Abstract
Objective
The aim of the study was to compare the cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate, and intramuscular (IM) interferon (IFN)-β1a as first-line therapies in the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).
Methods
A Markov model was developed to evaluate the cost effectiveness of disease-modifying drugs (DMDs) from a US societal perspective. The time horizon in the base case was 5 years. The primary outcome was incremental net monetary benefit (INMB), and the secondary outcome was incremental cost-effectiveness ratio (ICER). The base case INMB willingness-to-pay (WTP) threshold was assumed to be US$150,000 per quality-adjusted life year (QALY), and the costs were in 2012 US dollars. One-way sensitivity analyses and probabilistic sensitivity analysis were conducted to test the robustness of the model results.
Results
Dimethyl fumarate dominated all other therapies over the range of WTPs, from US$0 to US$180,000. Compared with IM IFN-β1a, at a WTP of US$150,000, INMBs were estimated at US$36,567, US$49,780, and US$80,611 for fingolimod, teriflunomide, and dimethyl fumarate, respectively. The ICER of fingolimod versus teriflunomide was US$3,201,672. One-way sensitivity analyses demonstrated the model results were sensitive to the acquisition costs of DMDs and the time horizon, but in most scenarios, cost-effectiveness rankings remained stable. Probabilistic sensitivity analysis showed that for more than 90 % of the simulations, dimethyl fumarate was the optimal therapy across all WTP values.
Conclusion
The three oral therapies were favored in the cost-effectiveness analysis. Of the four DMDs, dimethyl fumarate was a dominant therapy to manage RRMS. Apart from dimethyl fumarate, teriflunomide was the most cost-effective therapy compared with IM IFN-β1a, with an ICER of US$7,115.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first cost-effectiveness analysis in relapsing-remitting multiple sclerosis to (1) make comprehensive comparisons between the three new oral disease-modifying drugs and the established therapy intramuscular (IM) interferon (IFN)-β1a, (2) incorporate second-line therapy in the model, and (3) present results in terms of incremental net monetary benefit (INMB) |
Dimethyl fumarate dominated all other therapies over the range of willingness-to-pay (WTP) values, from US$0 to US$180,000. Compared with IM IFN-β1a, at a WTP of US$150,000, INMBs were estimated at US$36,567, US$49,780, and US$80,611 for fingolimod, teriflunomide, and dimethyl fumarate, respectively. The three oral therapies were favored in the cost-effectiveness analysis |
After dimethyl fumarate, teriflunomide was the most cost-effective therapy compared with IM IFN-β1a, with an incremental cost-effectiveness ratio of US$7,115. When the monthly cost is below US$5,132, fingolimod is cost effective compared with IM IFN-β1a. However, fingolimod is not cost effective compared with teriflunomide |
1 Introduction
Before the introduction of oral fingolimod (Gilenya™, Novartis, East Hanover, NJ, USA), over half of the patients with relapsing-remitting multiple sclerosis (RRMS) who were treated with disease-modifying drugs (DMDs) were using injected interferons (IFNs) [1], and intramuscular (IM) IFN-β1a (Avonex®, Biogen Idec, Weston, MA, USA) had the largest market share in 2010 [2]. However, IM IFN-β1a and other traditional DMDs require long-term parenteral administration, which imposes a burden on patients and may have a significant impact on medication adherence. Over the past few years, three new oral DMDs, namely fingolimod, teriflunomide (Aubagio®, Sanofi Aventis, Cambridge, MA, USA), and dimethyl fumarate (Tecfidera®, Biogen Idec, Weston, MA, USA), were approved by the FDA in 2010, 2012, and 2013, respectively. Fingolimod was the first oral therapy approved, and the Trial Assessing Injectable Interferon versus FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS) showed that fingolimod appeared to be more effective than IM IFN-β1a in reducing the frequency of relapses [3]. The large-scale phase III clinical trials the Teriflunomide Multiple Sclerosis Oral (TEMSO) trial and the Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS (DEFINE) trial also demonstrated that teriflunomide and dimethyl fumarate, respectively, significantly reduced annualized relapse rates, slowed disability progression, and reduced the number of lesions on magnetic resonance imaging [4, 5]. Although these new oral therapies were thought to contribute to the growth of the total costs of multiple sclerosis (MS), so far there is no comprehensive evidence on either the cost effectiveness of the new oral DMDs compared with the established treatment IM IFN-β1a, or incremental cost effectiveness among the oral therapies. For these reasons, this paper compares the cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate, and IM IFN-β1a as first-line therapies in the treatment of patients diagnosed with RRMS.
2 Materials and Methods
2.1 Model Overview
The cost-effectiveness analysis was conducted from a US societal perspective over a 5-year time horizon. We chose 5-year rather than 10-year or life time as the time horizon because (1) extrapolating a 1- or 2-year randomized controlled trial (RCT) over long time horizons requires more unreliable assumptions on model extrapolations [6] and (2) high discontinuation rates imply that a large proportion of patients will discontinue or develop secondary-progressive multiple sclerosis (SPMS) over time [3–5, 7]. Costs were reported in 2012 US dollars, and both costs and outcomes were discounted at a 3 % annual rate in the base case scenario. The primary outcome was incremental net monetary benefit (INMB), and the secondary outcome was incremental cost-effectiveness ratios (ICERs). INMB was chosen as the primary outcome since, when comparing multiple treatment options, it more clearly delineates treatments with dominance or extended dominance [8, 9]. The willingness-to-pay (WTP) threshold was assumed to be US$150,000 per quality-adjusted life year (QALY), which is three times the 2012 US gross domestic product (GDP) per capita, as recommended by the World Health Organization [10, 11]. The choice of US$150,000 as the WTP threshold rather than the antiquated US$50,000 value in the US context is also supported by the study of Braithwaite et al. [12] and is used in numerous previous studies [13–16].
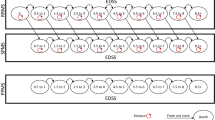
A Markov model was developed in Microsoft® Excel to simulate the disease progression of patients with RRMS (Fig. 1). The cycle is 1 month. The comparators included oral fingolimod at a daily dose of 0.5 mg, oral teriflunomide 14 mg once daily, oral dimethyl fumarate 120 mg twice a day for the first 7 days and 240 mg twice a day after 7 days, and IM IFN-β1a at a weekly dose of 30 μg [17]. The disease progression was modeled by the Expanded Disability Status Scale (EDSS), which is most widely used in the measurement of MS [18]. Specifically, health states were divided as EDSS 0.0–2.5 (no or mild disability), EDSS 3.0–5.5 (moderate disability, ambulatory without aid), EDSS 6.0–7.5 (waking aid required), EDSS 8.0–9.5 (restricted to bed), EDSS 10.0 (death) and another two relapse states. Since MS is a progressive disease, patients were assumed to only progress to a more severe health state or a relapse state.
A cohort of 1,000 patients was assumed to enter the model. Consistent with the clinical trials, all patients were initially distributed to EDSS 0.0–2.5 and 3.0–5.5 states and treated with first-line DMDs [3–5]. The EDSS distribution ratio between the two states was estimated from a national cross-sectional survey [19]. In any cycle during the simulation, patients could discontinue the drug and then transition to a second-line treatment, natalizumab, or to the symptom management (SM) arm without active drug therapy. Moreover, patients could also discontinue natalizumab due to insufficient response or adverse events and then switch to SM treatment.
The decision to choose natalizumab as the second-line therapy was based on the fact that (1) natalizumab was specifically indicated for use when previous DMDs failed, as recommended by American Academy of Neurology [20]; (2) a retrospective cohort study found that approximately 10 % of patients who were initially treated with IFN-β or glatiramer acetate (GA) experienced breakthrough disease and either switched to natalizumab or an immunosuppressant (e.g., mitoxantrone) or declined new therapy [21] (however, according to another study, which followed a cohort from 2000 to 2008, only 1 % of the first-line and second-line DMD populations used mitoxantrone [22]); and (3) other first-line drugs are often used as second-line therapies, despite not being indicated after failure of a previous DMD, and they are actually similar in efficacy; however, there is evidence that switching to natalizumab is more effective than switching to other first-line drugs [23]. Therefore, patients were assumed to receive natalizumab as second-line therapy.
Patients in EDSS 0.0–2.5 and 3.0–5.5 states would likely transition to a temporary state of relapse and stay for a cycle (1 month). Following a relapse, patients could transition back to the previous state or progress to a next more severe health state. According to a recent natural history study of SPMS, for patients initially diagnosed with RRMS, 80.0 % reached SPMS at or before EDSS 6.0 and 99.5 % at or before EDSS 8.0 [24]. That is to say, for those transitioned to EDSS 6.0, at least 80 % of the patients would have already reached SPMS, and so would almost all of the patients who progressed to EDSS 8.0. Therefore, it was assumed that patients in EDSS 6.0–7.5 and EDSS 8.0–9.5 had developed SPMS and thus were not associated with further relapses. Since these DMDs are indicated for relapse forms of MS, patients transitioned to EDSS 6.0–7.5 and EDSS 8.0–9.5 would stop DMD treatment and then be treated with SM.
The model design in this paper was consistent with previous cost-effectiveness studies comparing DMDs in that the same health states were classified and the same disease progression path was defined [2, 25–27]. The health states were decided in a way that the transition points (EDSS 3.0, 6.0, 8.0, and 10) reflected key disability levels in the natural history of MS and are critical in defining clinical course [7, 28–30]. In our model, we also allowed the patients to switch to second-line DMD treatment when they discontinued the first-line therapy, to better reflect clinical practice [20, 31]. In addition, we had each author verify the model equations and computations independently to ensure the internal validity [32].
2.2 Patient Characteristics
The baseline characteristics of the modeled patients were very similar in the phase III clinical trials across the four DMDs (Table 1) [3–5, 33]. Generally, patients were between 18 and 55 years old, had a diagnosis of RRMS, had had at least two relapses during the previous 2 years or at least one relapse during the previous year before randomization, and had an EDSS score of 0–5.5. Based on a national survey study, the initial proportions of patients distributed in EDSS 0.0–2.5 and EDSS 3.0–5.5 were estimated at 41.3 and 58.7 %, respectively [19].
2.3 Transition Probabilities
Transition probabilities for disease progression, relapses, and discontinuation were obtained from the literature and modeled using the DEALE method (Table 2) [3–5, 33–36]. For patients in SM, the EDSS progression probabilities were estimated from the London Ontario natural history study of MS [7]. The London Ontario data were used because, unlike in other studies, the patients in the study did not receive disease-modifying therapies and the database was subjected to a rigorous quality review in 2009 [37]. There were 806 RRMS-onset patients in the database, and the shortest follow-up was 16 years. Since the patients were similar in demographics and clinical characteristics, for patients treated with fingolimod, teriflunomide, dimethyl fumarate, and natalizumab, the hazard ratios of disease progression for DMDs compared with placebo reported in phase III placebo-controlled trials were used to derive the 1-month transition probabilities for each DMD. For the IFN-β1a arm, the hazard ratio from the head-to-head trial TRANSFORMS between fingolimod and IFN-β1a was also used to estimate transition probabilities [3].
The transition probabilities of relapses for patients in SM were obtained from the placebo group in the FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) trial [33]. Hazard ratios of relapses between DMDs (teriflunomide, dimethyl fumarate, and natalizumab) and placebo were used to derive the transition probabilities to relapse state for the DMDs. For patients treated with fingolimod and IFN-β1a, relapse probabilities were estimated by using the data in the TRANSFORMS trial [3]. All discontinuation rates were extracted from the corresponding phase III clinical trials. After discontinuation of the first-line therapy, the assignment ratio between natalizumab and SM was assumed to be equal in the base case scenario, and extreme cases were tested in sensitivity analyses. Since the mortality rate due to MS is very low, survival probabilities were based on the mortality rates of the general population [38]. The age-specific mortality rates were estimated from the life expectancy data in national vital statistics reports using the DEALE method [35, 36, 39].
2.4 Utilities
Since utilities were not available in the pivotal RCTs, we reviewed the literature and identified the best available evidence to support the utility estimates. The utilities for each health state from EDSS 0.0 to EDSS 9.5 and the disutility for IFN-β1a were obtained from the Prosser et al. [40] quality-of-life study. The study used the standard-gamble method to measure patient and community preferences for treatments and health states in patients with RRMS. The Prosser et al. [41] study was used because standard-gamble was thought to be the gold standard in preference elicitation since it is the only method that estimates Von-Neumann–Morgenstern utility (preference measured under uncertainty) [41]. Also, since this study was performed from a societal perspective, use of community preferences was more appropriate as it reflected the society’s preference on the resource allocation [42]. Disutility for relapses was based on the Kobelt et al. study [19]. For the effects of fingolimod and natalizumab, though there was evidence that fingolimod and natalizumab could improve the quality of life of MS patients significantly [43–45], no study on utility impacts was available. Therefore, to be conservative, the disutility for fingolimod and natalizumab was assumed to be 0 in the base case scenario. Changes in assumed base case utility were explored in sensitivity analyses. For teriflunomide, one study has demonstrated that there was no disutility associated with administration of teriflunomide, so the impact of teriflunomide on utility was assumed to be 0 in the base case analysis [46]. Dimethyl fumarate has been reported to have significant improvements in physical and mental aspects of health and functioning, where the change in EQ-5D value was 0.01 [47, 48]. The base case utilities and the effects of DMDs on utilities are shown in Table 2.
2.5 Costs
Costs in the model were mainly obtained from the cost-of-illness study by Kobelt et al. [19] and inflated to 2012 dollars (Table 2). We applied the results from the Kobelt et al. [19] study because the costs were reported on the basis of stratified EDSS score, which corresponded to each health state in our model. The costs included costs of hospital inpatient care, ambulatory care, tests, drugs (DMDs and other drugs), services, adaptations and costs of informal care. The productivity losses were not included, because the costs associated with productivity were captured in the QALYs [49]. All drug costs were obtained from the Federal Supply Schedule drug prices [50]. Costs of relapses were estimated from a cross-sectional, web-based survey that investigated the impacts of relapses on costs and quality of life for patients with RRMS in the USA [51].
2.6 Sensitivity Analysis
One-way sensitivity analyses were conducted to test the robustness of the pairwise comparisons of three oral therapies versus IM IFN-β1a and the robustness of the optimal therapy selection by using INMB as the outcome. The base case inputs of the parameters were varied by 25 % in both positive and negative directions, unless indicated otherwise (Table 2). We varied the parameters by 25 % so that the upper and lower bound of the sensitivity analysis range differed markedly from the base case inputs. The 25 % variation ranges of the parameters were also comparable to their corresponding 95 % confidence intervals where available. Key parameters that may affect the disease progression, utilities and costs were included in the analysis. In addition, a threshold analysis was conducted if a parameter variation resulted in a major change in conclusion [49]. Tornado diagrams were plotted in the order from most sensitive parameter to least sensitive. Moreover, sensitivity to time horizon was specifically tested by varying the time horizon from 2 to 30 years under both discounted and non-discounted scenarios.
The robustness of the base case results was also tested by probabilistic sensitivity analysis based on a second order Monte Carlo simulation (1,000 times). Choice of the distribution for the model inputs was based on the recommendations and reflected how the confidence interval of each parameter was estimated [52]. The distributions of hazard ratios and annual relapse rates were assumed to be log-normal [53]. Utilities were assumed to follow a beta distribution which is confined between 0 and 1 [53]. Health care costs for each health state and drug acquisition costs were assumed to follow a gamma distribution [53]. The result of the probabilistic sensitivity analysis was reported as the probability of each drug maximizing the net monetary benefits (NMBs) over the range of WTPs [54]. That is the probability of each drug being the optimal therapy.
3 Results
3.1 Base-Case Scenario
Over 5 years, the total costs per patient were estimated at US$223,606, US$239,947, US$226,085, and US$200,145 for IM IFN-β1a, fingolimod, teriflunomide, and dimethyl fumarate, respectively (Table 3). The accumulated QALYs were 3.34, 3.69, 3.68, and 3.72 for IM IFN-β1a, fingolimod, teriflunomide, and dimethyl fumarate, respectively. Assuming a WTP at US$150,000, the NMBs were at US$276,745, US$313,312, US$326,525, and US$357,356 for each of the DMDs above. Having the lowest costs and highest QALYs, dimethyl fumarate dominated all other drugs.
Compared with IM IFN-β1a, the INMBs were US$36,567, US$49,780, and US$80,611 for fingolimod, teriflunomide, and dimethyl fumarate, respectively (Table 3). The NMBs of the four DMDs over a range of WTPs are shown in Fig. 2. As long as the WTP was greater than US$100,000, NMBs of all the drugs would be greater than zero. Dimethyl fumarate dominated all other drugs across the range of WTPs.
The ICERs for fingolimod and teriflunomide compared with IM IFN-β1a were US$46,328 and US$7,115, respectively, so both fingolimod and teriflunomide are cost effective compared with IM IFN-β1a. In addition, the ICER of fingolimod compared against teriflunomide was US$3,201,672 (Table 3). Therefore, apart from dimethyl fumarate, teriflunomide was the most cost effective therapy at a WTP threshold of US$150,000.
3.2 One-Way Sensitivity Analysis
The ten most sensitive parameters are shown in Fig. 3. Generally, the results for base case analysis were stable to the change in most parameters. For the comparison between fingolimod and IM IFN-β1a (Fig. 3a), the monthly costs for fingolimod and IM IFN-β1a were the most sensitive parameters. When the monthly cost for fingolimod exceeded US$5,132, fingolimod was no longer cost effective. A decrease in the cost of IM IFN-β1a substantially reduced the INMB, but fingolimod still remained cost effective. For the sensitivity analysis of teriflunomide and dimethyl fumarate compared with IM IFN-β1a (Fig. 3b, c), the INMBs were consistently greater than 0. For all of the three oral therapies versus IM IFN-β1a, INMB increased as the time horizon became longer under both discounted and non-discounted cases (Fig. 4), indicating oral therapies are associated with greater benefits for long-term care.
For the sensitivity analysis of therapy selection, dimethyl fumarate remained the optimal therapy in almost all of the cases. The result was only sensitive to the monthly cost for teriflunomide. If the monthly cost for teriflunomide was lower than US$2,908, teriflunomide would be the most cost-effective therapy.
3.3 Probabilistic Sensitivity Analysis
Figure 5 shows the probability that each drug maximized the NMBs. Over the range of WTPs, the probability that dimethyl fumarate was the highest value therapy always exceeded 90 %. As a result, both teriflunomide and fingolimod had a less than 10 % chance of having the highest value. However, the probability for teriflunomide being preferred was constantly higher than that for fingolimod. Finally, IM IFN-β1a had a negligible probability of being the highest value treatment.
4 Discussion
This paper evaluated the cost effectiveness of three oral therapies, fingolimod, teriflunomide, and dimethyl fumarate, compared with IM IFN-β1a as first-line therapies in the treatment of RRMS patients. A Markov model based on EDSS disability level was developed to simulate disease progression over a 5-year time horizon.
To our knowledge, this is the first paper to compare the cost effectiveness of the new oral DMDs comprehensively and incorporate second-line therapy in the model. Model results favored oral therapies in economic and health benefits compared with IM IFN-β1a. Over a range of time horizons and WTPs, dimethyl fumarate was always the dominant strategy because of high QALY gained and low total costs. Leaving aside the dominance of dimethyl fumarate, given a WTP threshold of US$150,000, fingolimod and teriflunomide were cost effective compared with IM IFN-β1a, with ICERs of US$46,328 and US$7,115, respectively. However, fingolimod was not cost effective compared with teriflunomide, and thus teriflunomide was the most cost-effective therapy after dimethyl fumarate. One-way sensitivity analyses indicated that model results were most sensitive to acquisition costs for DMDs and time horizon, yet the results were rarely reversed and the decision-making rankings based on the results were robust in one-way sensitivity analyses. Probabilistic sensitivity analysis also showed dimethyl fumarate was the optimal therapy most of the time, whereas teriflunomide was the second best choice.
In prior studies, fingolimod was the only oral therapy that was evaluated using cost-effectiveness analysis. Lee et al. [2] compared fingolimod with IM IFN-β1a by building a Markov model on a 10-year time horizon. They estimated the ICER for fingolimod at US$73,975 compared with US$46,328 in this paper. However, given the annual discontinuation rate of about 10 %, a 10-year time horizon would imply that essentially all patients would have discontinued by the end of the simulation. Moreover, since Lee et al. [2] ignored second-line treatments, their results may not be realistic. O’Day et al. [55] compared fingolimod with natalizumab in the treatment of RRMS, with incremental cost per relapse avoided as the outcome. The study found that natalizumab dominated fingolimod since it was less costly and more effective in reducing relapses. Natalizumab was not included as a comparator in this study, because it is recommended for use after alternative therapies failed, while fingolimod has been approved as a first-line therapy.
This study is subject to some limitations. First, all data on the effects of the DMDs, such as hazard ratio of disease progression, annual relapse rates, and discontinuation rates, were taken from the corresponding phase III RCTs. Though RCTs are thought to have strong internal validity, their external validity is often limited. Also, hazard ratios extracted from RCTs that do not compare all of the active comparators are not an ideal data. However, since the oral DMDs have been available in the US market for about 4 years, there is a limited number of RCTs and no high-quality meta-analyses are available. Therefore, the large-scale phase III pivotal clinical trials with each drug compared with placebo constitute the best available evidence we could have at this point. Finally, as mentioned above, given the clinical trial follow-up periods of 1–2 years, extrapolating the time horizon to 5 years required modeling assumptions. For example, we assumed that the drug treatment effects would persist over a 5-year time horizon. However, our model sensitivity analysis demonstrated that the results were stable at a 2-year time horizon.
Second, the disease progression parameters for the SM were estimated from the London Ontario natural history study data, which were collected decades ago [7]. The disease progression of patients today may be different from the progression rate when the data were collected. Also, the data collected in Canada may not be generalizable to the US population. However, other natural study data are either inaccessible or are not proper for this analysis. For example, The Olmsted County study had patients on DMD treatment and the British Columbia study did not report information required for this analysis [56, 57]. Therefore, the London Ontario study is still the best source we could use to estimate the probabilities for the SM arm.
Third, while adverse event impacts on QALYs were included, adverse event costs were not included in the analysis. However, adverse event costs would not likely affect the results significantly, because the most common adverse effects are similar among the drugs and are often mild and need no treatment, while the incidence of serious adverse events was too low to have a substantial impact on total costs [58], and patients were assumed to discontinue and switch treatments if subject to serious adverse events.
Finally, we did not include GA or other first-line injected DMDs as comparators in our study. One reason that we only included IM IFN-β1a was because the TRANSFORMS trial was a recent head-to-head clinical trial and we were able to check that the patients treated with IM IFN-β1a and fingolimod were comparable. Moreover, the efficacy of fingolimod in the TRANSFORMS trial was similar to its efficacy in the placebo-controlled trial (FREEDOMS). As a result, the disease progression rate derived for the IM IFN-β1a arm was assumed comparable to that for other oral DMDs. Unfortunately, there are no head-to-head trials available between other injected therapies and oral DMDs. In addition, as mentioned, IM IFN-β1a has a large market share, so using only IM INF-β1a as the primary injectable comparator is still informative for decision makers regarding how cost effective the new oral therapies are compared with established injected treatments. Nonetheless, future studies should incorporate GA and other newer DMDs as better data are made available.
5 Conclusion
The three new oral therapies are favored in cost-effectiveness analyses. Of the four DMDs, dimethyl fumarate is a dominant strategy to manage RRMS. Apart from dimethyl fumarate, teriflunomide is most cost effective compared with IM IFN-β1a.
References
Stavnitser A, Patel N, Miller, A, et al. Impact of new oral therapies on multiple sclerosis cost and utilization trends. http://www.ajmc.com. Accessed 05 Aug 2013.
Lee S, Baxter DC, Limone B, et al. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ. 2012;15:1088–96.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303.
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Hay JW. Evaluation and review of pharmacoeconomic models. Expert Opin Pharmacother. 2004;5:1867–80.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain. 2010;133:1914–29.
Drummond MF, Schulper MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. New York: Oxford University Press; 2007.
World Health Organization. Cost-effectiveness thresholds, http://www.who.int/choice/costs/CER_thresholds/en/. Accessed 19 Aug 2014.
The World Bank. GDP per capita, http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 01 June 2013.
Braithwaite SR, Meltzer D, King J, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life year decision rule? Med Care. 2008;46:349–56.
Villacorta R, Hay JW, Messali A. Cost effectiveness of moderate to severe psoriasis therapy with etanercept and ustekinumab in the United States. PharmacoEconomics. 2013;31:823–39.
Mehta D, Hay JW. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol Oncol. 2014;132:677–83.
Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States: a literature review and Markov Model. Neuro Oncol. 2013;15:1532–42.
Hay JW. Where’s the value in health care? Value Health. 2006;9:11–4.
National Multiple Sclerosis Society. Treatments, http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/treatments/index.aspx. Accessed 10 May 2013.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology. 1983;33:1444.
Kobelt G, Berg J, Atherley D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the USA. Neurology. 2006;66:1696–702.
Goodin DS, Cohen BA, O’Connor P, et al. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–73.
Castillo-Trivino T, Mowry EM, Gajofatto A, et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One. 2011;6:e16664.
Halpern R, Agarwal S, Borton L, et al. Adherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysis. Adv Ther. 2011;28:761–75.
Prosperini L, Gianni C, Leonardi L, et al. Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler. 2012;18:64–71.
Tremlett H, Zhao Y, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler. 2008;14:314–24.
Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13:245–61.
Earnshaw SR, Graham J, Oleen-Burkey M, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy. 2009;7:91–108.
Jankovic SM, Kostic M, Radosavljevic M, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on data a Balkan country in socioeconomic transition. Vojnosanit Pregl. 2009;66:556–62.
Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I: clinical course and disability. Brain. 1989;112:133–46.
Kremenchutzky M, Rice GP, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129:584–94.
Scalfari A, Neuhaus A, Daumer M, et al. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 2013;70:214–22.
Guo S, Pelligra C, Thibault CSL, et al. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics. 2014;32:559–72.
Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value Health. 2012;15:843–50.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”): I. Validation of the method. Am J Med. 1982;73:883–8.
Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the “DEALE”): II. Use in medical decision-making. Am J Med. 1982;73:889–97.
Scalfari A, Neuhaus A, Daumer M, et al. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77:1246–52.
Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26:102–7.
Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–65.
Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler. 2003;9:311–9.
Torrance GW, Furlong W, Feeny D. Health utility estimation. Expert Rev Pharmacoecon Outcomes Res. 2002;2:99–108.
Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5:1–30.
Crayton H. Improved quality of life after therapy change to fingolimod. In: 27th Annual Meeting of the CMSC and the 5th Cooperative Meeting of the CMSC-ACTRIMS. Orlando, USA, 29 May-01 June 2013, Hackensack: CMSC.
Montalban X, Comi G, O’Connor P, et al. Oral fingolimod (FTY720) in relapsing multiple sclerosis: impact on health-related quality of life in a phase II study. Mult Scler. 2011;17:1341–50.
Rudick RA, Miller D, Hutchinson M, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–46.
O’Connor P, Briggs A, Carita P, et al. Impact on health-related quality of life of teriflunomide treatment by estimating utilities in patients with relapsing multiple sclerosis: results from TEMSO post hoc analysis. J Neurol. 2012;259:S107.
Kita M, Fox RJ, Phillips JT, et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: findings from the CONFIRM study. Mult Scler. 2014;20:253–7.
Kappos L, Gold R, Arnold DL. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: the DEFINE study. Mult Scler. 2014;20:243–52.
Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine: report of the panel on cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 250.
U.S. Department of Veterans Affairs. Drug pharmaceutical prices, http://www.pbm.va.gov/PharmaceuticalPrices.asp. Accessed 22 Aug 2013.
Oleen-Burkey M, Castelli-Haley J, Lage MJ, et al. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5:57–69.
Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8:1–2.
Briggs A, Schulper MJ, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–87.
O’Day K, Meyer K, Miller RM, et al. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14:617–27.
Pittock SJ, Mayr WT, McClelland RL, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004;62:601–6.
Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66:172–7.
Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15:543–55.
Acknowledgments
Xinke Zhang, Dr. Hay and Xiaoli Niu have no conflicts of interest to declare. No funding was received for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Hay, J.W. & Niu, X. Cost Effectiveness of Fingolimod, Teriflunomide, Dimethyl Fumarate and Intramuscular Interferon-β1a in Relapsing-Remitting Multiple Sclerosis. CNS Drugs 29, 71–81 (2015). https://doi.org/10.1007/s40263-014-0207-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0207-x