Abstract
Background
Schizophrenia imposes a substantial economic burden on society. This updated systematic review aims to collate the latest societal cost of schizophrenia across countries by reviewing recent cost-of-illness (COI) studies.
Methods
An electronic search was conducted across several databases (MEDLINE, Embase, PsycINFO, Cochrane Database of Systematic Reviews, Health Management Information Consortium, and System for Information on Grey Literature) to identify COI studies published from 2016 to 2022. Two independent reviewers selected studies for inclusion. The cost components and estimates reported by included studies were descriptively summarised. All costs were converted to US dollars (2022 values). Study quality was assessed using a checklist adapted from Larg & Moss.
Results
Twenty-four studies were included (5 from the update review and 19 from the original review), of which only two were conducted for low- and middle-income countries (LMICs). Widespread methodological heterogeneity among included studies was observed. The annual societal cost per person varied from US$819 in Nigeria to US$94,587 in Norway. Productivity losses accounted for 32–83% of the overall societal cost, whilst direct healthcare cost made up 11–87%. The reporting quality of included studies varied.
Conclusion
This review highlights the substantial economic burden of schizophrenia and a lack of COI studies for LMICs. Recommendations on future research, and good practices on improving the methodological and reporting quality of COI research for schizophrenia are provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
This updated review highlights the substantial economic burden of schizophrenia across countries. The annual societal cost of schizophrenia per person varied from US$819 in Nigeria to US$94,587 in Norway. |
Productivity losses accounted for 32–83% of the overall societal cost. Substantial savings could potentially be achieved by providing vocational rehabilitation to schizophrenia patients and support to their caregivers. |
There is a lack of COI studies of schizophrenia in low- and middle-income countries (LMICs). Since the results of economic studies may not be transferable between different countries, it is recommended that more COI studies be conducted for LMICs. |
Recommendations on future research, and good practices for improving the methodological and reporting quality of future COI studies are provided. |
1 Introduction
Schizophrenia is a long-term illness that obstructs one's capacity to reason rationally, manage emotions, make decisions, and interact with others. Compared with the general population, patients with schizophrenia are at a higher risk of developing weight gain, cardiovascular disease, diabetes mellitus and substance use disorders [1, 2]. In addition, compared with the general population, people with schizophrenia are more likely to be homeless, jobless or in poverty [3]. As a result, the economic burden associated with schizophrenia is substantial not only for the patients but also for their families, caregivers and the larger society [4]. For example, in the US, the societal cost of schizophrenia was reported to be US$62.7 billion in 2002 [5] and US$281.6 billion in 2020 [6].
Cost-of-illness (COI) studies provide a summary of the economic burden of a specific disease to the healthcare system or society. A COI study can be conducted from different costing perspectives, such as the healthcare system, third-party payer (e.g., insurance company), or the society as a whole. The choice of the costing perspective may have a substantial impact on the cost estimates. COI studies can be prevalence-based or incidence-based. The prevalence-based method calculates the financial impact of an illness over a given time frame, often 6–12 months; whilst the incidence-based method calculates the lifetime cost from the start of an illness to conclusion. There are several methods to estimate expenses in COI studies. The bottom-up method (‘person-based’) estimates the average cost per patient and multiplies it by the disease prevalence; whilst the top-down approach (‘population-based’) uses a population-attributable fraction to assign a percentage of the total expenditure to patients with the disease of interest. The econometric approach compares the cost difference in patients with and without the disease of interest. A COI study can consider three different categories of productivity losses including caregivers’ productivity losses, patients’ productivity losses due to morbidity, and patients’ productivity losses due to premature mortality. Two approaches have been commonly used for estimating carers’ productivity losses: replacement method and opportunity cost method. Whilst the replacement method values unpaid labour at the market price that would need to be paid for a substitute to complete the task, the opportunity cost method estimates the paid or unpaid work it disrupts as measured by the salary the individual would make if in paid employment. Three main methods have been commonly used for estimating patient productivity losses due to morbidity or premature mortality: the human capital approach (HCA), the friction cost approach (FCA), and the willingness-to-pay (WTP) method. The HCA assigns a monetary value to the predicted productivity losses of an illness that are avoided as a result of a health intervention. The FCA takes into account the projected productivity losses during the ‘friction period’, or the time required to replace an employee that is ill. The WTP method measures the amount an individual or the society would pay to reduce the incidence or the mortality of the disease of interest. There is no consensus on which method is superior to the others.
A previous systematic review of COI studies for schizophrenia conducted by Jin and Mosweu [7] summarised the societal cost of schizophrenia across countries. That review identified 19 COI studies and reported that the societal cost of schizophrenia per patient ranges from US$5818 in Thailand [8] to US$94,587 in Norway [9]. However, the literature searches of Jin and Mosweu were conducted in 2016 and all cost estimates were reported in 2015 US dollars. Several new COI studies have been published since 2016, thus warranting a new systematic review of the subject and an uplift of the cost estimates to the current year value. In addition, Jin and Mosweu did not assess the quality of identified COI studies.
To fill this gap, our study aims to conduct an updated systematic review of COI studies for schizophrenia. Specific objectives were as follows:
-
1.
To identify new COI studies that report the societal cost of schizophrenia published from 2016 to 2022;
-
2.
To uplift the cost estimates reported by COI studies included in the original review to the current year value;
-
3.
To assess the reporting quality of all COI studies and to provide recommendations for good practices for future COI studies.
2 Methods
This review was conducted according to the PRISMA standards for reporting systematic reviews and meta-analyses of studies evaluating healthcare treatments [10]. The protocol for this review was registered on PROSPERO (CRD42022328723).
2.1 Inclusion/Exclusion Criteria
The inclusion and exclusion criteria were reported in detail in the original review [7] and are briefly summarised below. Studies were included if they met both of the following criteria: (1) original COI studies which adopted a societal perspective (including both direct costs and productivity losses); (2) children/young people/adults with a diagnosis of schizophrenia or psychosis. Studies were excluded if they met any of the following criteria: (1) cost-effectiveness analysis; (2) the cost of schizophrenia was not reported and could not be derived; (3) focusing on the cost of just one health state of schizophrenia, such as relapse; (4) reviews, commentaries, letters, editorials, or abstracts; (5) published before 1996; and (6) not reported in English. All studies included in the original review [7] were retained in the current review.
2.2 Search Strategy
A literature search was conducted on 22 June 2022 to identify COI studies of schizophrenia published since 2016. Six databases were searched, including MEDLINE, Embase, PsycINFO, the Cochrane Database of Systematic Reviews, the Health Management Information Consortium (HMIC) and OpenGrey. We used the same search strategy as the original review [7], which included medical terms such as schizophrenia, psychosis, delusion, hallucination, catatonia as well as health economic terms such as cost of illness, healthcare cost, hospital cost, productivity loss and burden. The detailed search strategy is reported in Online Resource 1, Appendix 1 (see Electronic Supplementary Material).
2.3 Selection of Studies
The initial screening of the literature search results was carried out by two independent reviewers (CL and XZ) by comparing the titles and abstracts to the inclusion criteria. Final inclusion of studies in the review was determined by agreement of both reviewers, with any disagreements resolved by discussion with a third reviewer (HJ).
To confirm that all pertinent COI studies had been found by the search approach employed, the bibliographies of published review/overview articles obtained from the search were also examined.
2.4 Data Extraction and Analysis
Data were extracted by one reviewer (CL) and checked by a second reviewer (XZ), with disagreements resolved by discussion. The following information was extracted from all included studies: author; year; country; patient/disease specification; costing perspective and methods; cost estimates by component; and information on quality criteria set out by the COI checklist (see the next section for details). It was noted that different studies employed different definitions for ‘direct healthcare costs’, ‘direct non-healthcare costs’ and ‘informal care/productivity losses’. For example, informal care (productivity losses for the carer) was considered as direct non-healthcare costs by some studies but was considered as indirect costs by other studies. To keep consistency, the cost components reported by included studies were reclassified by the authors of this review, according to the following definitions:
-
Direct healthcare costs include the cost of inpatient, outpatient, and community service, as well as the medicine costs and any other healthcare system-related costs.
-
Direct non-healthcare costs include the costs of sheltered housing, legal costs, the expense of administering social welfare payments, transportation expenditures, private expenses, and any other direct non-healthcare costs.
-
Productivity losses include productivity losses for the schizophrenia patients due to morbidity or premature mortality, and productivity losses for the caregivers.
Social welfare benefits were excluded by the authors, as such costs are considered as transfers from one group of people (taxpayers) to another group of people (social welfare beneficiaries), and thus do not impose any cost on society.
The cost estimates reported by all included studies were converted to 2022 US dollars using the Campbell and Cochrane Economics Methods Group Evidence for Policy and Practice Information and Coordination (CCMEMG-EPPI) Centre cost converter (http://eppi.ioe.ac.uk/costconversion/default.aspx) [11].
2.5 Quality Assessment
The reporting quality of all included studies was assessed using a checklist adapted from Larg and Moss's guide to critical evaluation of COI studies [12]. Elements explored include the reporting of the costing perspective, epidemiological approach, study question, methods of valuation of different cost components, estimation of intangible cost, description of statistical analyses, inclusion of sensitivity analysis and reporting of the detailed cost components. The quality assessment was conducted by two independent reviewers (CL and XZ), with any disagreements resolved by discussion with a third reviewer (HJ).
3 Results
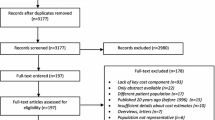
After de-duplication, the updated search identified 1699 titles and abstracts, of which full texts were obtained for 152 of them. Five newly identified studies met the requirements for inclusion and were added to the 19 studies which were included in the original review. The Cohen's kappa value for the inter-reviewer agreement was 0.76, which is considered to be a substantial level of agreement [13]. The PRISMA flow chart [14] is shown in Fig. 1.
3.1 Study Characteristics
The characteristics of all included studies (including five newly identified studies [15,16,17,18,19] and 19 studies included in the original review) are presented in Table 1. Half of included studies (12/24) originated in Europe, followed by 25% (6/24) in America, 17% (4/24) in Asia, 4% (1/24) in Africa and 4% (1/24) in Australia. All studies were conducted for high-income countries except two: one for Nigeria [18] and one for Malaysia [19]; both of which were identified during the update review. A range of data sources were used such as patient registries, including hospital/clinic databases and published literature. All included studies were retrospective, except Hastrup et al. [15], Mangalore and Knapp [20], and Sarlon et al. [21]. Of the included studies, 92% (22/24) were prevalence-based; the rest are incidence-based (2/24, 8%) [22, 23] and activity-costing based (4%) [16]. Among all included studies, 14/24 (58%) used a bottom-up approach, 6/24 (25%) used a mixed approach, 2/24 (8%) used an econometric approach [24, 25], and 2/24 (8%) used a top-down approach [26, 27].
Nine studies (9/24, 38%) did not include or report caregivers’ productivity losses. In the other 15 studies, the methods used to estimate caregivers’ productivity losses varied, including the opportunity cost approach (9/15, 60%), human capital approach (HCA) (4/15, 27%) [15, 17,18,19], the mixed approach (1/15, 7%) [28], and the replacement approach (1/15, 7%) [20].
All included studies considered patients’ lost productivity due to morbidity, and all studies used HCA to estimate patients’ lost productivity due to morbidity except one study which used the friction cost approach (FCA) (1/24, 4%) [24]. Ten studies (10/24, 42%) did not include or report patients’ lost productivity due to premature mortality. In the other 14 studies, the methods used to estimate patients’ lost productivity due to premature mortality include HCA (9/14, 64%), FCA (4/14, 29%) [27, 29, 30], and the willingness to pay method (1/14, 7%) [31]. Only 58% (14/24) of included studies reported results of sensitivity analysis. More details about the included studies, including year of valuation, sample size, and details about patients’ diagnosis, age and sex are reported in the ESM, Appendix 2.
3.2 Cost Estimates
The annual healthcare cost and societal cost of schizophrenia per patient by country are shown in Figs. 2 and 3, respectively. The annual healthcare cost of schizophrenia per patient varied from US$350 in Nigeria [18] to US$76,019 in Norway [9]. The annual societal cost of schizophrenia per patient varied from US$819 in Nigeria [18] to US$118,595 in Norway [9]. Generally speaking, north Europe incurred the highest societal cost, followed by the UK, the US, Japan, central Europe, and Canada. The ratio of societal cost per patient to 2022 GDP per capita varied from 35% in Nigeria [18] to 237% in the UK [20]. The healthcare and societal costs reported by individual studies are presented in the ESM, Appendix 3, Table 2.
Figure 4 shows the societal cost of schizophrenia by cost component. Productivity losses accounted for 32% [16] to 83% [40] of the overall societal cost, whilst direct healthcare costs made up 11% [16] to 87% [15]. The proportion of direct non-healthcare costs ranges from 0.3% [20] to 57% [16]. The cost estimates of each cost component are briefly summarised below and presented in detail in the ESM, Appendix 3, Tables 3–5.
3.2.1 Direct Healthcare Costs
The annual direct healthcare costs ranged from US$350 in Nigeria [18] to US$76,019 in Norway [9]. The cost of inpatient service is higher than the cost of outpatient/community service in all countries assessed except the US [25, 32], Ireland [31], and France [21]. The medication cost takes up <30% of direct healthcare costs in all countries except Italy (35%) [16], the US (37%) [32], and Nigeria (51%) [18]. The annual medication cost was lowest in Nigeria [18] (US$179) and highest in the US [5] (US$4455).
3.2.2 Direct Non-Healthcare Costs
Direct non-healthcare costs varied from US$113 in Malaysia [19] to US$23,857 in Italy [16]. The types of direct non-healthcare costs of schizophrenia considered by COI studies include legal costs, sheltered home, programme monitoring and evaluation, data analysis, and repair to property. Legal cost has been more commonly reported in COI studies conducted for western countries (e.g., the US, UK, and Canada) compared with those COI studies conducted for Asian countries (e.g., Japan and Malaysia).
3.2.3 Productivity Losses
The annual productivity losses due to schizophrenia ranged from US$346 in Nigeria [18] to US$62,431 in the UK [20]. For those 15 studies which considered carer’s lost productivity, the ratio of carer’s lost productivity to the total productivity loss ranged from 2% in the UK [20] to 95% in Italy [16]. The annual productivity losses for carers varied from US$120 in Nigeria [18] to US$12,542 in Italy [16]. Patients’ productivity losses varied from US$226 in Nigeria [18] to US$48,648 in the UK [20]. Of those 14 studies which considered a patient’s lost productivity to premature mortality, the estimated productivity losses due to premature mortality ranged from US$1 in Canada [34] to US$17,333 in the UK [20].
3.3 Quality Assessment
The performance of included studies on all items of the checklist is shown in Fig. 5. Items where all studies performed well (24/24, 100%) included reporting of the costing perspective, study question, and valuation methods of healthcare resource. Items where studies performed particularly badly included reporting of included intangible cost (only one study reported it, 1/24, 4%), results of sensitivity analysis (14/24, 58%), and valuation methods of productivity losses (17/24, 71%). The quality assessment results of each individual study are reported in ESM 1, Appendix 4.
4 Discussion
4.1 Interpretation of Results
This review provides an update on the societal cost of schizophrenia per person, ranging from US$819 in Nigeria to US$94,587 in Norway (the range reported by the original review is US$5818 in Thailand to US$94,587 in Norway). Of the 19 COI studies included in the original review, all of them were conducted for high-income countries. Of the five newly identified COI studies, two of them were conducted for low- and middle-income countries (LMICs). As expected, the societal cost of schizophrenia in high-income countries is much higher than the cost in LMICs: the annual societal cost of schizophrenia in Norway [9] is 144 times higher than the cost in Nigeria [18]. However, even in LMICs, the economic burden of schizophrenia is still substantial: in Nigeria and Malaysia, the ratio of societal cost per schizophrenia patient to 2022 local GDP per capita is 35% [18] and 50% [19], respectively.
Generally speaking, productivity losses are the driver of the societal cost of schizophrenia, followed by direct healthcare cost, and direct non-healthcare cost. Whilst all included studies considered patients’ productivity losses due to morbidity, only about 40% of them also considered patients’ productivity losses due to premature mortality and productivity losses for the caregivers. If all relevant productivity losses were included in the analysis, productivity losses could take up an even higher proportion of the societal cost of schizophrenia.
Within the direct healthcare cost, inpatient care remains the single most expensive component in most countries. However, in some western countries where deinstitutionalisation (i.e. releasing institutionalised individuals with mental health disorders from institutional care, such as a psychiatric hospital to care in the community) has been introduced since the 1950s, such as the US [25, 32], Ireland [31], and France [21], the cost of outpatient care/community service started to exceed the cost of inpatient care.
A quarter of the included studies did not include any direct non-healthcare costs, despite the fact that all of them claimed to use a societal costing perspective. Of those studies which did consider direct non-healthcare costs, there is great variation in terms of what cost components were included. This might be caused by the diversity of cultures, social structures and health-care systems across countries. For example, it was noticed that legal cost has been more commonly reported in COI studies conducted in western countries (such as the US, UK, and Canada) where deinstitutionalisation has been widely implemented, compared with those countries where few attempts have been made, such as Japan and Malaysia. This might be because, compared with those institutionalised patients, patients who reside in the community may be at an increased risk of both committing and being the victim of a violent crime, such as rape/sexual assault, personal and property theft, and other violent attacks, all of which would increase the cost to the criminal justice system. In the US, it was estimated that deinstitutionalisation resulted in 3.2 million people with untreated serious mental illness living in the community, who are responsible for 10% of all homicides and 50% of all mass killings [41]. Another study from the US reported that for those people with severe mental illness using community mental health services in an inner-city area, over 25% of them were victims of at least one violent crime per year, a proportion which was eleven times higher than the inner-city average [42].
Great variance in cost estimates were also observed in COI studies conducted for the same country. For example, both Latorre et al. [16] and Marcellusi et al. [17] used modelling methods to assess the societal cost of schizophrenia in Italy during overlapping years (2010–2018 vs 2002–2016). However, the annual societal cost reported by Latorre et al. [16] is about three times as high as the cost reported by Marcellusi et al. [17] (US$41,827 vs US$13,022). This was mainly because the costs of sheltered home (US$23,109) and carer’s lost productivity (US$12,542) reported by Latorre et al. [16] were much higher than the costs of sheltered home (US$4184) and carer’s lost productivity (US$700) reported by Marcellusi et al. [17]. Latorre et al. [16] did not report the details of the data sources used to estimate the cost of sheltered home or carer’s lost productivity; it only reported that “Goods and services use was assessed from medical records (e.g., patient's usage of a specific drug) or focus group (i.e., a team of five experts including the director of the practice and psychiatrists) indication when objective data were not available.” The medical records used in Latorre et al. [16] were obtained from 523 schizophrenia patients in south Italy, with a mean age of 51.5 years (±13.3 years) and a mean duration of illness of 19.7 years (±13.3 years). Marcellusi et al. [17] reported that their cost of sheltered home and carer’s lost productivity were estimated based on the results of a longitudinal study [43]. It was noticed that the patients recruited in the longitudinal study [43] were on average 20 years younger and with 16 years shorter duration of illness compared with the patients recruited by Latorre et al. [16]. This might explain why the costs of sheltered home and carer’s lost productivity reported by Latorre et al. [16] were much higher than the costs reported by Marcellusi et al. [17]. In addition, methodological heterogeneity might also contribute to the great variation in cost estimate. For example, Latorre et al. [16] used a mix of top-down and bottom-up approaches for estimating resource use, whilst Marcellusi et al. [17] used the bottom-up approach. Whilst there is no consensus on which method is superior, there is evidence showing that use of different approaches could result in very different cost estimates [44]. Therefore, it is important for COI studies to clearly report their costing methods, data sources, and patient characteristics to help with interpretation of their results.
4.2 Implications for Policy Making and Future Research
This review found that in most countries, productivity losses started to overtake direct healthcare cost to become the single most expensive component of the societal cost of schizophrenia. It was reported that up to 97.5% of schizophrenia patients may want some type of work role (e.g. volunteering or paid employment) [45]. However, the employment rate of schizophrenia in most western countries is only around 10–20% [46]; and 53% of schizophrenia patients stated they had not received any support in obtaining work [45]. Caregivers’ productivity is also affected as they often have to reduce their working hours or take a leave of absence to look after the patients. In some of the included studies, the productivity losses for caregivers have been shown to be higher than the cost of productivity loss borne by schizophrenic patients themselves. Substantial savings could potentially be achieved by providing vocational rehabilitation to the schizophrenia patients and support to their caregivers.
It should be noted that whilst COI studies are helpful in highlighting the magnitude of economic burden of an illness and identifying the cost drivers, they do not consider the health outcomes of an intervention and therefore cannot be used directly to inform resource allocation decisions for a particular intervention, that is, which vocational rehabilitation is most cost effective for schizophrenia patients and thus should be funded [47]. Such decisions need to be informed by cost-effectiveness analysis (CEA), which examines both the cost and health outcomes of one or more interventions. It is recommended that more CEAs need to be conducted to assess the cost effectiveness of interventions that can improve the employment status for schizophrenia patients and their caregivers. The cost estimates reported by the COI studies identified in this review can be used to parametrise such CEAs.
This review also highlights a lack of COI studies of schizophrenia in LMICs. Due to the differences in local economic situations and healthcare systems, it is generally agreed among economists that the results of economic studies may not be transferable between different countries [48]. Therefore, the societal cost reported by high-income countries cannot be used to directly inform policy making or parametrise CEAs in LMICs. It is recommended that more COI and CEA studies of schizophrenia needed to be conducted for LMICs.
4.3 Recommendations for Good Practice for COI Studies of Schizophrenia
Based on the results of quality assessment conducted as part of this updated review, as well as the guide for COI studies suggested by Larg and Moss [12], good practices for conducting and reporting COI studies of schizophrenia are derived and summarised in Table 2. The suggested good practices cover both costing methods and reporting of COI studies. It is recommended that the suggested good practices should be used by health economists in conjunction with their own judgement, taking into consideration of the local context and practical resource constrains.
4.4 Strengths and Limitations
This updated review has several strengths. Firstly, it identifies the latest COI studies published since 2016. Secondly, the cost estimates reported by all included studies were uplifted and converted to 2022 US dollars and presented graphically on maps. Thirdly, the original review did not assess the quality of identified COI studies. In this updated review, the reporting quality all of 24 included studies was assessed using a checklist adapted from Larg and Moss [44]. Finally, based on the findings of this review and results of the quality assessment, recommendations on future research, and good practice for improving the methodological and reporting quality of future COI studies are provided.
This review is subject to two main limitations. Firstly, as an updated review, we used the same inclusion/exclusion criteria as the original review and therefore only included studies that undertook a societal costing perspective. A societal perspective is often favoured by economists as it is most comprehensive and can provide useful information to decision makers from different sectors. However, COI studies which are conducted for a narrower perspective (e.g., healthcare system) could also provide valuable information to decision makers from a particular sector. Secondly, where there is more than one study reporting the cost of schizophrenia for the same country, we used the cost estimate reported by the most recent study when plotting the cost of schizophrenia across different countries on a map. It was acknowledged that the cost reported by the most recent study might not be more accurate than the cost reported by older studies. However, considering the rapid changes in treatment options, care pathways and health policies for schizophrenia across the world, we believe the costs reported by the most recent studies are more likely to reflect the economic burden of schizophrenia in current practice. In addition, our result of quality assessment indicate that the reporting quality of more recent studies is generally better than older studies.
5 Conclusion
This review highlights the substantial economic burden of schizophrenia across countries and a lack of COI studies for LMICs. Productivity losses accounted for 32–83% of the overall societal cost of schizophrenia. Great cost variation has been observed both across and within countries, which might be caused by differences in local economic state and healthcare systems, and widespread methodological heterogeneity among COI studies. Recommendations on future research, and good practices for improving the methodological and reporting quality of future COI studies are provided.
References
Wander C. Schizophrenia: opportunities to improve outcomes and reduce economic burden through managed care. Am J Manag Care. 2020;26:S62–8.
Abdullah HM, Azeb Shahul H, Hwang MY, Ferrando S. Comorbidity in Schizophrenia: conceptual issues and clinical management. Focus (Madison). 2020;18:386–90.
Verbeeck W. Risk of homelessness was increased in schizophrenia when 3 factors were present. Evid Based Ment Health. 1999;2:122–122.
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of Schizophrenia. PLoS Med. 2005;2:e141.
Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, Moulis M, et al. The economic burden of Schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66:1122–9.
Canady VA. New reports reveals billions in cost of schizophrenia. Ment Heal Wkly. 2021;31:7–8.
Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics. 2017;35:25–42.
Phanthunane P, Whiteford H, Vos T, Bertram M. Economic burden of schizophrenia: empirical analyses from a survey in Thailand. J Ment Health Policy Econ. 2012;15:25–32.
Evensen S, Wisløff T, Lystad JU, Bull H, Ueland T, Falkum E. Prevalence, employment rate, and cost of schizophrenia in a high-income welfare society: a population-based study using comprehensive health and welfare registers. Schizophr Bull. 2016;42:476–83.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535.
Shemilt I, James T, Marcello M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6:51–9.
Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29:653–71.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–82.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71.
Hastrup LH, Simonsen E, Ibsen R, Kjellberg J, Jennum P. Societal costs of Schizophrenia in Denmark: a nationwide matched controlled study of patients and spouses before and after initial diagnosis. Schizophr Bull. 2020;46:68–77.
Latorre V, Messeni Petruzzelli A, Uva AE, Ranaudo C, Semisa D. Unveiling the actual cost of Schizophrenia: an activity-based costing (ABC) approach. Int J Health Plan Manag. 2022;37:1366–80.
Marcellusi A, Fabiano G, Viti R, Francesa Morel PC, Nicolò G, Siracusano A, et al. Economic burden of schizophrenia in Italy: a probabilistic cost of illness analysis. BMJ Open. 2018;8: e018359.
Oloniniyi IO, Akinsulore A, Aloba OO, Mapayi BM, Oginni OA, Makanjuola R. Economic cost of Schizophrenia in a Nigerian Teaching Hospital. J Neurosci Rural Pract. 2019;10:39–47.
Teoh SL, Chong HY, Abdul Aziz S, Chemi N, Othman AR, Md Zaki N, et al. The economic burden of schizophrenia in Malaysia. Neuropsychiatr Dis Treat. 2017;13:1979–87.
Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10:23–41.
Sarlon E, Heider D, Millier A, Azorin J-M, König H-H, Hansen K, et al. A prospective study of health care resource utilisation and selected costs of schizophrenia in France. BMC Health Serv Res. 2012;12:269.
Guest JF, Cookson RF. Cost of Schizophrenia to UK Society. Pharmacoeconomics. 1999;15:597–610.
Langley-Hawthorne C. Modeling the lifetime costs of treating schizophrenia in Australia. Clin Ther. 1997;19:1470–95.
Frey S. The economic burden of schizophrenia in Germany: a population-based retrospective cohort study using genetic matching. Eur Psychiatry [Internet]. 2014;29:479–89. https://www.cambridge.org/core/product/identifier/S0924933800244659/type/journal_article
Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, Moulis M, et al. The Economic Burden of Schizophrenia in the United States in 2002. J Clin Psychiatry [Internet]. 2005;66:1122–9. Available from: http://article.psychiatrist.com/?ContentType=START&ID=10001442
Evensen S, Wisløff T, Lystad JU, Bull H, Ueland T, Falkum E. Prevalence, employment rate, and cost of schizophrenia in a high-income welfare society: a population-based study using comprehensive health and welfare registers. Schizophr Bull [Internet]. 2016;42:476–83. https://doi.org/10.1093/schbul/sbv141.
Sung MC, Cho S-J, Jeon HJ, Hahm B-J, Lee HJ, Park J-I, et al. Economic burden of Schizophrenia in South Korea. J Korean Med Sci [Internet]. 2008;23:167. https://doi.org/10.3346/jkms.2008.23.2.167.
Oliva-Moreno J, López-Bastida J, Osuna-Guerrero R, Montejo-González AL, Duque-González B. The costs of schizophrenia in Spain. Eur J Health Econ. 2006;7:182–8.
Frey S. The economic burden of schizophrenia in Germany: a population-based retrospective cohort study using genetic matching. Eur Psychiatry. 2014;29:479–89.
Goeree R, Farahati F, Burke N, Blackhouse G, O’Reilly D, Pyne J, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin [Internet]. 2005;21:2017–28. https://doi.org/10.1185/030079905X75087.
Behan C, Kennelly B, O’Callaghan E. The economic cost of schizophrenia in Ireland: a cost of illness study. Ir J Psychol Med. 2008;25:80–7.
Desai PR, Lawson KA, Barner JC, Rascati KL. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. J Pharm Health Serv Res. 2013;4:187–94.
Ekman M, Granstrom O, Omerov S, Jacob J, Landen M. The societal cost of schizophrenia in Sweden. J Ment Health Policy Econ. 2013;16:13–25.
Goeree R, Farahati F, Burke N, Blackhouse G, O’Reilly D, Pyne J, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin. 2005;21:2017–28.
Goeree R, O’Brien BJ, Goering P, Blackhouse G, Agro K, Rhodes A, et al. The economic burden of Schizophrenia in Canada. Can J Psychiatry. 1999;44:464–72.
Pletscher M, Mattli R, von Wyl A, Reich O, Wieser S. The societal costs of Schizophrenia in Switzerland. J Ment Health Policy Econ. 2015;18:93–103.
Rice DP, Miller LS. Health economics and cost implications of anxiety and other mental disorders in the United States. Br J Psychiatry. 1998;173:4–9.
Rice DP, Miller LS. The economic burden of schizophrenia: Conceptual and methodological issues, and cost estimates. Handbook of mental health economics and health policy. 1996.
Sado M, Inagaki A, Koreki A, Knapp, Kissane LA, Mimura, et al. The cost of schizophrenia in Japan. Neuropsychiatr Dis Treat. 2013;787.
Sung MC, Cho S-J, Jeon HJ, Hahm B-J, Lee HJ, Park J-I, et al. Economic Burden of Schizophrenia in South Korea. J Korean Med Sci. 2008;23:167.
Fuller TE. Deinstitutionalization and the rise of violence. CNS Spectr. 2015;20:207–14.
Teplin LA, McClelland GM, Abram KM, Weiner DA. Crime victimization in adults with severe mental illness. Arch Gen Psychiatry. 2005;62:911.
Cortesi PA, Mencacci C, Luigi F, Pirfo E, Berto P, Sturkenboom MC, et al. Compliance, persistence, costs and quality of life in young patients treated with antipsychotic drugs: results from the COMETA study. BMC Psychiatry. 2013;13:98.
Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics [Internet]. 2011;29:653–71. http://www.ncbi.nlm.nih.gov/pubmed/21604822
Seebohm P, Secker J. New Thinking about Mental Health and Employment. What do service users want? Oxford: Radcliffe Publishing; 2005.
Marwaha S, Johnson S. Schizophrenia and employment. Soc Psychiatry Psychiatr Epidemiol. 2004;39:337–49.
Byford S. Economic note: cost of illness studies. BMJ [Internet]. 2000;320:1335. https://doi.org/10.1136/bmj.320.7245.1335.
Jin H, Li X. Combining cost-effectiveness results into a single measurement: What is the value? EClinicalMedicine [Internet]. 2022. https://doi.org/10.1016/j.eclinm.2022.101563.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
HJ conceived the idea of this review. CL and XZ performed the first round (title and abstract) screening and second round (full-text) sifting, data extraction and quality assessment. HJ advised on the overall plan and implementation of the systematic review. CL and HJ wrote the first draft of the paper, which was subsequently edited by all authors who have approved the final version. CL will serve as a guarantor for the overall content of the manuscript.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability statement
All data generated or analysed during this study are included in this published article.
Funding
No funding was received for the preparation of this study.
Conflict of interest
CL, XZ and HJ declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, C., Zhang, X. & Jin, H. The Societal Cost of Schizophrenia: An Updated Systematic Review of Cost-of-Illness Studies. PharmacoEconomics 41, 139–153 (2023). https://doi.org/10.1007/s40273-022-01217-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01217-8