded that the drug presents a favorable economic profile measured as cost per relapse avoided. Three analyses assessed NTZ, the first two (Bakhshai et al. [27] and Chiao and Meyer [32]) came to the same conclusion that the cost per relapse avoided was lowest for NTZ at $US56,594, followed by $US87,791 for IFNβ-1b, $US93,306 for IFNβ-1a 30 μg, $US96,178 for IFNβ-1a 44 μg, and $US103,665 for GA. O’Day et al. compared NTZ with FGM and found that the first was dominant in the incremental cost-effectiveness analysis, as it was less costly and more effective in reducing relapses [49]. Goldberg et al. [37] and Becker and Dembek [28] assessed the cost per relapse avoided ratio of IFNs and GA, using the same model but effectiveness data from a different selection of clinical trials, and reported similar results ($US88,310 and $US87,767 for GA, $US80,589 and $US80,121 for IFNβ-1a 44 μg, and $US87,061 and $US86,572 for IFNβ-1b 250 μg), with the exception of IFNβ-1a 30 μg ($US141,721 in the first study and $US77,980 in the second). Guo et al. [38] compared IFNβ-1a 44 μg with IFNβ-1a 30 μg sourcing most of the effectiveness data from the EVIDENCE head-to-head trial, and finding that the first IFNβ-1a 44 μg was more expensive but more effective in reducing relapses, with an ICER of $US10,755/relapses avoided. Bozkaya et al. compared the cost effectiveness of NTZ versus FGM, DMF versus GA, and pegIFNβ-1a versus IFNβ-1a 44 μg, showing that the first DMT in each comparison was dominant over the second DMT in each comparison in terms of cost per relapse avoided [6].
3.3 Funding Sources
Funding sources were categorized as from pharmaceutical companies (pharma), academic or governmental sources, or no external source of funding. Ten studies from the US, UK, Serbia, and Iran were funded from academic or governmental agencies. Three studies from the US received no external funding and the remaining 24 studies from Western Europe, the UK, and the USA identified pharma sources of funding. Overall, the source of funding did not impact the results of the cost-effective analyses and the drug of interest in most cases was determined to be cost effective.
3.4 Drivers of the Cost Effectiveness
An analysis was conducted to identify the most important drivers of the cost effectiveness in the studies included in the review. These were identified by analyzing the reported results and the parameters that most influenced the sensitivity analysis results of the different papers, following the discussion and the ranking of importance (i.e., tornado diagram) presented by the authors. Moreover, the discussion section was reviewed for reports of the main drivers of the cost effectiveness. The drivers were then grouped into ten categories, as reported in Table 3. Three studies did not report information on the drivers of the ICERs and were therefore not included in this analysis [33, 41, 50]. The driver that was most cited was ‘Effectiveness’ [23/37 papers, corresponding to 62% of the cases) followed by ‘Drug prices’ (n = 20, 54%), ‘QoL utilities’ (n = 9, 24%), ‘Tx duration’ and ‘Time horizon’ (n = 8, 22%), ‘Baseline characteristics’ (n = 7, 19%), ‘Long-term effect’ and ‘Direct costs’ (n = 4, 11%), ‘Social costs’ (n = 3, 8%), and ‘NAbs’ (n = 1, 3%).
3.5 Quality of Reporting
We assessed the reporting quality of 33 studies using the CHEERS statement (Table 4). In general, the level of compliance with reporting standards was high. All studies were identifiable as economic evaluations based on the title, presented a structured abstract, and an explicit statement of the broader context for the study. Studies generally reported the target population and subgroups well (n = 26 of 33, 79%). In most analyses with a time horizon longer than 2 years, a statement on the choice of discount rate was reported (n = 23 of 24, 96%). The description of the sources for effectiveness was generally sufficiently detailed (n = 30 of 33, 91%). The information on the population and methods used to elicit preferences for outcomes was available in almost all the cost-utility analyses (n = 16 of 18, 89%). The year of costing was available in all but five studies (n = 28 of 33, 85%). In almost all studies, conflicts of interests were reported (n = 30 of 33, 91%).
4 Discussion and Conclusions
We systematically reviewed the literature with the objective of assembling and analyzing recent published evidence on cost effectiveness of DMTs in MS patients. The strength of the systematic review is to provide a replicable overview of the state of research in a particular field and to enable an assessment of the quality of individual studies [57]. As such, the main focus of the systematic review was the assessment of the quality of the studies, using the CHEERS checklist, and on the identification of the main drivers for cost effectiveness. Among the 33 papers and four HTA reports that were included in the review, almost all were based on a health economic model, with only one study that was completely based on observational data. Modeling studies broadly belonged to two main groups: the first applied a simple approach to estimate a short-term measure of cost consequence (mostly cost per relapse avoided), while the second group relied on a more standard cost per QALY estimate over patients’ lifetime. The conclusions reported in the reviewed studies were difficult to classify and synthesize, for the most part due to the different geographic areas covered.
It is widely recognized that cost-effectiveness results are rarely transferable between different countries [56]. The 37 studies included for review were developed in 10 different countries, including seven European countries, Canada, the US, and Iran. However, about half of the studies were in the US setting. Even within the same country, the level of discrepancy between results was high. For example, in Iran, three separate analyses compared the same set of treatments and arrived at contradictory conclusions. It was thus not possible to summarize the results of all the analyses, providing a clear indication on what group of DMTs could be considered more cost effective in MS.
Although a summary of overall analysis was not possible due to the variance in study inputs, general trends of interest were identified. Overall, pegIFN and DMF were almost always cost effective in studies in which they were analyzed. With the exception of three US studies, oral treatments were generally cost effective when compared with injection treatments.
The studies’ category of funding (i.e., pharma, academic/governmental, and no funding) did not seem to have an impact on the results of the individual analyses and the drugs of interest were mostly determined to be cost effective.
More informative was the analysis of the drivers of the cost effectiveness. Not surprisingly, the most cited driver was the effectiveness of the treatment, encompassing all parameters related to the effectiveness of the study drug such as the annualized relapse rate and the disability progression hazard ratio. The second parameter that was cited by more than 50% as a key driver of the cost effectiveness was the price of the drugs involved. Efficacy is always of utmost importance in patient care; however, it is not surprising that the second most cited driver was the drug cost. Drug acquisition costs vary greatly between countries. For instance, in Serbia, no DMTs were found to be cost effective [42]. Noyes et al. found that DMTs represent a considerable fraction of health-related costs for patients with MS in the US [47]. While there is no uniform threshold in the US, some studies found the estimated ICER(s) higher than many accepted cost-effectiveness thresholds for chronic diseases, but comparable to some other immunomodulatory therapies [29, 47]. The variation in ICERs for DMTs indicates the field would benefit from further analyses of drivers both within countries and internationally.
An important consideration was the analysis of the quality of reporting in the primary studies. Unlike clinical studies that report only the efficacy and safety of an intervention, economic evaluations require many additional inputs including resource use, direct and indirect costs, treatment preference information, and treatment effectiveness. The magnitude and complexity of model inputs create a challenge for journal editors, peer reviewers, and anyone who wishes to evaluate the findings of a cost-effectiveness study. As such, the quality of reporting of economic evaluations has been shown to vary [58]. Transparency of reporting on inputs and model structure is of utmost importance with health economic evaluations, as there are significant implications from policy decisions which may be based on misleading study findings. As the number of published economic evaluations continues to increase, the lack of systemic accountability makes it especially important to assess the quality of reporting in a systematic fashion [23]. The CHEERS statement checklist developed by the ISPOR Health Economic Evaluation Publication Guidelines Task Force provides recommendations in a checklist to evaluate reporting in health economic evaluations [23]. The included studies were checked against the CHEERS statement. In general, the level of compliance with reporting standards was very high, with only a limited amount of the prescribed information missing. We believe this could be to the result of a general improvement in the quality of reporting in health economics papers seen in the recent years.
In an effort to capture the maximum available HTA (unpublished) results, the systematic literature review (SLR) included HTA reports for all agencies which disclosed the full data set for each input that was considered and details on the methods and analyses that were performed. This limited the inclusion of reports from agencies/countries that only publish the results of the cost-effectiveness analysis and at the end only the reports from the UK’s NICE met the criteria and were included. This, however, represents a limit to our analysis. In addition to HTA reports, the SLR was limited to full, published journal articles in English and excluded abstracts or other unpublished results.
In conclusion, recent years have seen the publication of a large number of health economic assessments of DMTs in RRMS in several different countries. While the methods employed were not dissimilar and the quality of the reporting was generally high, the conclusions reached were not homogeneous and in some cases even conflicting. These discrepancies were likely due to different input data or to non-homogeneous assumptions. This study provides a valuable analysis of the primary literature to identify and review the main drivers, recognize trends in cost effectiveness of DMTs, and a systematic evaluation of the quality of primary studies identified in the review. As such, the paper provides an important essential base for policymakers and stakeholders when considering the quality of results. As well, it provides a strong repository of information on the main drivers and trends in cost effectiveness of DMTs in RRMS that can be drawn on for comparison or to fuel in-depth research with a narrower scope to answer questions on the effect of driver variance.
Data Availability Statement
The evidence abstraction database synthesizing the information from the papers included in the review is made available as electronic supplementary material.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Kieseier BC, Jeffery DR. Chemotherapeutics in the treatment of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(5):277–91.
Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–94.
Multiple Sclerosis International Federation. Atlas of MS. 2013. http://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf.
Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl 1):S5–48.
Bozkaya D, Livingston T, Migliaccio-Walle K, Odom T. The cost-effectiveness of disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis. J Med Econ. 2017;20(3):297–302.
Lee S, Baxter DC, Limone B, Roberts MS, Coleman CI. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ. 2012;15(6):1088–96.
National Institute for Health and Care Excellence. NICE TA254. Fingolimod for the treatment of highly active relapsing–remitting multiple sclerosis. 2012. https://www.nice.org.uk/guidance/ta254.
National Institute for Health and Care Excellence. NICE TA320. Dimethyl fumarate for treating relapsing–remitting multiple sclerosis. 2014. https://www.nice.org.uk/guidance/ta320.
Su W, Kansal A, Vicente C, Deniz B, Sarda S. The cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis in Canada. J Med Econ. 2016;19(7):718–27.
Zhang X, Hay JW, Niu X. Cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate and intramuscular interferon-beta1a in relapsing-remitting multiple sclerosis. CNS Drugs. 2015;29(1):71–81.
Noyes K, Weinstock-Guttman B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am J Manag Care. 2013;19(17 Suppl):s321–31.
Hartung DM. Economics and cost-effectiveness of multiple sclerosis therapies in the USA. Neurotherapeutics. 2017;1–9. doi:10.1007/s13311-017-0566-3.
Raftery J. Multiple sclerosis risk sharing scheme: a costly failure. BMJ. 2010;340:c1672.
Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81.
Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune Dis. 2012;2012:784364.
Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41(1):32–44.
Koeser L, McCrone P. Cost-effectiveness of natalizumab in multiple sclerosis: an updated systematic review. Expert Rev Pharmacoecon Outcomes Res. 2013;13(2):171–82.
Hawton A, Shearer J, Goodwin E, Green C. Squinting through layers of fog: assessing the cost effectiveness of treatments for multiple sclerosis. Appl Health Econ Health Policy. 2013;11(4):331–41.
Thompson JP, Abdolahi A, Noyes K. Modelling the cost effectiveness of disease-modifying treatments for multiple sclerosis: issues to consider. Pharmacoeconomics. 2013;31(6):455–69.
Guo S, Pelligra C, Saint-Laurent Thibault C, Hernandez L, Kansal A. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics. 2014;32(6):559–72.
Allen F, Montgomery S, Maruszczak M, Kusel J, Adlard N. Convergence yet continued complexity: a systematic review and critique of health economic models of relapsing-remitting multiple sclerosis in the United Kingdom. Value Health. 2015;18(6):925–38.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Agashivala N, Kim E. Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clin Ther. 2012;34(7):1583–90.
Agashivala NV, Dastani HB, Carlton R, Sarnes E. Cost-effectiveness of fingolimod in treating patients with relapsing-remitting multiple sclerosis. Am J Pharm Benefits. 2011;3(6):320–8.
Bakhshai J, Bleu-Laine R, Jung M, Lim J, Reyes C, Sun L, et al. The cost effectiveness and budget impact of natalizumab for formulary inclusion. J Med Econ. 2010;13(1):63–9.
Becker RV 3rd, Dembek C. Effects of cohort selection on the results of cost-effectiveness analysis of disease-modifying drugs for relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2011;17(5):377–81.
Bell C, Graham J, Earnshaw S, Oleen-Burkey M, Castelli-Haley J, Johnson K. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13(3):245–61.
Brandes DW, Raimundo K, Agashivala N, Kim E. Implications of real-world adherence on cost-effectiveness analysis in multiple sclerosis. J Med Econ. 2013;16(4):547–51.
Chevalier J, Chamoux C, Hammes F, Chicoye A. Cost-effectiveness of treatments for relapsing remitting multiple sclerosis: a french societal perspective. PLoS One. 2016;11(3):e0150703.
Chiao E, Meyer K. Cost effectiveness and budget impact of natalizumab in patients with relapsing multiple sclerosis. Curr Med Res Opin. 2009;25(6):1445–54.
Darbà J, Kaskens L, Sanchez-de la Rosa R. Cost-effectiveness of glatiramer acetate and interferon beta-1a for relapsing-remitting multiple sclerosis, based on the CombiRx study. J Med Econ. 2014;17(3):215–22.
Dembek C, White LA, Quach J, Szkurhan A, Rashid N, Blasco MR. Cost-effectiveness of injectable disease-modifying therapies for the treatment of relapsing forms of multiple sclerosis in Spain. Eur J Health Econ. 2014;15(4):353–62.
Earnshaw SR, Graham J, Oleen-Burkey M, Castelli-Haley J, Johnson K. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy. 2009;7(2):91–108.
Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617–27.
Goldberg LD, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15(7):543–55.
Guo S, Bozkaya D, Ward A, O’Brien JA, Ishak K, Bennett R, et al. Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a: modelling the clinical and economic implications. Pharmacoeconomics. 2009;27(1):39–53.
Hernandez L, Guo S, Kinter E, Fay M. Cost-effectiveness analysis of peginterferon beta-1a compared with interferon beta-1a and glatiramer acetate in the treatment of relapsing-remitting multiple sclerosis in the United States. J Med Econ. 2016;19(7):684–95.
Hernandez L, Guo S, Toro-Diaz H, Carroll S, Syed Farooq SF. Peginterferon beta-1a versus other self-injectable disease-modifying therapies in the treatment of relapsing-remitting multiple sclerosis in Scotland: a cost-effectiveness analysis. J Med Econ. 2017;20(3):228–38.
Imani A, Golestani M. Cost-utility analysis of disease-modifying drugs in relapsing-remitting multiple sclerosis in Iran. Iran J Neurol. 2012;11(3):87–90.
Jankovic SM, Kostic M, Radosavljevic M, Tesic D, Stefanovic-Stoimenov N, Stevanovic I, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on data a Balkan country in socioeconomic transition. Vojnosanit Pregl. 2009;66(7):556–62.
Kobelt G, Berg J, Lindgren P, Jonsson B, Stawiarz L, Hillert J. Modeling the cost-effectiveness of a new treatment for MS (natalizumab) compared with current standard practice in Sweden. Mult Scler. 2008;14(5):679–90.
Maruszczak MJ, Montgomery SM, Griffiths MJ, Bergvall N, Adlard N. Cost-utility of fingolimod compared with dimethyl fumarate in highly active relapsing-remitting multiple sclerosis (RRMS) in England. J Med Econ. 2015;18(11):874–85.
Najafi B, Ghaderi H, Jafari M, Najafi S, Ahmad Kiadaliri A. Cost effectiveness analysis of avonex and cinnovex in relapsing remitting MS. Glob J Health Sci. 2014;7(2):139–47.
Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. Daru. 2013;21(1):50.
Noyes K, Bajorska A, Chappel A, Schwid SR, Mehta LR, Weinstock-Guttman B, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011;77(4):355–63.
Nuijten M, Mittendorf T. A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German societal perspective. Clin Ther. 2010;32(4):717–28.
O’Day K, Meyer K, Miller RM, Agarwal S, Franklin M. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14(5):617–27.
O’Day K, Meyer K, Stafkey-Mailey D, Watson C. Cost-effectiveness of natalizumab vs fingolimod for the treatment of relapsing-remitting multiple sclerosis: analyses in Sweden. J Med Econ. 2015;18(4):295–302.
Pan F, Goh JW, Cutter G, Su W, Pleimes D, Wang C. Long-term cost-effectiveness model of interferon beta-1b in the early treatment of multiple sclerosis in the United States. Clin Ther. 2012;34(9):1966–76.
Sánchez-de la Rosa R, Sabater E, Casado MA, Arroyo R. Cost-effectiveness analysis of disease modifiying drugs (interferons and glatiramer acetate) as first line treatments in remitting-relapsing multiple sclerosis patients. J Med Econ. 2012;15(3):424–33.
Tappenden P, McCabe C, Chilcott J, Simpson E, Nixon R, Madan J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health. 2009;12(5):657–65.
National Institute for Health and Care Excellence. NICE TA127. Natalizumab for the treatment of adults with highly active relapsing–remitting multiple sclerosis. 2007. https://www.nice.org.uk/Guidance/TA127.
National Institute for Health and Care Excellence. NICE TA312. Alemtuzumab for treating relapsing-remitting multiple sclerosis. 2014. https://www.nice.org.uk/guidance/ta312.
Barbieri M, Drummond M, Willke R, Chancellor J, Jolain B, Towse A. Variability of cost-effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability. Value Health. 2005;8(1):10–23.
Ressing M, Blettner M, Klug SJ. Systematic literature reviews and meta-analyses: part 6 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(27):456–63.
Rennie D, Luft HS. Pharmacoeconomic analyses: making them transparent, making them credible. JAMA. 2000;283(16):2158–60.
Acknowledgements
The authors thanks Dagmara Chojecki for the support in defining and executing the search strategy and Nicole Tunstall for the assistance in the copy-editing and revision of the manuscript.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
Corresponding author
Ethics declarations
The study was free from financial support of external sources. S. Iannazzo received consulting fees from Biogen, not related to the present study. L. Perrault and A.C. Iliza were free from conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iannazzo, S., Iliza, AC. & Perrault, L. Disease-Modifying Therapies for Multiple Sclerosis: A Systematic Literature Review of Cost-Effectiveness Studies. PharmacoEconomics 36, 189–204 (2018). https://doi.org/10.1007/s40273-017-0577-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0577-2