Abstract
Objective
To compare the cost-effectiveness of injectable disease-modifying therapies (DMTs) for the first-line treatment of relapsing-remitting multiple sclerosis (RRMS) in Spain.
Methods
A Markov model was developed to estimate the cost-effectiveness of intramuscular interferon beta-1a (IM IFNβ-1a), subcutaneous interferon beta-1a (SC IFNβ-1a), interferon beta-1b (IFNβ-1b) and glatiramer acetate (GA) relative to best supportive care in a hypothetical cohort of 1,000 RRMS patients in Spain. The model was developed from a societal perspective with a time horizon of 30 years. Natural history and clinical trial data were used to model relapse rates and disease progression. Cost and utility data were obtained from a published survey of multiple sclerosis patients in Spain. The primary outcome measure was cost per quality-adjusted life year (QALY) gained. Univariate and probabilistic sensitivity analyses were performed.
Results
Compared to best supportive care, the base case cost-effectiveness was €168,629 per QALY gained for IM IFNβ-1a, €231,853 per QALY gained for IFNβ-1b, €295,638 per QALY gained for SC IFNβ-1a, and €318,818 per QALY gained for GA. Results were most sensitive to changes in DMT cost, utility values and treatment effect.
Conclusions
In our cost-effectiveness analysis of first-line injectable DMTs in Spain, we found IM IFNβ-1a to be more cost-effective than SC IFNβ-1a, IFNβ-1b or GA. Sensitivity analyses confirmed the robustness of these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system. The disease results in injury to the myelin sheaths, the oligodendrocytes and, to a lesser degree, the axons and nerve cells themselves. Four clinical courses of MS have been identified with the most common being relapsing-remitting MS (RRMS). RRMS is characterized by self-limited attacks of neurologic dysfunction followed by partial or complete recovery [1]. MS is twice as common in women as in men with most patients being diagnosed between 20 and 50 years of age [2].
An estimated 2.5 million people worldwide are affected by MS [2]. MS affects approximately 350,000 people in Europe including over 30,000 people in Spain [3–5]. The prevalence of MS in Spain has been reported as 32–65 per 100,000 [6–10]. The annual incidence of MS in Spain has been estimated as 3.8 per 100,000 [7].
The economic burden of RRMS is considerable, due in part to the early age of onset and the progressive disabling course of the disease. A recent cost-of-illness study in Spain estimated the mean societal cost of MS to be €33,456 per patient per year [10]. Consistent with other studies, costs were found to be correlated significantly with disease severity. In Spain, the mean total cost for patients with mild disease and expanded disability status scale (EDSS) of 2.0 was approximately €19,604 per patient per year while patients with more progressive disease (EDSS 6.5) had annual per patient costs of over €44,000 [11]. In early years, disease-modifying therapies (DMTs) comprise a significant portion of total costs; while in later years, informal home care accounts for the largest component of cost. Productivity loss is another significant component of the societal burden of MS. In Spain, 34 % of MS patients reported early retirement due to their MS. The annual cost of early retirement and long-term sick leave was estimated at almost €9,000 per patient or 26 % of the total annual per patient cost [10].
Over the last two decades, the introduction of injectable DMTs has transformed the treatment of RRMS. Injectable DMTs for first-line treatment of RRMS include intramuscular interferon beta-1a (IM IFNβ-1a–Avonex), subcutaneous interferon beta-1a (SC IFNβ-1a–Rebif), interferon beta-1b (IFNβ-1b–Betaseron, Extavia) and glatiramer acetate (GA–Copaxone). Randomized, placebo-controlled clinical trials have shown DMTs to reduce the number of relapses and slow the progression of the disease [12–17].
Since the introduction of injectable DMTs in the 1990s, a number of economic models have been developed to estimate their cost-effectiveness as the first-line treatment of RRMS [18–27]. Several models were developed from a US managed care perspective and report cost per relapse avoided [20, 21]. Limitations of these models include short time horizons—less than 5 years—and the exclusion of indirect costs. Other models have been developed from the societal perspective with time horizons ranging from 10 years to lifetime [18, 24]. The primary outcome measure of these models was cost per quality-adjusted life year (QALY) gained. Few cost-effectiveness models have been developed from the societal perspective of a European country [19, 22, 25, 26]. The purpose of this model was to estimate the cost-effectiveness of first-line injectable therapies for the treatment of RRMS from the Spanish societal perspective. The primary outcome measure was cost per QALY gained.
Materials and methods
Model overview
A Markov model was developed to simulate the treatment effects and costs of five therapeutic strategies for RRMS, which included; IM IFNβ-1a (30 μg administered once weekly), SC IFNβ-1a (44 μg administered every other day), IFNβ-1b (125 μg administered thrice weekly), GA (20 mg administered daily) and best supportive care. Best supportive care consisted of symptomatic treatment and treatment of relapses. The model was developed from a societal perspective with a time horizon of 30 years measured in monthly cycles. The 30-year time horizon was selected to reflect the chronic progressive nature of the disease and to capture the long-term costs associated with RRMS and the benefits of disease-modifying therapy.
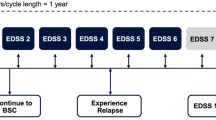
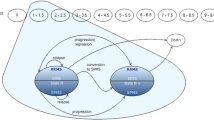
The Markov model diagram is presented in Fig. 1. Health states were defined using the Kurtzke EDSS [28]. EDSS is a widely used method of quantifying disability in MS. Scores range from 0 (normal neurologic examination) to 10 (death due to MS) and increase in half-point increments. Natural history studies have grouped EDSS levels into categories based on key levels of disability where EDSS levels 0–2.5 represent no or minimal disability, 3.0–5.5 moderate disability but fully ambulatory, 6.0–7.5 assistance required for ambulation, 8.0–9.5 restricted to a wheelchair or bed and, 10.0 death from MS [29]. These EDSS health state groupings have been used in previous cost-effectiveness models in MS [18, 24, 30].
A hypothetical cohort of 1,000 patients entered the model with no or minimal disability. The distribution of patients by EDSS level (57 % with EDSS scores of 1–1.5 and 43 % with EDSS scores of 2–2.5) was based on natural history data from patients at disease onset [29]. Patients were assumed to enter the model at 30 years of age which is the average age of MS diagnosis in Spain.
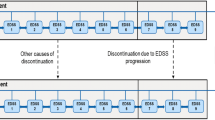
All patients entered the model in the EDSS 0.0–2.5 level. As shown in Fig. 1, during each 1 month cycle, patients in the model experienced one of the following events: (1) remained in the same EDSS level with no relapse; (2) relapsed and remained in the same EDSS level; (3) progressed to the next EDSS level with no relapse; (4) relapsed and moved to the next EDSS level; or (5) death. Patients progressed through these different health state possibilities until model end. Consistent with the natural history of the disease, patients in the model were unable to return to a less disabled state after progressing beyond.
Patients initiated DMTs upon entry into the model. Consistent with clinical practice in Spain, treatment with DMTs was assumed to end when a patient reached EDSS 7.0. Once DMTs were terminated, patients in the model progressed according to the natural history of the disease and no longer accrued the benefits of therapy.
Disease progression
Data from natural history studies were used to model disease progression for patients receiving best supportive care [29, 31]. Transition probability estimates used in the current model have been used in prior MS cost-effectiveness models [18, 24]. Disease progression for patients treated with DMTs were modeled using data from the pivotal clinical trials [12–17]. Spanish age-specific and gender-specific general mortality rates were used for all patients and treatments [32]. Patients were assumed to have no additional likelihood of death due to MS.
Relapse
Relapse rates for best supportive care were obtained from natural history studies while data from the pivotal clinical trials were used to model relapses for DMTs [12–17, 29, 33]. Relapses were assumed to have a duration of 1 month. Natural history studies have demonstrated that the risk of relapse in MS is time dependent and decreases over time [33, 35]. To account for this time dependency of relapse rates, we incorporated a predictive regression formula that was published in a recent risk-benefit model [36]. The formula was based on data from four prospective natural history studies [33–35]. In addition, to address the time dependency of relapse and to be consistent with other RRMS models, we assumed that no relapses occurred after EDSS 6.0 [18, 24, 29, 36].
Model inputs
Treatment effects
Treatment effects for IM IFNβ-1a, SC IFNβ-1a, IFNβ-1b and GA were defined as a percent reduction in the probabilities of relapse and disease progression. Treatment effects were obtained from pivotal clinical trials [12–17] (Table 1) and applied to the natural transition probabilities (Table 2). All studies were randomized double-blind placebo-controlled trials. Effectiveness data for all studies reflected the experience of patients who had received at least 2 years of therapy. Treatment effects were discounted at 3 % annually.
Costs
The current model included direct and indirect costs to reflect the societal burden of MS. Treatment costs for IM IFNβ-1a, SC IFNβ-1a (44 μg), IFNβ-1b and GA were based on Spanish ex-factory prices as of July 2010 [37] and are listed in Table 3. Direct costs and indirect costs were obtained from a published study of the economic burden and quality of life of MS patients in Spain [11]. Costs were stratified by EDSS level (Table 2).
Direct medical costs consisted of inpatient and outpatient admissions, visits to physicians or other health-care professionals, laboratory tests and procedures. Direct non-medical costs included those costs related to walking aids, social services, informal care and transportation assistance. Indirect costs included productivity loss due to short-term absence, long-term sick leave and early retirement due to MS. The human capital method was used to estimate cost. The Spanish consumer price index (CPI) was used to inflate all cost data to 2010 euros, and an annual discount rate of 3 % was applied to costs in the model [38].
Utilities
Utilities are used to measure preference for different health states and range from 1.0 (perfect health) to 0 (death). Our model incorporated mean annual utilities by EDSS level from a published survey of over 1,800 Spanish patients with MS [11] that were adjusted to reflect mean monthly utility by EDSS level for each cycle (see Table 2). The survey used the EQ-5D (EuroQol), a validated generic instrument, to elicit utility values [39]. Relapse effects were assigned a utility decrement of 0.1 which was subtracted from the mean utility score for the corresponding EDSS level of the patient. Relapses were accrued on a per event basis.
Model outputs
Model results were reported as total costs, total QALYs and incremental cost-effectiveness ratios (ICERs) using cost per QALY gained as the effectiveness measure. ICERs were calculated for each DMT versus best supportive care.
Both univariate and multivariate sensitivity analyses were performed to evaluate the impact of uncertainty on model results. For the univariate sensitivity analysis the parameters varied were: natural disease progression rates; cost of relapse; cost of EDSS state; indirect costs; EDSS health utilities and relapse disutility; DMT efficacy; initial patient EDSS distribution; discount rate; time horizon; DMT costs; and SC IFNβ-1a dose.
Multivariate probabilistic sensitivity analysis (PSA) using Monte Carlo methods (1,000 simulations) was based on the variances of costs; natural disease progression rates; relapse rates and utility values. Results for the four DMTs versus best supportive care were plotted on cost-effectiveness acceptability curves (CEACs). The cost-effectiveness acceptability frontier (CEAF) was also assessed, assuming the decision to use a DMT had been made.
Results
Base case
The results of the base case analysis are presented in Table 4. Best supportive care produced a total of 13.07 QALYs gained per patient. The average total cost for patients treated with best supportive care was €299,922 per patient.
Treatment with all four DMTs provided additional benefit when compared to best supportive care. Total QALYs gained per patient were greatest with IM IFNβ-1a (13.94), followed by SC IFNβ-1a (13.85), IFNβ-1b (13.78) and GA (13.57). The mean per patient costs were lowest with IM IFNβ-1a (€446,832), followed by GA (€459,199), IFNβ-1b (€465,400), and SC IFNβ-1a (€530,832). The ICERs for IM IFNβ-1a, SC IFNβ-1b, SC IFNβ-1a and GA each compared to best supportive care were €168,629, €231,853, €295,638, and €318,818 per QALY gained, respectively.
Sensitivity analysis
Sensitivity analyses indicated that for the comparison of IM IFNβ-1a, SC IFNβ-1a and IFNβ-1b versus best supportive care, results were most sensitive to variation in DMT costs, cycle utilities, and relapse severity as measured by the disutility assigned to relapse events (Fig. 2a–d). For the comparison of GA versus best supportive care, results were most sensitive to variations in relapse severity, DMT costs, and the reduction in relapse rate. Notably, the ICER for IM IFNβ-1a versus best supportive care exhibited the least sensitivity to one-way variation in input parameters while the ICER for GA versus best supportive care exhibited the most sensitivity. A scenario analysis showed that when the 22 μg form of SC IFNβ-1a was used with a monthly price of €778.15, a percentage reduction in disease progression of 22.35 %, and a percentage reduction in relapse rate of 28.91 %, the ICER versus best supportive care was €233,594 per QALY.
The CEACs indicate that results of the current model were robust (Fig. 3). There was a 50 % probability that IM IFNβ-1a would be cost-effective versus best supportive care at a cost per QALY threshold of €170,000 per QALY, as opposed to a 0, 6, and 0 % probability that SC IFNβ-1a, SC IFNβ-1b or GA, respectively, would be cost-effective compared to best supportive care at this threshold. The CEAF results (not shown) indicated that IM IFNβ-1a represented the optimal treatment option across all ceiling values tested, conditional on using a DMT, with results ranging from it being most cost-effective in 79–97 % of simulations tested.
Discussion
Based on the model results presented here, IM IFNβ-1a was more cost-effective than SC IFNβ-1a, IFNβ-1b or GA as measured by cost per QALY gained. Sensitivity analyses confirmed the robustness of these results and demonstrated that IM IFNβ-1a was the most cost-effective option in 79–97 % of all the simulations performed.
We are aware of five cost-effectiveness studies that have reported incremental cost per QALY gained in first-line injectable DMTs [18, 19, 23, 24, 26]. Prosser et al. [24] compared the cost-effectiveness of DMTs using pivotal trial data and a 10-year time horizon. The base case results from the model were US $1.8 million per QALY gained for IM IFNβ-1a. The results from this latter study were substantially higher than ours due to several assumptions in their model. The first is that the Prosser study applied a disutility to effectiveness to account for side effects. Other differences include a shorter time horizon, adjustments for discontinuation and different sources for utility values and cost. Despite these differences in data sources and assumptions, when Prosser extended the time horizon to 40 years in sensitivity analyses, the results were similar to our model with an incremental cost per QALY gained for IM IFNβ-1a of US $250,000 for women and $235,000 for men.
Another cost-effectiveness study by Chilcott et al. [19] was commissioned by the National Institute for Clinical Excellence and reported cost per QALY gained. This model used a time horizon of 20 years and was conducted from the perspective of the United Kingdom’s National Health Service. The base case results in this model ranged from £42,000 per QALY gained for IM IFNβ-1a to £98,000 per QALY gained for GA. While the cost per QALYs gained in the Chilcott model were lower than those reported in the current model, the rank order of results were the same.
A model published by Noyes and colleagues from the University of Rochester reported cost per QALY gained for first-line injectable DMTs [23]. Results from this model were considerably higher than in our study and most others with cost per QALY gained ranging from US $901,319 per QALY gained for IM IFNβ-1a to US $2.1 million per QALY gained for GA. The difference in results between the Noyes model and the current model can be explained by several factors. The first is that the University of Rochester model used data that included patients with secondary progressive multiple sclerosis (SPMS) which would be expected to have higher costs than RRMS patients. Interestingly, IM IFNβ-1a, SC IFNβ-1a, IFNβ-1b and GA are not indicated for use in SPMS in the United States so the rationale for including these patients in the University of Rochester model is not clear. Using a more disabled population would increase cost and decrease QALYs contributing to the higher cost per QALYs gained in the Rochester model. Time horizons used in the models also contributed to the differing results. The current model incorporated a 30-year time horizon while the Noyes model used a time horizon of 10 years. Lack of transparency is another limitation of the Noyes model. The paper, published in 2011, does not report how health states were defined or how treatment effects were incorporated into the model. The paper also does not report the utility values that were used in the model. Because of this lack of transparency, it is difficult to compare the results of this model with other cost-effectiveness models in MS. Of note, in sensitivity analyses, the Noyes model found improved cost-effectiveness ratios when DMTs were initiated earlier in the course of disease compared to waiting until patients were more disabled.
Results in the current model are most consistent with a cost-utility model developed by Bell et al. [18], which was based on a US payer perspective and a lifetime time horizon. Results in this model ranged from US $258,000 to $416,000 per QALY gained compared to best supportive care. The Bell model was similar to ours in model structure, cycle length and assumptions that limited the occurrence of relapse and DMT use to patients in EDSS 0.0–5.5. There are also several differences in the models. Bell adjusted the probabilities of relapse for the interferons to account for the development of neutralizing antibodies. This assumption may partially explain why GA, which is not associated with neutralizing antibodies, had the lowest cost per QALY in the Bell model.
The results of our model appear to be consistent with another model developed in Spain [26]. The Sanchez de la Rosa model was developed from the societal perspective with 1 month cycle lengths and a time horizon of 10 years. Total cost was reported as €322,509 for GA, €329,595 for IM IFNβ-1a, €333,925 for IFNβ-1b and €348,208 for SC IFNβ-1a. Similar to our model, total QALYs gained were highest for IM IFNβ-1a and lowest for GA. The Sanchez de la Rosa model found IM IFNβ-1a to be the dominant treatment strategy (lower cost and higher QALY) compared to SC IFNβ-1a and IFNβ-1b. The cost per QALY gained for IM IFNβ-1a compared to GA was €117,914.
In our model, univariate sensitivity analyses indicated that DMT cost was a major driver of cost-effectiveness. In Spain, at the time our model was developed, the cost of SC IFNβ-1a (44 μg) was 40 % higher than IM IFNβ-1a. However, reducing the cost of SC IFNβ-1a to the level of IM IFNβ-1a resulted in a cost per QALY gained of €233,594 compared to €168,629 for IM IFNβ-1a.
Strengths of our model include the structure, which has been used in several other cost-effectiveness studies in RRMS [18, 24, 30]. The model incorporates health states based on the widely accepted EDSS and uses a 1 month cycle length, which has been used in previous RRMS models [18, 20, 30]. Another strength is our use of a 30-year time horizon. Weinstein and colleagues recommend that model time horizons should be long enough to reflect important and valued differences between long-term consequences and costs of alternative treatment strategies [40]. Given the chronic, progressive nature of MS, the differences among DMTs in slowing disease progression, and the substantial costs associated with more disabled disease states, we believe a 30-year time horizon is appropriate for RRMS. Finally, consistent with good practice guidelines, we conducted both deterministic and probabilistic sensitivity analyses to explore parameter uncertainty [40, 41].
There are several limitations to the current study. First, the effectiveness inputs are based on efficacy data from randomized controlled clinical trials with strict inclusion and exclusion criteria. As such, the efficacy results from the trials may not accurately reflect the treatment effects in the general RRMS population. Ideally, our model would incorporate “real world data” to better reflect the performance of these therapies in clinical practice. In the absence of these data and to be consistent with previous cost-effectiveness studies in RRMS, we used efficacy data from the randomized placebo-controlled trials for our effectiveness inputs. Another limitation of our model is the lack of long-term comparative data for the DMTs. As commonly done in cost-effectiveness studies, we used efficacy results from 2-year clinical trials and assumed a constant treatment effect over a time horizon beyond the 2-year trial length. Another limitation of our model is that we did not consider the impact of adverse events or common treatment side effects such as flu-like symptoms or injection site reactions. Inclusion of these transient events would likely reduce QALYs slightly across all DMTs but would not likely have a significant effect on results. Lastly, the model assumes that the risk of death is equivalent for the MS and general populations. Evidence on whether MS patients have a lower life expectancy than the general population is mixed. Some research has suggested that MS patients have a slightly lower life expectancy but the magnitude of the difference is small. Therefore while the model could have adjusted the probability of death, it would not likely have had a significant effect on results.
As new therapies become available for the treatment of MS, it will be important to consider both the clinical and economic value of all therapies. Cost-effectiveness models provide useful data to compare the cost and effectiveness of different therapies. Our model suggests that, in Spain, IM IFNβ-1a appears to be a more cost-effective treatment option than SC IFNβ-1a, IFNβ-1b or GA. These results have important implications for Spanish payers and physicians as the number of treatment options for RRMS continue to increase. Further research is needed to compare the cost-effectiveness of injectable DMTs with the new oral agents.
References
Goodin, D.S., Frohman, E.M., Garmany, G.P., et al.: Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for clinical practice guidelines. Neurology 58(2), 169–178 (2002)
National Multiple Sclerosis Society. Multiple sclerosis: just the facts. MS Soc 2010: available at http://nationalmssociety.org (2011). Accessed 13 July 2011
Multiple Sclerosis International Federation. About MS. Available at http://www.msif.org/en/about_ms (2011). Accessed 10 August 2011
Asociacion Espanola de Esclerosis Multiple. Multiple Sclerosis: living with multiple sclerosis. Available at http://www.aedem.info/portal/ (2011). Accessed 10 August 2011
Pozzilli, C., Romano, S., Cannoni, S.: Epidemiology and current treatment of multiple sclerosis in Europe today. J. Rehabil. Res. Dev. 39(2), 175–185 (2002)
Bufill, E., Blesa, R., Galan, I., et al.: Prevalence of multiple sclerosis in the region of Osona, Catalonia, northern Spain. J. Neurol. Neurosurg. Psychiatry 58, 577–581 (1995)
Benito-Leon, J., Martin, E., Vela, L., et al.: Multiple sclerosis in Mostoles, central Spain. Acta Neurol. Scand. 98, 238–242 (1998)
Tola, M., Yugueros, M., Fernandez-Buey, N., et al.: Prevalence of multiple sclerosis in Vallaloid, northern Spain. J. Neurol. 246, 170–174 (1999)
Pugliatti, M., Rosati, G., Carton, H., et al.: The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 13, 700–722 (2006)
Ares, B., Prieto, J.M., Lema, M., et al.: Prevalence of multiple sclerosis in Santiago de Compostela (Galicia, Spain). Mult. Scler. 13, 262–264 (2007)
Kobelt, G., Berg, J., Lindgren, P., et al.: Costs and quality of life of multiple sclerosis in Spain. Eur. J. Health Econ. 7(Suppl 2), S65–S74 (2006)
Jacobs, L.D., Cookfair, D.L., Rudick, R.A., et al.: Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann. Neurol. 39(3), 285–294 (1996)
Johnson, K., Brooks, B., Cohen, J., et al.: Copolymer 1 reduces relapse rates and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 45, 1268–1276 (1995)
PRISMS: Randomised double-blind placebo controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 52, 1498–1504 (1998)
The PRISMS Study Group: PRISMS-4: long-term efficacy of interferon beta-1a in relapsing MS. Neurology 56, 1628–1636 (2001)
The IFNB Multiple Sclerosis Group and the University of British Colombia MS/MRI Analysis Group: Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 45, 1277–1285 (1995)
INFB Multiple Sclerosis Study Group: Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. Neurology 43, 655–661 (1993)
Bell, C., Graham, J., Earnshaw, S., et al.: Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J. Manag. Care Pharm. 13(3), 245–261 (2007)
Chilcott, J., McCabe, C., Tappenden, P., et al.: Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ. 326(7388), 522 (2003). (discussion p 22)
Goldberg, L.D., Edwards, N.C., Fincher, C., et al.: Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J. Manag. Care Pharm. 15(7), 543–555 (2009)
Guo, S., Bozkaya, D., Ward, A., et al.: Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a: modelling the clinical and economic implications. Pharmacoeconomics 27(1), 39–53 (2009)
Kobelt, G., Texier-Richard, B., Lindgen, P.: The long-term cost of multiple sclerosis in France and potential changes with disease-modifying interventions. Mult. Scler. 15, 741–751 (2009)
Noyes, K., Bajorska, A., Chappel, A., et al.: Cost-effectiveness of disease-modifying therapy for multiple sclerosis. Neurology 77, 355–363 (2011)
Prosser, L.A., Kuntz, K.M., Bar-Or, A., et al.: Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 7(5), 554–568 (2004)
Nuijten, M., Mittendorf, T.: A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German Societal Perspective. Clin. Ther. 32(4), 717–728 (2010)
Sanchez-de la Rosa, R., Sabater, E., Casado, M.A. et al.: Cost-effectiveness analysis of disease modifying drugs (interferons and glatiramer acetate) as first line treatments in remitting-relapsing multiple sclerosis patients. J. Med. Econ. 15(3), 424–433 (2012)
Tappenden, P., McCabe, C., Chilcott, J., et al.: Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the medicare population. Value Health 12(5), 657–665 (2009)
Kurtzke, J.: Rating neurologic impairment in multiple sclerosis: an expanded disability status scale. Neurology 33, 1444–1452 (1983)
Weinshenker, B.G., Bass, B., Rice, G.P., et al.: The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112(Pt 1), 133–146 (1989)
Earnshaw, S.R., Graham, J., Oleen-Burkey, M.K.: Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl. Health Econ. Health Policy 7(2), 91–108 (2009)
Runmarker, B., Andersen, O.: Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116(Pt 1), 117–134 (1993)
OECD/European Union: “Mortality from all Causes”, in Health at a Glance: Europe 2010, OECD Publishing. (2010). http://dx.doi.org/10.1787/health_glance-2010-en
Broman, T., Andersen, O., Bergmann, L.: Clinical studies on multiple sclerosis. I. Presentation of an incidence material from Gothenburg. Acta Neurol. Scand. 63(1), 6–33 (1981)
Fog, T., Linnemann, F.: The course of multiple sclerosis in 73 cases with computer-designed curves. Acta Neurol Scand Suppl 47, 3–175 (1970)
Patzold, U., Pocklinton, P.: Course of multiple sclerosis: first results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand 65, 248–266 (1982)
Thompson, J.P., Noyes, K., Dorsey, E.R., Schwid, S.R., Holloway, R.G.: Quantitative risk-benefit analysis of natalizumab. Neurology 71(5), 357–364 (2008)
IHS Global Insight: Ex-factory Spanish Prices as of July 2010 (2011)
Spanish Consumer Price Index from the Instituto Nacional de Estadistica
The EuroQol Group: EuroQol—a new facility of the measurement of health-related quality of life. Health Policy 16, 199-208 (1990)
Weinstein, M., O’Brien, B., Hornberger, J., et al.: Principles of good practice for decision analytic modeling in health care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health 6(1), 9–17 (2003)
Philips, Z., Bojke, L., Sculpher, M., et al.: Good practice guidelines for decision-analytic modelling in health technology assessment. Pharmacoeconomics 24(4), 355–371 (2006)
Acknowledgments
This research was funded by Biogen Idec.
Conflict of interest
Carole Dembek and Leigh Ann White are employees and shareholders of Biogen Idec. Andrea Szkurhan, Jayson Quach and Nazia Rashid were paid consultants on the study. M.R. Blasco received consulting fees from Biogen Idec for participation in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dembek, C., White, L.A., Quach, J. et al. Cost-effectiveness of injectable disease-modifying therapies for the treatment of relapsing forms of multiple sclerosis in Spain. Eur J Health Econ 15, 353–362 (2014). https://doi.org/10.1007/s10198-013-0478-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-013-0478-z