Abstract
Background
Cost-of-illness (COI) studies provide useful information on the economic burden that schizophrenia imposes on a society.
Objectives
This study aims to give a general overview of COI studies for schizophrenia and to compare the societal cost of schizophrenia across countries. It also aims to identify the main cost components of schizophrenia and factors associated with higher societal cost to improve the quality and reporting of COI studies for schizophrenia.
Methods
We performed an electronic search on multiple databases (MEDLINE, Embase, PsycINFO, Cochrane Database of Systematic Reviews, Health Management Information Consortium [HMIC] and the System for Information on Grey Literature [openSIGLE]) to identify COI studies of schizophrenia published between 1996 and 2016. The primary outcome of this review was societal cost per schizophrenia patient, by cost component. All costs were converted to $US, year 2015 values.
Results
We included 19 studies in this review. The annual societal cost per patient varied from $US5818 in Thailand to $US94,587 in Norway; whereas the lifetime societal cost per patient was estimated to be $US988,264 in Australia (all year 2015 values). The main cost drivers were direct healthcare costs and productivity losses. Factors associated with higher individual costs included patient demographics, severity of disease and methods used to calculate the costs of productivity losses and comorbidities.
Conclusions
This review highlights the large economic burden of schizophrenia. The magnitude of the cost estimates differs considerably across countries, which might be caused by different economic conditions and healthcare systems and widespread methodological heterogeneity among COI studies. Proposed recommendations based on this review can be used to improve the consistency and comparability of COI studies for schizophrenia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Schizophrenia is associated with a large economic burden, which is mainly driven by direct healthcare costs and productivity losses. |
The societal costs of schizophrenia vary greatly between countries, which might be caused by differences in the state of the economy and healthcare systems across countries and widespread methodological heterogeneity in cost-of-illness studies. |
Substantial savings could potentially be achieved by investing in occupational therapy for schizophrenia patients, service and support for carers, transferring treatment from psychiatric hospitals to the community and preventing crimes. |
1 Introduction
1.1 Schizophrenia
Schizophrenia is a chronic, severe and disabling disease characterized by psychotic symptoms that alter a person’s perception, thoughts, affect and behaviour. A systematic review of 188 studies showed that the median lifetime prevalence of schizophrenia is 0.4 % worldwide (10–90 % quantiles: 0.16–1.21 %) [1]. People with schizophrenia are at high risk of physical morbidity and premature mortality, including cardiovascular disease and type II diabetes mellitus, compared with the general population. Life expectancy for patients with schizophrenia is reduced by up to 20 years compared with the general population [2].
Schizophrenia places a great financial burden on health systems, families and society. The overall societal cost for schizophrenia is estimated to be $US62.7 billion (year 2002 values) in the USA [3], $Can6.9 billion (year 2004 values) in Canada [4] and £6.1 billion (UK pounds, year 2004 values) in the UK [5].
1.2 Cost-of-Illness Studies (COIs)
Cost-of-illness (COI) studies provide useful information on the magnitude of the financial impact of an illness on society or a part of society. This information can highlight aspects of the disease and processes of care where improvements are needed and thus inform planning of healthcare services and the prioritisation of research [6]. Moreover, COI studies provide important information for economic evaluations of healthcare interventions, such as cost-effectiveness, cost-utility and cost-benefit analyses. The key methodological points to consider when reviewing a COI study are briefly summarised below.
1.2.1 Perspective of the Analysis and Costs Assessed
Depending on the purpose of a COI study, it can be conducted from different perspectives such as a societal or a healthcare system perspective. The choice of perspective can have a large effect on the actual cost estimates [7]. Generally speaking, a COI study will take the perspective of the organisation that is sponsoring it [8]. On the other hand, the societal perspective is preferred by economists [9–11] and minimizes the potential biases of narrower views [12].
1.2.2 Epidemiological Approach
There are two basic epidemiological approaches for COI studies: the prevalence-based approach and the incidence-based approach. The former approach can be used to measure the costs of disease over a certain period of time, usually 1 year, regardless of the date of illness onset. Alternatively, the incidence-based approach can be used to estimate lifetime costs of disease from onset to conclusion for patients diagnosed within the period of the study.
1.2.3 Estimating Resource Consumption
There are three main approaches to estimating resource consumption: the top-down approach, the bottom-up approach and the econometric approach. The top-down approach uses aggregated data along with a population-attributable fraction (PAF) to assign a percentage of total expenditure (the attributable costs) to the disease of interest. The bottom-up approach assesses the average cost of treatment per patient and multiplies it by the prevalence of the illness [8]. The econometric approach compares the costs in a cohort of the population with the estimated counterfactual costs in this cohort in the absence of the disease. The choice of approach can sometimes have a dramatic effect on the cost estimates [13]; however, as there is no clear consensus on which approach is best, each is defensible. The appropriateness of approaches to estimating resource consumption largely depends on the disease or risk factor under investigation, the study question and the data available [8]. The top-down approach normally uses aggregated data (such as total expenditure in a healthcare system) along with PAF to calculate the attributable costs. The bottom-up approach usually requires patient-level resource use data and unit costs from multiple sources. The econometric approach is the least data demanding, as it often only requires one dataset about the resource use for individuals who have the disease and those who do not [7].
1.2.4 Prospective or Retrospective COI Studies
In a retrospective COI study, all events will have already occurred by the time the study is initiated. Past resource use data will be collected, and their cost will be adjusted to the base year price. By contrast, in a prospective COI study, the relevant events will have not yet occurred when the study is initiated. Therefore, the researchers can design the data-collection methods and then follow patients over time to collect the data.
1.2.5 Cost Components
A comprehensive COI study should consider all cost consequences of the disease, including direct healthcare costs, direct non-healthcare costs and indirect costs (productivity losses). Direct healthcare costs are those that arise from treating the disease and its consequences; they normally include hospital inpatient, outpatient and community care; nursing home care; rehabilitation care; diagnostic tests; and medications. Direct non-healthcare costs include supported living, legal costs (e.g. incarceration, policing, legal and costs to victims of crime), transportation, and private expenditure. Indirect costs include losses in productivity of patients or their carers (informal care) associated with the disease, treatment, any disability incurred as a result of disease (morbidity costs) and premature death such as suicide (mortality costs). Recently there has been a movement away from the term ‘indirect cost’ to the term ‘productivity losses’ in COI studies, as ‘indirect cost’ is also commonly used for overheads and other shared costs in patient-level costing of healthcare services within an accounting framework [8].
1.2.6 Estimating Productivity Losses
Three types of productivity losses can be considered in a COI study: carer productivity loss, patient productivity loss due to morbidity, and patient productivity loss due to mortality.
The methods most commonly used to estimate carer productivity losses are the replacement approach and the opportunity cost approach [8]. The former values the unpaid labour at the market price that would need to be paid to find a replacement from the market to do the work, whereas the latter approach is based on the paid or unpaid work it displaces, as measured by the wage the person would earn if in paid employment. Each method is defensible.
In terms of patient productivity losses due to morbidity or mortality, there are three primary approaches: the human capital approach (HCA), the friction cost approach (FCA) and the willingness-to-pay (WTP) method [7]. The HCA aims to reflect lost productive potential by multiplying the earnings lost for different age and sex groups by the corresponding number of patients in that group. The FCA assumes that patients who stop working because of illness will be replaced by someone who was previously unemployed and therefore only measures the productivity losses during the time it takes to replace a worker [14]. While the HCA may overstate actual productivity losses, the FCA is relatively difficult to implement as it would require detailed information about the labour market conditions and behaviours. The WTP method measures the amount an individual would pay to reduce the probability of illness or mortality. However, this approach often requires extensive surveys of people’s preferences and is thus difficult to implement. There is no consensus on which approach is superior to the others.
1.2.7 Sensitivity Analysis (SA)
Sensitivity analysis (SA) involves recalculating the cost estimate with different values for selected input values to compare the results with the original estimate. Therefore, SA can be useful to help the reader assess the confidence they can place in the conclusions of the study, identify those variables for which a more precise estimate is most needed and determine which parameters/assumptions are key cost drivers. It is recommended that, for COI studies, SA be conducted for all important parameters and key assumptions, and the results of such analyses must always be reported and evaluated [15].
1.3 Aims and Objectives
The aim of this study is to systematically review all published COI studies for schizophrenia. More specifically, this review aims to address the following questions:
-
What is the societal cost of schizophrenia per patient in different countries?
-
What are the main cost components of schizophrenia?
-
What factors are associated with higher societal cost for schizophrenia?
Based on the results of this review, we also aim to provide recommendations to improve the quality and reporting of COI studies for schizophrenia.
2 Methods
This systematic review was conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) recommendations for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions [16].
2.1 Search Methodology
On 13 October 2015, we conducted a literature search to identify COI studies of schizophrenia in the following electronic databases: MEDLINE (including in-Process and other non-indexed), Embase, PsycINFO, the Cochrane Database of Systematic Reviews, the System for Information on Grey Literature (openSIGLE) and the Health Management Information Consortium (HMIC). The search strategy included the medical subject heading terms and text words ‘schizophrenia’, ‘psychosis’, ‘delusion’, ‘hallucination’, ‘thinking disorder’, ‘hebephrenia’, ‘catatonia’, ‘oligophrenia’ and ‘paranoia’ in combination with the following health economics terms: cost of illness, cost analysis, healthcare cost, hospital cost, productivity loss and burden. We applied no language, date or country restrictions to the search. The detailed search strategy is reported in Table 1 of the Electronic Supplementary Material (ESM).
2.2 Selection of Studies
We defined the inclusion and exclusion criteria a priori in accordance with the COI evaluation checklist developed by Larg et al. [8]. Studies were included if the population of interest was children/young people/adults with a clinical diagnosis of schizophrenia or psychosis and the study adopted a societal perspective (included both direct costs and productivity losses). The rationale and limitations of choosing the societal perspective is discussed in Sects. (4.7.2, 4.7.3). The different cost components were described in sufficient detail. As a minimum, the direct costs needed to include at least inpatient and outpatient or community costs; the productivity losses need to include productivity loss for patients due to morbidity. The following exclusion criteria were applied: (1) studies compared the cost effectiveness of different interventions for schizophrenia or referred to costs of interventions only; (2) the costs of interest were not reported and could not be derived; (3) study participants were not representative of the general schizophrenia population, i.e. studies in which patients received one particular type of intervention, were in a particular disease phase (e.g. only patients in relapse) or were being treated in a particular setting (e.g. outpatient only); (4) studies focusing on just one disease phase of schizophrenia, such as relapse; (5) studies published 20 years ago (before 1996) as they were unlikely to be relevant to current practice and costs; (6) reviews, commentaries, letters, editorials, or abstracts; and (7) studies not reported in English (although no language restrictions were imposed on the search itself, only papers published in English language were included in the review).
Two reviewers (HJ and IM) performed the first screening of the literature search results by comparing titles and abstracts against the inclusion criteria. The full articles were then obtained for possibly useful studies and checked against the inclusion criteria. Final inclusion of studies in the review was determined by agreement of both reviewers.
The bibliographies of published review/overview papers identified from the search were checked to ensure all relevant COI studies had been retrieved by the search strategy used.
2.3 Presentation of Cost Estimates
The primary outcome for prevalence-based studies was annual societal cost per patient (we converted costs reported by studies with different time horizons to annual costs). The primary outcome for incidence-based studies was lifetime cost per patient. When cost per patient was not reported, we calculated it when data allowed. Secondary outcomes included patient/disease specification, basis of analysis, costing methods and factors associated with higher costs.
The cost estimates of different cost components were reported separately. Studies included in this review employed different definitions for ‘direct healthcare costs’, ‘direct non-healthcare costs’ and ‘informal care/productivity losses’. For example, some studies included informal care (productivity losses for the carer) as a direct non-healthcare cost, whereas other studies considered it an indirect cost. To maintain consistency, we reclassified all cost components reported by included studies according to the following definitions:
-
Direct healthcare costs: inpatient costs, outpatient/community costs, medicine costs and any other costs to the healthcare system.
-
Direct non-healthcare costs: costs of sheltered accommodation, legal costs, administration costs of social welfare benefits, transport costs, private expenditure and any other direct non-healthcare costs.
-
Productivity losses: productivity losses for the carer, productivity losses for the patients due to morbidity or mortality.
Some studies included social welfare benefits in the total societal cost. Since social welfare benefits are considered transfers from one group of people (taxpayers) to another group of people (social welfare beneficiaries), they do not impose any resource cost on society. Therefore, for those studies, we excluded social welfare benefits. However, we did include the administration cost of social welfare benefits, if reported separately, as this is an add-on cost caused by schizophrenia.
Sometimes, (a portion of) carer’s productivity losses can also be considered a transfer cost if carers are employed and entitled to paid leave to care for sick family members. However, since COI studies tend to not report details about carers’ employment status and their entitlements to a carer’s allowance, it was impossible for us to calculate the proportion of carers’ productivity losses that can be offset against social welfare. The impact of this limitation is discussed in Sect. 4.7.2.
We converted all cost estimates reported by included studies to $US, year 2015 values, using the Campbell and Cochrane Economics Methods Group Evidence for Policy and Practice Information and Coordination (CCMEMG-EPPI) Centre cost converter (http://eppi.ioe.ac.uk/costconversion/default.aspx) [17]. The price–year adjustment utilises a gross domestic product (GDP) deflator index, and the currency conversion utilises purchasing power parities (PPPs) for GDP. The GDP deflator index was used instead of a health service-specific deflator because the primary outcome of this review was the societal cost of schizophrenia, a large proportion of which is non-healthcare costs such as productivity losses.
2.4 Identification of Factors Associated with Higher Individual Costs
Factors associated with higher societal costs were extracted from the following parts of included studies: results of sensitivity analyses, subgroup analyses and regression analyses. Only generalizable factors were extracted. We excluded study-specific factors such as methods used to match the control group, inclusion of uninsured patients, stricter criteria for identifying schizophrenia cases from claim databases, resource use and transitional probabilities of each disease state (for modelling studies only). We also excluded the prevalence of schizophrenia as it does not affect the societal cost per patient.
3 Results
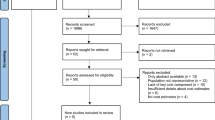
Table 2 in the ESM shows the detailed results of the literature search. After de-duplication, 3177 titles and abstracts were reviewed, and 197 full articles were retrieved. Of these, 19 satisfied predefined inclusion criteria and were included. The inter-reviewer agreement, measured by Cohen’s kappa was 0.32, which indicates fair agreement. Figure 1 illustrates the literature selection process.
We checked the bibliographies of nine published review/overview papers [18–26] to ensure all relevant COI studies were included in this review.
3.1 Study Characteristics
Table 1 summarizes the characteristics of included studies.
In terms of year of valuation, 68.4 % (13/19) of included studies evaluated the societal costs of schizophrenia after the year 2000. For the earlier six studies, five evaluated the cost of schizophrenia between year 1990 and 1999, and one evaluated the costs in 1985 [27].
A total of 47.4 % (9/19) of included studies were from Europe, 21.1 % (4/19) were from the USA, 15.8 % (3/19) were from Asia, 10.5 % (2/19) were Canada and 5.3 % (1/19) were from Australia.
The studies reported different diagnoses: schizophrenia (13 studies) [3, 4, 32–42]; schizophrenia and schizoaffective disorder (two studies) [28, 29]; schizophrenic disorder (one study) [30]; schizophrenia, schizophrenic disorder or other non-organic psychoses (one study) [31]; schizophrenia and schizophreniform (one study) [27]; and schizophrenia/schizophreniform and schizoaffective disorder (one study) [5].
The sample size of schizophrenia patients for cost calculation ranged from 288 to 15,164.
3.2 Data Sources and Methods Employed by Included Studies
Table 2 shows the data sources and methods employed by included studies.
A total of 15 studies used Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases and Related Health Problems (ICD) codes as diagnostic criteria for schizophrenia, whereas four studies [4, 35–37] did not specify the diagnostic criteria used.
The data sources for most included studies were survey results, national registries, hospital databases, claims databases and published literature.
All included studies were retrospective, except those by Mangalore and Knapp [5] and Sarlon et al. [41]. A total of 89.5 % (17/19) of included studies were prevalence-based studies, whereas only two (Guest and Cookson [36] and Langley-Hawthorn [37]) were incidence-based studies.
Of the included studies, 57.9 % (11/19) adopted the bottom-up approach, 21.1 % (4/19) adopted a mixed approach, 10.5 % (2/19) adopted an econometric approach and 10.5 % (2/19) adopted the top-down approach.
A total of 68.4 % (13/19) of included studies included carer productivity losses: nine adopted the opportunity cost approach [3, 28, 31, 32, 34, 36, 37, 39, 42], one adopted the replacement approach [5] and one adopted a mixed approach [38]. Two studies did not provide details about the method they used [27, 30].
All included studies considered patient lost productivity due to morbidity. All except one study [34] (which used the FCA) adopted the HCA.
Of the included studies, 68.4 % (13/19) included patient lost productivity due to mortality: eight adopted the HCA [3, 5, 27, 30, 31, 36, 38, 40] and four adopted the FCA [4, 34, 35, 42] and only one study [32] adopted the WTP approach.
A total of 63.2 % (12/19) reported results of SA.
3.3 Cost Estimates
Table 3 presents the societal costs of included studies by different cost components.
One included study [37] reported the lifetime cost for a newly diagnosed schizophrenia patient. The total lifetime cost was $US988,264 in Australia, which consisted of $US595,537 (60.3 %) in productivity losses, $US302,812 (30.6 %) direct healthcare costs, and $US89,915 (9.1 %) direct non-healthcare costs. The rest of the studies (n = 18) reported the annual societal cost per schizophrenia patient. The total societal cost varied from $US5818 in Thailand [28] to $US94,587 in Norway [29]. The ratio of societal cost per patient to 2015 GDP per capita varied from 37 % in Switzerland [39] to 214 % in the UK [5].
Direct healthcare costs contributed 13.5 % [42] to 64.1 % of the total societal cost [29]; whereas productivity losses made up 35.9 % [29] to 83.0 % [42]. The proportion of direct non-healthcare is the smallest, varying from 0.3 % [5] to 18.2 % [30].
In terms of absolute cost, the annual direct healthcare cost varied from $US1445 in Thailand [28] to $US60,630 in Norway [29]. Twelve studies reported annual direct non-healthcare costs, which varied from $US113 in Thailand [28] to US8237 in the USA [3]. The annual productivity losses varied from $US4,260 in Thailand [28] to $US62,431 in the UK [5].
The costing details for direct healthcare costs, direct non-healthcare costs and productivity losses are reported in Tables 3, 4, and 5, respectively.
3.3.1 Direct Health Costs
Table 4 reports the costing details of direct healthcare costs.
The annual inpatient cost was lowest in Thailand ($US732) [28] and highest in Norway ($US36,577) [29]. The annual outpatient/community cost was lowest in Spain ($US200) [38] and highest in Norway ($US21,569) [29], and the medicine cost was lowest in South Korea ($US100) [42] and highest in the USA ($US4455) [3].
3.3.2 Direct Non-Healthcare Costs
Table 5 reports the costing details of direct non-healthcare costs.
Of the included studies, 68.4 % (13/19) reported direct non-healthcare costs. The most commonly reported direct non-healthcare costs were sheltered home and legal costs. The annual cost of sheltered homes varied from $US173 in South Korea [42] to $US6056 in the UK [36]; whereas annual legal costs varied from $US17 in the UK [5] to $US2330 in the USA [3].
3.3.3 Productivity Losses
Table 6 reports the costing details of productivity losses.
Of the 19 included studies, 13 included carer lost productivity. The ratio of carer lost productivity to total productivity loss varied from 2.2 % in the UK [36] to 87.9 % in Spain [38]. Nine studies employed opportunity cost. The annual productivity losses for carers estimated by these nine studies varied from $US538 [36] in the UK to $US9263 in Germany [34], whereas the lifetime cost was estimated to be $US23,919 in Australia [37]. Only one UK study used replacement costs; they reported the highest productivity losses for carers to be $US9805 [5]. Two US studies did not specify they method used but reported that productivity losses for carers ranged from $US1422 [30] to $US1555 [27].
All included studies reported patient productivity losses due to morbidity. Only one study adopted the FCA; it reported the productivity loss for both morbidity and mortality as $US4188 (no separate results are reported) [34]. The other 18 studies used HCA; productivity losses due to morbidity varied from $US228 in Spain [38] to $US38,800 in the UK [5].
Of the 19 included studies, 13 reported patient productivity losses due to mortality. Productivity losses due to mortality reported by HCA studies varied from $US541 in Spain [38] to $US13,824 in the UK [5], whereas those estimated by FCA studies were much lower, varying from $US1 in Canada [4] to $US2039 in South Korea [42]. The only WTP study [32] reported productivity losses due to mortality of $US7,656 in Ireland.
3.4 Factors Associated with Higher Societal Costs
3.4.1 Factors Identified by Results of Subgroup Analysis
Four of the 19 included studies conducted subgroup analysis. Frey [34] reported that the societal cost of schizophrenia was highest in patients aged ≥65 years (€24,112), followed by those aged 44–65 years (€21,304), ≤25 years (€20,147) and 26–45 years (€19,930) (all year 2008 values). Mangalore and Knapp [5] explored the impact of setting on societal costs and reported that the annual societal costs per patient (in descending order) were institutional (£71,162), household (£42,342), homeless (£39,320) and prisons (£2,086) (year 2004 values). Ekman et al. [33] reported that patients with the lowest global functioning (global assessment of functioning score [GAF] <50) were associated with costs that were almost three times as large as those for patients in the highest GAF class (GAF ≥70). Other factors identified by Ekman et al. [33] included male sex and patients who had an inpatient stay during the costing year. One modelling study [36] examined the annual cost of newly diagnosed patients by ten disease courses and reported that the top three most expensive disease courses were long-term high-dependency course (£249,756), high-dependency followed by stable course (£222,679) and high-dependency followed by episodic course (£207,024). The least expensive disease course was first episode followed by recovery (£5596) (all 1997 values).
3.4.2 Factors Identified by Results of One-Way SA
Nine of the 19 included studies reported results of one-way SA. One study reported that their cost estimates were robust to all variables tested [31], whereas another study reported that their costs were robust to all variables tested except prevalence of schizophrenia [35]. One modelling study only tested study-specific factors, such as resource use and transitional probabilities of different disease states [36]. The factors identified by the other six studies are as follows:
-
Use of HCA instead of FCA [34].
-
Longer duration of friction period for work replacement (for FCA studies only) [42].
-
Higher unemployment rate for schizophrenia patients [42].
-
Use of market wage instead of minimum wage [28].
-
Higher productivity reduction ratio [42].
-
Higher family caregiver contact rate [3].
-
Zero or lower discount rate (which was used to discount projected future earnings lost due to premature mortality) [3, 42].
-
Inclusion of treatment cost for comorbidities of schizophrenia [39].
3.4.3 Factors Identified by Results of Regression Analysis
Two included studies [33, 41] reported factors associated with higher costs identified by regression analysis. Sarlon et al. [41] used between-effects regression models to examine the impact of socio-demographic and clinical variables on direct medical mental healthcare costs. The following factors were found to be significantly (p ≤ 0.05) related to higher direct medical costs: patient satisfaction with social relations, patient satisfaction with finance situations and relapse of symptoms during the follow-up period. However, these results should be interpreted with caution for several reasons. First, the R 2 value of Sarlon’s regression model was only 0.22, which means only 22 % of the total variance of the response data around its mean can be explained by the model. Second, comorbidities were not taken into account. Third, factors associated with higher direct medical costs are not necessarily associated with higher societal costs. In another study, Ekman et al. [33] used a multiple regression model to identify several factors associated with higher societal costs, including decrease in GAF, male sex, and unmarried status. However, the adjusted R 2 value of Ekman’s multiple regression was only 16 %; therefore, the results must be interpreted with caution.
4 Discussion
4.1 Summary of Results
This review highlighted a large economic burden of schizophrenia. The ratio of societal cost per schizophrenia patient to 2015 GDP per capita varied from 37 % in Switzerland [39] to 214 % in the UK [5]. This review also identified the main cost components of schizophrenia as well as factors associated with higher societal costs. Based on these findings, substantial savings could potentially be achieved by increasing investment in the following areas:
-
Occupational therapy for schizophrenia patients.
-
Service and support for carers.
-
Reducing patients’ hospital admission rates or length of stay (e.g. by transferring treatment from psychiatric hospitals to the community).
-
Preventing crimes (e.g. by providing community treatment programmes).
-
Preventing relapse.
-
Improving patients’ GAF scores.
Finally, this study reveals huge variations in the societal costs of schizophrenia among different countries. For example, the annual societal cost of schizophrenia per patient in Norway is 16.3 times as high as in Thailand. One reason for this variation might be differences in the state of the economy and differences in healthcare systems across countries, especially the availability of healthcare services. Another important reason is the wide range of methodological heterogeneity in COI studies, especially for productivity losses and direct non-healthcare costs. In the following sections, we focus on methodological heterogeneity and discuss its impact on societal cost estimates. Recommendations to improve the consistency and comparability of COI studies are provided in the last section of the Discussion.
4.2 Methodological Heterogeneity in Estimating Productivity Losses
Schizophrenia is associated with high rates of unemployment and job-related difficulties. From this review, it became apparent that productivity losses represent a large part of societal costs. However, two types of heterogeneity were identified among included studies: the first relates to the type of productivity loss being included; the second relates to how costs for each type of productivity losses are estimated. Ideally, COI studies for schizophrenia need to include three types of productivity loss: carer productivity losses, patient productivity loss due to morbidity, and patient productivity loss due to mortality. All included COI studies considered patient lost productivity due to morbidity; however, many ignored carer lost productivity or patient lost productivity due to mortality. According to a survey of 982 carers of schizophrenia patients conducted by the World Federation for Mental Health in Australia, Canada, Germany, France, Italy, Spain, the UK and the USA, carers can spend more than 10 h per week caring for their relative [43]. Sometimes carers are forced to give up their job or take time off work to provide care for a family member [5]. Therefore, it is very important to account for this ‘hidden’ work in COI studies. Patient productivity loss due to mortality is another type of cost component that is often ignored in COI studies. Schizophrenia is severely disabling and can sometimes result in a lifetime of lost productivity. Moreover, compared with the general population, the life expectancy for a schizophrenia patients is reduced by up to 20 years [2]. Therefore, ignoring patient productivity losses due to mortality may lead to an underestimation of the productivity losses.
Great variations were also identified for methods used in estimating productivity losses. Patient productivity loss due to morbidity or mortality can be estimated by HCA, FCA or WTP. Each method, if conducted properly, might be appropriate in some cases. However, the choice of method could have a dramatic impact on the cost estimates. For example, Goeree et al. [44] compared the results of the HCA and the FCA for schizophrenia patients and found the productivity losses estimated by the HCA to be nearly 70 times those estimated using the FCA. This is because the HCA counts the salary loss for the entire costing period, whereas the FCA only counts the salary loss for the friction period (the time to replace the former employee and train a new employee). Because of the potential for such wide variation, the method used to estimate indirect costs should be clearly reported. When data allow, SA should be conducted to examine the impact of costing methods on the final results.
Different approaches can also be used to estimate carer productivity loss, such as opportunity cost, replacement cost or market value and self-valuation, etc. Again, there is no clear consensus on which method is better than the others, but the method used in the study needs to be reported clearly.
Other than costing methods, the value of all three types of productivity losses are highly sensitive to the data and assumptions upon which they are based, such as employment rates for schizophrenia patents, which salary value to use (minimum salary, average salary, or real salary reported by the patients or their carers) and duration of friction period, all of which are subject to a high degree of uncertainty. Therefore, it is suggested that all productivity losses be reported in adequate detail, and be subject to SA.
4.3 Heterogeneity in Estimating Direct Non-Healthcare Cost
Although all included studies claimed to employ a societal perspective, six studies did not include any direct non-healthcare costs. For the 13 studies that did consider direct non-healthcare costs, there was great variation in the type of non-healthcare costs included. There are two potential explanations for this variation. The first explanation is omission of relevant cost components. The second is that direct non-healthcare costs of schizophrenia are very much context bound. Given the diversity of cultures, social structures and healthcare systems across countries, the structure of direct non-healthcare costs is likely to vary considerably. For example, in the past decades, many western countries have initiated a ‘deinstitutionalisation’ process by transferring curative treatment for the mentally ill from psychiatric hospitals to the community. A negative consequence of this process has been that patients with severe mental illness (SMI) who live in the community are at increased risk of violent crime. In the USA, it is reported that over a one-quarter of individuals with SMI accessing community mental health services in an inner-city area are victims of at least one violent crime per year, a proportion 11 times higher than the inner-city average [45]. The elevated victim rate holds for every category of crime, including rape/sexual assault, other violent assaults and personal and property theft. This could potentially explain why legal costs have been more commonly reported in COI studies of schizophrenia conducted in western countries (such as the USA and the UK) than in countries in which few attempts at deinstitutionalisation have been made, such as Japan [46].
Since direct non-healthcare costs are so context bound, it is not possible to provide a list of all important direct non-healthcare costs that is valid for all countries. For researchers working on COI studies for schizophrenia, it is important that all non-trivial direct non-healthcare costs be properly identified and included, based on the local context.
4.4 Heterogeneity in Conducting SA
In this review, the results of SAs reported by included COI studies have been used to determine factors associated with higher costs for schizophrenia. However, these results need to be interpreted with caution because of variations in how the SA has been conducted in the included studies.
Although it is recommended that SA be conducted for all important parameters and assumptions used in COI studies [8], only 63.2 % of included studies (12/19) reported results of SA. Even for those studies that did report results of SA, many only tested a few parameters or assumptions. For example, Frey [34] only conducted SA for two assumptions (use of HCA instead of FCA, and alternative methods of matching schizophrenia patients with control subjects) without testing the value for any parameters. Therefore, it is possible that some important cost drivers have been missed by this review, as they have not been tested by SA in included studies.
Another problem in the included studies was the different robustness criteria used when reporting SA results. It is generally considered that, if a small change in the value of a cost element’s parameter or assumption yields a large change in the overall cost estimate, then the results are considered sensitive to that parameter or assumption. However, there is no consensus on what percentage of change can be considered ‘small’ or ‘large’ for COI studies. Therefore, for this review, we only recorded factors reported to be associated with a large increase in the overall cost estimate. This approach could potentially cause inconsistent interpretation of SA results, as some parameters or assumptions that are reported to be cost drivers in one study might not meet the criteria for a cost driver in another study.
4.5 Recommendations for Good Practice for COI Studies
In terms of costing methodologies for COI studies, we recommend the following:
-
Social welfare benefits should not be included in societal costs. However, administration fees of social welfare benefits should be included.
-
Productivity losses should include patient productivity loss due to morbidity, patient productivity loss due to mortality and carer productivity loss.
-
All non-trivial direct non-healthcare costs should be properly identified and included in COI studies for schizophrenia, based on the local context. A non-exhaustive list includes the following: sheltered home, legal costs (incarceration, policing, legal costs and costs to victims of crime), administration fee for social welfare benefits, transport costs and private expenditure.
-
SA should be conducted to test all important parameters and key assumptions used in COI studies. A non-exhaustive list includes the following: different costing methods for productivity losses (HCA vs. FCA, opportunity cost vs. replacement cost, etc.); duration of friction period (for FCA studies only); different sources of employment rate and salary; and inclusion/exclusion of costs for treating comorbidities.
When reporting the results of COI studies, we recommend the following are reported clearly:
-
Disease specification. Ideally, relevant ICD or DSM codes should be provided.
-
Data sources and methods used to estimate costs for each cost component.
-
Carers’ employment status and their entitlements to carer’s allowance.
-
A detailed breakdown of each cost component that allows for recalculation and different levels of disaggregation.
4.6 Future Research
Of the 19 included studies, 17 adopted a prevalence-based approach, whereas only two included studies adopted an incidence-based approach [36, 37]. This might be because prevalence-based approaches only need to collect data for a fixed time period (usually 1 year), and nothing needs to be known or assumed about the future course of schizophrenia. If decision makers are only interested in taking a snapshot of the current disease burden of schizophrenia, then the prevalence-based approach would be sufficient. However, for decision makers interested in knowing how costs vary at different stages, and the potential savings that can be made from preventing schizophrenia, the incidence approach would be more appropriate [6, 47]. The two incidence-based COI studies identified by this review were both conducted before the year 2000 [36, 37]. Therefore, a new incidence-based COI study that uses the most recent epidemiological data and takes different disease courses into consideration is needed for schizophrenia.
4.7 Limitations
4.7.1 Limitations of COI Studies in General
For decision makers who are interested in reducing the financial burden of schizophrenia, it should be noted that, although COI studies are useful in highlighting the magnitude of financial burden of schizophrenia and attracting public attention, they do not provide information about efficiency of resource allocation. However, not all costs reported by COI studies are amenable to current available treatment [48]. Therefore, decision makers interested in reducing the financial burden of schizophrenia need to refer to other types of evidence, such as cost-effectiveness, cost-utility and cost-benefit analysis.
4.7.2 Limitations of this Review
This review only included studies that adopted a societal perspective, for two reasons. First, the societal perspective is the most comprehensive perspective [8] as it includes healthcare costs, non-healthcare direct costs and indirect costs. Therefore, COI studies conducted from a societal perspective are not limited to one particular group of decision makers, but can be useful to decision makers from different agencies or other social organisations. Since a societal perspective allows a complete analysis of all of the opportunity costs attributable to a disease, it can minimize the potential biases of narrower views [12]. Therefore, a societal perspective is often favoured by economists [9–11]. However, by excluding COI studies conducted from other perspectives, this review is likely to lose some information that might be valuable to particular groups, such as healthcare or third-party payers.
Another limitation of this review is that we did not deduct paid leave for carers from the total societal cost, because information about carers’ employment status and their entitlements to a carer’s allowance was lacking. This could lead to an overestimation of the total societal cost for schizophrenia. For example, in the USA, employees are entitled to use up to 12 weeks (480 h) of sick leave each leave year to provide care for a family member with a serious health condition. It is important that COI studies report the proportion of carer’s productivity losses that can be covered by social welfare, so this cost can be deducted from the total societal cost.
4.7.3 Limitations of Included COI Studies
The main methodological limitations of included studies and their impact on the total cost estimates were discussed in detail in Sects. 4.2–4.4. Other than those limitations, we note that some included studies were conducted a long time ago: five studies evaluated the cost of schizophrenia between 1990 and 1999, and one evaluated costs in 1985 [27]. Great changes have happened in recent years and in the wider economy, including diagnostic criteria for schizophrenia, treatment options and settings and mental health policies. All of these factors will affect the size and composition of the cost of schizophrenia. Therefore, the results of older studies need to be interpreted with great caution.
5 Conclusion
This study highlights the large economic burden and cost drivers of schizophrenia. It also brings to the fore the widespread methodological heterogeneity in published COI studies for schizophrenia. Based on the review findings, recommendations about good practice for schizophrenia COI studies have been suggested.
References
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141.
The Schizophrenia Commission. The abandoned illness: a report by the Schizophrenia Commission. London: Rethink Mental Illness; 2012.
Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, Moulis M, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–9.
Goeree R, Farahati F, Burke N, Blackhouse G, O’Reilly D, Pyne J, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin. 2005;21(12):2017–28.
Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10(1):23–41.
Rice DP. Cost of illness studies: what is good about them? Injury Prev. 2000;6(3):177–9.
Segel JE. Cost-of-illness studies: a primer. RTI-UNC Center of Excellence in Health Promotion Economics RTI International. 2006. p. 1–39.
Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29(8):653–71.
Drummond M, O’Brien B, Stoddart GL, et al. Methods for the economic evaluation of health care programmes, 2nd ed. Oxford: Oxford University Press; 1997
Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34(12 Suppl):DS197–199.
Sindelar J. Social costs of alcohol. J Drug Issues. 1998;28(3):763–80.
Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20.
Access Economics. Bipolar disorder: costs. An analysis of the burden of bipolar disorder and related suicide in Australia. Canberra: Report for SANE Australia; 2002.
Koopmanschap MA, Rutten FF, van Ineveld BM, van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ. 1995;14(2):171–89.
Gold MR. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535.
Ian S, Marcello M, James T. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6(1):51–9.
Andlin-Sobocki P, Rossler W. Cost of psychotic disorders in Europe. Eur J Neurol. 2005;12(Suppl 1):74–7.
Genduso LA, Haley JC. Cost of illness studies for schizophrenia: components, benefits, results, and implications. Am J Managed Care. 1997;3(6):873–7.
Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30(2):279–93.
Neil AL, Lewin TJ, Carr VJ. Allocation of resources and psychosis. Aust N Z J Psychiatry. 2003;37(1):15–23.
Tajima-Pozo K, de Castro Oller MJ, Lewczuk A, Montanes-Rada F. Understanding the direct and indirect costs of patients with schizophrenia. Version 2. F1000Res. 2015;4:182. doi:10.12688/f1000research.6699.2.
McEvoy JP. The costs of schizophrenia. J Clin Psychiatry. 2007;14:4–7.
Blomqvist AG, Leger PT, Hoch JS. The cost of schizophrenia: lessons from an international comparison. J Ment Health Policy Econ. 2006;9(4):177–83.
Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2015;29(2):63–76.
Rice DP. The economic impact of schizophrenia. J Clin Psychiatry. 1999;60(suppl. 1):4–6.
Rice DP, Miller LS. The economic burden of schizophrenia: Conceptual and methodological issues, and cost estimates. In: Moscarelli M, Rupp A, Sartorius N, editors. Handbook of mental health economics and health policy. Volume 1: schizophrenia. Oxford: Wiley; 1996. p. 321–334.
Phanthunane P, Whiteford H, Vos T, Bertram M. Economic burden of schizophrenia: empirical analyses from a survey in Thailand. J Ment Health Policy Econ. 2012;15(1):25–32.
Evensen S, Wisløff T, Lystad J, Bull H, Ueland T, Falkum E. Prevalence, employment rate, and cost of schizophrenia in a high-income welfare society: a population-based study using comprehensive health and welfare registers. Schizophr Bull. 2016;42(2):476–83.
Rice DP, Miller LS. Health economics and cost implications of anxiety and other mental disorders in the United States. Br J Psychiatry Suppl. 1998;34:4–9.
Desai PR, Lawson KA, Barner JC, Rascati KL. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. J Pharm Health Serv Res. 2013;4(4):187–94.
Behan C, Kennelly B, O’Callaghan E. The economic cost of schizophrenia in Ireland: a cost of illness study. Irish J Psychol Med. 2008;25(3):80–7.
Ekman M, Granstrom O, Omerov S, Jacob J, Landen M. The societal cost of schizophrenia in Sweden. J Ment Health Policy Econ. 2013;16(1):13–25.
Frey S. The economic burden of schizophrenia in Germany: a population-based retrospective cohort study using genetic matching. Eur Psychiatry. 2014;29(8):479–89. doi:10.1016/j.eurpsy.2014.04.003.
Goeree R, O’Brien BJ, Goering P, Blackhouse G, Agro K, Rhodes A, et al. The economic burden of schizophrenia in Canada. Can J Psychiatry. 1999;44(5):464–72.
Guest JF, Cookson RF. Cost of schizophrenia to UK Society. An incidence-based cost-of-illness model for the first 5 years following diagnosis. Pharmacoeconomics. 1999;15(6):597–10.
Langley-Hawthorne C. Modeling the lifetime costs of treating schizophrenia in Australia. Clin Ther. 1997;19(6):1470–95.
Oliva-Moreno J, Lopez-Bastida J, Osuna-Guerrero R, Montejo-Gonzalez AL, Duque-Gonzalez B. The costs of schizophrenia in Spain. Eur J Health Econ. 2006;7(3):182–8.
Pletscher M, Mattli R, von Wyl A, Reich O, Wieser S. The societal costs of schizophrenia in Switzerland. J Ment Health Policy Econ. 2015;18(2):93–103.
Sado M, Inagaki A, Koreki A, Knapp M, Kissane LA, Mimura M, et al. The cost of schizophrenia in Japan. Neuropsychiatr Dis Treat. 2013;9:787–98.
Sarlon E, Heider D, Millier A, et al. A prospective study of health care resource utilisation and selected costs of schizophrenia in France. BMC Health Serv Res. 2012;12:269–76.
Sung MC, Cho SJ, Hong JJ, Hahm BJ, Hyo JL, Park JI, et al. Economic burden of schizophrenia in South Korea. J Korean Med Sci. 2008;2:167–75.
World Federation for Mental Health. Keeping care complete fact sheet: international findings. 2013. Available from: http://www.supportingfamilies.org.nz/Libraries/Documents/Keeping_Care_Complete_Survey.sflb.ashx. Accessed Aug 2016.
Goeree R, O’Brien BJ, Blackhouse G, Agro K, Goering P. The valuation of productivity costs due to premature mortality: a comparison of the human-capital and friction-cost methods for schizophrenia. Can J Psychiatry. 1999;44(5):455–63.
Teplin LA, McClelland GM, Abram KM, Weiner DA. Crime victimization in adults with severe mental illness: comparison with the national crime victimization survey. Arch Gen Psychiatry. 2005;62(8):911–21. doi:10.1001/archpsyc.62.8.911.
Oshima I, Mino Y, Inomata Y. Institutionalisation and schizophrenia in Japan: social environments and negative symptoms. Nationwide survey of in-patients. Br J Psychiatry. 2003;183:50–6. doi:10.1192/bjp.183.1.50.
Drummond M. Cost-of-illness studies: a major headache? Pharmacoeconomics. 1992;2(1):1–4.
Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. BMJ. 2000;320(7245):1335.
Acknowledgments
The authors thank Dr. James Shearer for proof reading this manuscript.
Author contributions
Huajie Jin conducted the systematic review and led the writing of the paper. Iris Mosweu performed the first round (title and abstract) screening and the second round (full-text) sifting as the second reviewer, and contributed to the writing of the paper. Huajie Jin will serve as a guarantor for the overall content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this review.
Conflict of interest
HJ receives salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London. IM receives salary support from King’s College London. The views expressed are those of the authors and not necessarily those of the NIHR. HJ and IM have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, H., Mosweu, I. The Societal Cost of Schizophrenia: A Systematic Review. PharmacoEconomics 35, 25–42 (2017). https://doi.org/10.1007/s40273-016-0444-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0444-6