Abstract
Objectives
The aims of this study were to provide a systematic review and meta-analysis of the effects of atypical antipsychotics in children and adolescents on weight gain (primary objective) and other metabolic parameters (secondary objective).
Methods
A systematic literature review and meta-analysis of double-blind, randomized, controlled trials were conducted. The data sources used were as follows: EMBASE, PubMed, BIOSIS, International Pharmaceutical Abstracts, The Cochrane database (Clinical Trials), Clinical Trials Government Registry, The metaRegister of Controlled Trials, WHO (World Health Organization) Clinical Trials Registry Platform, and PsycINFO®. Hand searching was also carried out by examining the reference lists of identified studies. Double-blind, randomized, controlled trials investigating the metabolic adverse effects (weight gain, lipid, glucose, and prolactin level abnormalities) associated with atypical antipsychotic use in children and adolescents aged ≤18 years were included, irrespective of whether the investigation of adverse effects was a primary or secondary endpoint.
Results
We identified 21 studies of drug versus placebo that met the inclusion criteria, with a total of 2,455 patients, 14 studies for risperidone (1,331 patients), three for olanzapine (276 patients), and four for aripiprazole (848 patients). Compared with placebo, the mean weight increases for each drug were olanzapine 3.45 kg (95 % CI 2.93–3.98), risperidone 1.77 kg (95 % CI 1.35–2.20), and aripiprazole 0.94 kg (95 % CI 0.65–1.24). Regarding other metabolic abnormalities, eight studies reported statistically significant increases in prolactin with risperidone; two reported a statistically significant increase in glucose, total cholesterol, and prolactin with olanzapine; and three studies reported a statistically significant decrease in prolactin with aripiprazole. Data on lipid, glucose, and prolactin level changes were too limited to allow us to perform a meta-analysis.
Conclusions
Olanzapine, risperidone, and aripiprazole were all associated with statistically significant weight gain. Olanzapine was associated with the most weight gain and aripiprazole the least. For the secondary outcome, although a number of active comparator trials were identified, data were not available for meta-analysis and were too limited to allow firm conclusions to be drawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atypical antipsychotics (second-generation antipsychotics) have been regarded as a significant advance in psychopharmacotherapy because they have been reported as having a lower risk of extrapyramidal side effects (EPS) compared with the first-generation drugs [1–3]. In addition to their use in treating psychosis and mood disorder, mainly in adults and older teenagers, atypical antipsychotics have demonstrated their efficacy in the treatment of young people with disruptive behaviors in autism and intellectual impairment (mental retardation) in randomized controlled trials (RCTs) [1, 4, 5]. Although the use of atypical antipsychotics for the treatment of many psychiatric conditions in children and adolescents is growing, these drugs are often used off-label. A few short-term, placebo-controlled trials support the acute efficacy of risperidone, aripiprazole, olanzapine, or quetiapine in decreasing psychotic symptoms in adolescents and manic symptoms of bipolar disorder in children and adolescents [6–9].
In the last decade, the use of psychotropic drugs, especially atypical antipsychotics, has increased in children and adolescents [10–12]. A study using a research database in the USA showed that overall use of psychotropic medication in mental health patients aged 0–17 years increased from 59.5 % in 1997 to 62.3 % in 2000, with atypical antipsychotics having the highest change in utilization (138.9 %) over this period [13]. With this increased use there has been growing concern that certain drugs appear to be associated with metabolic dysfunction such as weight gain, diabetes mellitus, hyperglycemia and hyperlipidemia [11, 12]. These abnormalities may be at least partly due to the antagonism of atypical antipsychotics to various receptors in different neurotransmitter systems (serotonergic, dopaminergic, cholinergic, histaminergic, and others) [14–17].
Since childhood and adolescence involve important developmental periods of physical growth, together with motor, emotional, and cognitive development, the use of drugs that affect these aspects of development should be carefully considered and closely monitored [18]. These metabolic abnormalities are not only risk factors for increased morbidity and mortality, but may also impair patient adherence to treatment [19–22].
Several prospective studies have stated that weight gain and other metabolic abnormalities during childhood strongly predict obesity, metabolic syndrome, hypertension, cardiovascular disease, and osteoarthritis risk in adulthood [18–22].
The effects of atypical antipsychotics on glucose and lipid profiles in children and adolescents have been less well studied than have the effects in adults. Only a limited number of trials have evaluated the impact of these drugs in young patients [23]; in contrast, there have been several studies and meta-analyses in adults [24–26]. A meta-analysis by Allison et al. [25] in adults estimated that the mean weight gain after 10 weeks of treatment was 3.99 kg for clozapine, 3.51 kg for olanzapine, and 2.00 kg for risperidone. In another meta-analysis of adult data, Leucht et al. [26] found that clozapine and olanzapine were the most likely to be associated with weight gain, and abnormalities in glucose and lipids, followed by quetiapine and then risperidone. Regarding prolactin levels, risperidone and amisulpride were the most likely medications to be associated with an increase in these levels. However, data regarding weight gain and metabolic abnormalities in children and adolescents treated with antipsychotics are still limited [27, 28]. Our objective was to conduct a systematic review and meta-analysis of double-blind RCTs investigating the metabolic adverse effects of atypical antipsychotic medication prescribed to children and adolescents.
2 Methods
2.1 Literature Search
In order to identify RCTs and decrease location bias, we searched multiple databases including: EMBASE (1980–2010 Week 21), PubMed (1969–2010), BIOSIS (1969–2009 Week 27), International Pharmaceutical Abstracts (1970–May 2010), The Cochrane database (Clinical Trials), Clinical Trials Government Registry (http://www.clinicaltrials.gov), The metaRegister of Controlled Trials (www.controlled-trials.com), WHO (World Health Organization) Clinical Trials Registry Platform (http://www.who.int/ctrp/en/), and PsycINFO® (1978–2012) (http://www.apa.org/pubs/databases/psycinfo/index.aspx). In addition to this, the reference sections of all retrieved articles were manually searched for further relevant publications. The search strategy and terms are provided in Table 1.
2.2 Eligibility Criteria
We included double-blind RCTs investigating the metabolic adverse effects (weight gain, lipid, glucose, and prolactin level abnormalities) of atypical antipsychotics in children and adolescents aged ≤18 years. All such studies were included, irrespective of whether the investigation of adverse effects was a primary or secondary endpoint. All studies reporting the use of atypical antipsychotics, irrespective of the diagnosis or indication of drug used, were included, except studies of patients with anorexia nervosa, bulimia nervosa, or concurrent pre-existing medical conditions that might have affected weight gain (e.g., Cushing’s syndrome, renal disease, or diabetes). There was no language restriction. We excluded open-label trials, crossover design trials, reviews, case reports, observational cohort studies, editorials, and studies published only in abstract form.
2.3 Outcomes
The primary outcome of the study was to determine whether there was a significant mean weight gain associated with atypical antipsychotic drug treatment in children and adolescents. The secondary outcome was to determine whether there were any other significant reported metabolic adverse effects, including raised prolactin, lipid abnormalities, hyperglycemia, diabetes, or metabolic syndrome.
2.4 Data Extraction and Quality Assessment
Two reviewers (N.B.A., Y.L.) carried out the electronic searches and reviewed the articles independently. Any articles that did not meet the eligibility criteria were excluded on initial review. We also extracted information on the methodological quality of the studies, including, for example, whether the trials were described as double-blind and who was blinded. Articles marked for potential inclusion were obtained electronically or in paper copy and assessed again for inclusion. Disagreement was resolved by consensus. All available studies meeting the inclusion criteria were included and appraised. A standardized proforma was used to record the details of the papers reviewed. All search results were merged using Reference Manager® (Thomson Reuters, New York, NY, USA) and examined. Duplicates and irrelevant reports were removed.
Details recorded in the proforma included indication of atypical antipsychotic use, interventions, trial duration, study design, country of study, mean age of participants, participants’ sex, number of participants, and weight increase. The QUORUM (Quality of Reporting of Meta-analysis) was followed for reporting our review [29, 30]. For the assessment of the quality of the trials, the Jadad scale was used [31]. The scale is a 3-point questionnaire, each question to be answered with either ‘yes’ or ‘no’. Each ‘yes’ would score a single point, each ‘no’ no point; there were to be no fractional points. The questions were about randomization, blinding, withdrawals, and dropouts. Additional points were given if the method of randomization was described in the paper, and was appropriate, and the method of blinding was described, and was appropriate. Therefore, a paper reporting a clinical trial could receive a Jadad score between 0 and 5; trials with a score of <3 were considered to be of poor quality and hence excluded from the analysis.
2.5 Analysis
We used a random effects model with Review Manager (RevMan 5.0.20; The Cochrane Collaboration, Oxford, UK) [32]. This was used because a random effects model does not assume identical effects across studies, and therefore allows for between-study heterogeneity [32].
The primary outcome analysis (mean weight gain) was based upon intent-to-treat data. Data of secondary outcomes (other metabolic events) were taken from the same trials. The mean weight gain, standard deviation (SD), and sample size of all trials were extracted by N.B.A. Where SDs were not reported, they were obtained from standards errors, t values, or p values that related to the differences between means in two groups, or data were obtained directly from the study authors where necessary. The degree of heterogeneity between studies was assessed using the DerSimonian and Laird Q test, and the I2 statistic was used to describe the percentage of total variation across trials. A funnel plot was produced for the risperidone versus placebo group to assess publication bias, but could not be produced for the remaining groups because of the small number of studies identified.
3 Results
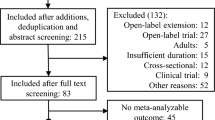
The initial electronic search identified 1,906 articles, of which 1,739 articles were excluded for the following reasons: duplicates (n = 400); study participants aged >18 (n = 162); open-label trials (n = 208); and other reasons, such as not relevant, case reports, reviews, comment, and editorial (n = 969) (Fig. 1). There were 167 articles that remained. Studies were classified into two groups: trials of drug versus placebo, and trials of drug versus drug.
3.1 Drug Versus Placebo
In this first group, initially 88 studies were included; however, 63 studies were excluded because they were either irrelevant or did not meet the inclusion criteria. The remaining 25 studies were further evaluated. An additional four studies were excluded for the following reasons: detailed data could not be obtained [33, 34]; treatment indication was anorexia nervosa [35]; and no direct comparison was made between the drug and placebo [36]. In total, 21 studies were included (Table 2).
3.2 Drug Versus Drug
For the second group, initially 79 studies were included; however, 68 studies were excluded because they were either irrelevant or did not meet the inclusion criteria. Only 11 studies were identified as relevant; however, they were further evaluated and all were excluded for the following reasons: weight was only reported at baseline [57], weight was not reported [58–62], or detailed data could not be obtained [63].
Therefore, no studies were left in the drug versus drug group, so we were unable to perform a meta-analysis of this comparison.
3.3 Results of Meta-Analysis
3.3.1 Primary Outcome
3.3.1.1 Risperidone Versus Placebo (14 Studies)
Compared with placebo, the mean weight gain associated with risperidone was 1.77 kg (95 % CI 1.35–2.20) (Fig. 2). Risperidone was therefore associated with a statistically significant weight gain compared with placebo (p < 0.00001).
Figure 3 shows a funnel plot, used to estimate publication bias in the studies of risperidone versus placebo. The plot is symmetrical, implying absence of publication bias, except for two studies: one at the bottom of the figure (Van Bellinghen and De Troch [39]) and the study at the far right end (Findling et al. [37]). Bias could be due to a small sample size in both studies, which gives effect estimates that scatter more widely in the graph (Fig. 3) [29].
3.3.1.2 Olanzapine Versus Placebo (3 Studies)
Compared with placebo, the mean weight gain associated with olanzapine was 3.45 kg (95 % CI 2.93–3.98) (Fig. 4). This implies that olanzapine is also associated with statistically significant weight gain compared with placebo (p < 0.00001).
3.3.1.3 Aripiprazole Versus Placebo (4 Studies)
Compared with placebo, the mean weight gain associated with aripiprazole was 0.94 kg (95 % CI 0.65–1.24) (Fig. 5). This implies that aripiprazole was also associated with statistically significant weight gain compared with placebo (p < 0.00001).
3.3.2 Secondary Outcomes
Regarding other metabolic abnormalities, eight studies reported statistically significant increases in prolactin with risperidone; two reported a statistically significant increase in glucose, total cholesterol, and prolactin with olanzapine, and three studies reported a statistically significant decrease in prolactin with aripiprazole. Changes in prolactin, glucose, and lipids in the included randomized trials are shown in Table 3. For the secondary outcomes, although a number of active comparator trials were identified, insufficient data were available for meta-analysis.
3.4 Heterogeneity
The p value of the Chi-squared test in Fig. 2 shows evidence of statistical variability between studies, with the I2 value showing considerable heterogeneity in the primary outcome variable (68 %). However, for the comparisons versus placebo shown in Figs. 4 and 5, there is no evidence of heterogeneity.
3.5 Quality of the Reports (Jadad Score)
In our meta-analysis, all trials included had a Jadad score of 4, except one study that had the full score of 5 (Table 2).
4 Discussion
The main finding from this review is that there is published evidence to indicate that treatment of young people aged ≤18 years with the atypical antipsychotic drugs risperidone, olanzapine, and aripiprazole is associated with statistically significant mean weight gain compared with placebo and that the effect appears to be greatest with olanzapine.
Regarding the other metabolic abnormalities, 13 studies reported statistically significant changes in lipid profile (triglyceride or cholesterol), glucose levels, and serum prolactin. In some of the included papers, it was not clear whether the authors were referring to clinical significance or statistical significance (Table 3). For serum prolactin, with long-term risperidone treatment in children and adolescents, levels tended to rise and peak in the beginning of treatment then steadily decline to values within or very close to normal range [64]. It was also noted that, in the aripiprazole study by Findling et al. [6], patients were required to discontinue prohibited medications, including mood stabilizers, antidepressants, and other psychotropics, at least 3 days before the initiation of treatment. For any discontinuing a medication that raised the prolactin level, this would contribute to the reduction in prolactin level on commencement of aripiprazole.
Recent studies suggest that hyperprolactinemia (at levels leading to hypogonadism) is associated with osteoporosis [65]. Childhood and adolescence involve important developmental periods of physical growth and bone mineralization, and hyperprolactinemia (which may lead to marked reduction in estrogen) can cause a decrease in bone density that may not improve later in life. Other unconfirmed potential risks from childhood hyperprolactinemia might include risk of breast cancer and pituitary tumors [23].
4.1 Comparison with Other Studies
Our meta-analysis revealed that olanzapine appeared to be the atypical antipsychotic associated with the greatest potential to induce weight gain compared with placebo. This finding is consistent with previous published studies of the use of atypical antipsychotics in adults [66–68] and young people [9, 24, 27, 28, 69–71].
In a study of 40 adult patients with borderline personality disorder randomly assigned to olanzapine or placebo, the mean baseline to endpoint weight gain was greater with olanzapine (3.71 kg) than with placebo (0.08 kg) [67]. In a prospective 12-week study assessing weight gain in 50 adolescents receiving olanzapine, risperidone, and haloperidol, the mean weight gain was 7.2 kg, 3.9 kg, and 1.1 kg, respectively, from baseline to endpoint [68].
4.2 Clinical Implications
The metabolic effects of antipsychotic drugs should be considered when planning the treatment strategy for individual patients. Baseline measurement of weight and height should be conducted, and any changes monitored. It has been recommended that plasma glucose, lipids, and prolactin should also be measured and regular follow-up should be individualized [65]. For example, according to NICE guidelines for mental health and behavioral disorders (2006) [72], baseline screening for weight and height should be monitored monthly for 6 months then every 6 months. Strategies for the management of drug-induced weight gain include therapeutic approaches, such as lifestyle change (diet, exercise) and pharmaceutical intervention. However, the prescription of additional medication to overcome the adverse effects of medication already prescribed should, if possible, be avoided.
The choice of the appropriate atypical antipsychotic drug should be based on treatment goals, the likely therapeutic benefit, the child’s condition, possible adverse effects, and medication cost. Cochrane systematic reviews are carried out using strictly defined criteria. One relevant Cochrane review was found, although it should be noted that it was not specifically in young people. This was an evaluation of the effects of aripiprazole compared with other atypical antipsychotic drugs for patients with schizophrenia and schizophrenia-like psychosis. Four trials were examined in this review: two comparing aripiprazole against placebo and two aripiprazole with risperidone. Aripiprazole was less effective than olanzapine, but was associated with fewer adverse effects, such as weight gain and sedation. Compared with risperidone, there were no differences in efficacy; limited data were available on EPS, cholesterol, glucose, and weight gain [73]. In an add-on or switching study of aripiprazole in adults with psychosis, a significant reduction in prolactin was found [74], which was associated with a significant improvement in quality of life [75]. The evidence was, however, limited. No similar analyses were found for young people, where the numbers would be even smaller; it is consequently not possible to make clear recommendations with regard to the antipsychotic drug of choice in children and teenagers on the basis of Cochrane reviews.
4.3 Limitations
This review is based on a limited number of studies, most of them of short duration. The majority of trials lasted for less than 10 weeks, which is sufficient to show change in weight gain, but may not be enough to show abnormalities in lipid profile. Although our results give a clear indication of a statistically significant mean weight gain associated with risperidone, olanzapine, and aripiprazole treatment in groups of young people, our mean weight gain results do not indicate changes in individuals, some of whom may gain large amounts of weight while others may gain none at all. As more data become available, it should be possible to deduce more definitive information on the metabolic adverse effects of these drugs.
5 Conclusions
This meta-analysis has demonstrated that there is a statistically significant association between mean weight gain and the administration of risperidone, olanzapine, and aripiprazole in young people. The mean weight gain appears to be greatest for olanzapine and least for aripiprazole. Weight gain can impair both physical health and psychological well-being; therefore, it will be important to determine factors that are associated with high risk of weight gain with atypical antipsychotics; these factors may include a genetic predisposition and lifestyle issues, particularly diet and exercise. The little data available on the secondary outcomes do not allow any firm conclusions to be drawn with regard to other metabolic changes. Although atypical antipsychotic medications have been studied for a range of psychiatric conditions in children and adolescents, the majority of these drugs are not licensed in children and many of the indications are for off-label use. This highlights a major gap in evidence-based psychiatric practice, especially as most trials involving children and adolescents were conducted on small sample sizes and with short treatment durations.
References
Jones P, Barnes TR, Davies L, et al. Randomised controlled trial of the effect of quality of life of second vs. first generation antipsychotic drugs in schizophrenia. Arch Gen Psychiatry. 2006;63:1079–87.
Malone RP, Sheikh R, Zito JM. Novel antipsychotic medications in treatment of children and adolescents. Psychiatr Serv. 1999;50:171–4.
Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behaviour disorders, and subaverage IQ. J Child Adolesc Psychopharmacol. 2004;14:243–54.
Glick I, Murray S, Hu R, et al. Treatment with atypical antipsychotics: new indications and new population. J Psychiatr Res. 2001;35:187–91.
Nasrallah N. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology. 2003;28:83–96.
Findling RL, Robb A, Nyilas M, et al. A multiple-centre, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–41.
Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of antipsychotics in early onset schizophrenia and schizoaffective disorder. Am J Psychiatry. 2008;165:1420–31.
Chang KD. The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry. 2008;69(suppl.4):4–8.
Kryzhanovskaya LA, Plouch CK, Xu W, et al. The safety of olanzapine in adolescents with schizophrenia or bipolar disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009;70:247–58.
Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systemic review and pooled analysis of short term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700.
Wong ICK, Murray ML, Camilleri-Novak D, et al. Increased prescribing trends of paediatric psychotropic medications. Arch Dis Child. 2004;89:1131–2.
Rani F, Byrne PJ, Murray ML, Wong ICK, et al. Epidemiological features of antipsychotics prescribing to children and adolescents in primary care in United Kingdom. Pediatrics. 2008;121:1002–9.
Martin A, Leslie D. Trends in psychotropic medications costs for children and adolescents, 1997–2000. Arch Pediatr Adolesc Med. 2003;157:997–1004.
Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. Clin Toxicol. 2001;39:1–14.
Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–91.
Kumra S, Oberstar JV, Sikich L, et al. Efficacy and tolerability of second generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71.
Fleischhaker C, Heister P, Hennighausen K, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol. 2006;16:308–16.
Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public health crisis common sense cure. Lancet. 2002;360:473–82.
Lissau I, Sorensen TI. Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet. 1994;343:324–7.
Srinivasan SR, Myers L, Berenson G. Predictability of childhood adiposity and insulin for developing Insulin resistance syndrome in young adulthood. The Bogalusa Heart Study. Diabetes. 2002;51:204–9.
Johonson JG, Cohen P, Kasen S, et al. Childhood adversities associated with the risk for eating disorder or weight problems during adolescents or early adulthood. Am J Psychiatry. 2002;159:394–400.
Correll C, Carlson H. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–91.
Almandil NB, Wong ICK. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Ed. 2011;96:192–6.
Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41.
Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;159:1686–96.
Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–63.
Pringsheim T, Lam D, Ching H, Pattens S. Metabolic and neurological complications of second generation antipsychotic use in children: a systematic review and meta-analysis of randomised controlled trials. Drug Saf. 2011;34(8):651–68.
De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26:144–58.
Egger M, Smith GB, Altman D. Systemic review in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. p. 23–66.
Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of randomised clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008.
Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18:140–56.
Scahill L, Leckman JF, Schultz RT, et al. A placebo controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130–5.
Spettigue W, Buchholz A, Henderson K, et al. Evaluation of the efficacy and safety of olanzapine as an adjunctive treatment for anorexia nervosa in adolescent females: a randomized, double-blind, placebo-controlled trial. BMC Pediatr. 2008;8:4.
Delbello MP, Schwiers ML, Rosenberg HL, et al. A double blind randomised placebo controlled study of quetiapine as adjunctive treatment of adolescent mania. Psychiatry. 2002;41:1216–23.
Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:509–16.
Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, et al. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry. 2001;62:239–48.
Van Bellinghen M, De Troch C. Risperidone in the treatment of disturbances in children and adolescents with borderline intellectual functioning: a double-blind placebo controlled trial. J Child Adolesc Psychopharmacol. 2001;11:5–13.
Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry. 2002;41:1026–36.
Aman MG, DeSmedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviours in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–46.
McCracken JT, McGough J, Shah B, etal.; Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21.
Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–41.
Reyes M, Buitelaar J, Toren P, et al. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry. 2006;163:402–10.
Nagaraj R, Shinghi P, Malhi P. Risperidone in children with autism: randomised, placebo-controlled, double blind study. J Child Neurol. 2006;21(6):450–5.
Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: a placebo-controlled pilot study. J Am Acad Child Adolesc Psychiatry. 2007;46:558–65.
Anderson GM, Scahill L, McCracken JT, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–50.
Luby J, Mrakotsky C, Stalets MM, et al. Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006;16:575–87.
Haas M, Unis AS, Armenteros J, et al. A 6 week randomized double blind placebo controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009;19(6):611–21.
Haas M, Delbello MP, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized double blind placebo controlled study. Bipolar Disord. 2009;11(11):687–700.
Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16:541–8.
Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164:1547–56.
Kryzhanovskaya L, Schulz SC, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: A 6 week randomised double blind placebo controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:60–70.
Tramontina S, Zeni CP, Ketzer CR, et al. Aripiprazole in children and adolescents with bipolar disorder co-morbid with attention-deficit/hyperactivity disorder: a pilot randomized clinical trial. J Clin Psychiatry. 2009;70:756–64.
Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124:1533–40.
Marcus RN, Owen R, Kamen L, et al. A placebo controlled fixed does study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110–9.
Barzman DH, Delbello MP, Adler CM, et al. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsive and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2006;16:665–70.
Keefe R, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;166:985–95.
Buchsbaum MS, Haznedar MM, Aronowitz J, et al. FDG-PET in never-previously medicated psychotic adolescents treated with olanzapine or haloperidol. Schizophr Res. 2007;94:293–305.
Emsley RA, Risperidone working group. Risperidone in the treatment of first episode psychotic patients: a double blind multicentre study. Schizophr Bull. 1999;25:721–9.
Emsley R, Rabinowitz J, Medori R, et al. Remission in early psychosis: rates, predictors and clinical and functional outcome correlates. Schizophr Res. 2007;89:129–39.
Facorro BC, Iglesias RP, Bonilla MR, et al. A practical clinical trial comparing haloperidol, risperidone and olanzapine for acute treatment of first episode nonaffective psychosis. J Clin Psychiatry. 2006;67:1511–21.
Kumra S, Kranzler H, Gerbino-Rosen G, et al. Clozapine versus “high-dose” olanzapine in refractory early-onset schizophrenia: an open-label extension study. J Child Adolesc Psychopharmacol. 2008;18:307–16.
Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long risperidone treatment in children and adolescents. J Clin Psychiatry. 2003;64:1362–9.
Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(suppl 4):26–36.
Butterfield MI, Becker ME, Connor KM, et al. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Inter Clin Psychopharmacol. 2001;16:197–203.
Bogenschutz MP, Nurnberg HG. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry. 2004;65:104–9.
Beasley CM, Sutton VK, Hamilton SH, et al. A double blind randomized placebo controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23:582–94.
Ratzoni G, Gothelf D, Brand-Gothelf A, et al. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41:337–43.
Ross RG, Novins D, Farley GK, et al. A 1-year open label trial of olanzapine in school age children with schizophrenia. J Child Adolesc Psychopharmacol. 2003;13:301–9.
Haapasalo-Pesu KM, Saarijärvi S. Olanzapine induces remarkable weight gain in adolescent patients. Eur Child Adolesc Psychiatry. 2001;10:205–8.
National Institute for Health and Clinical Excellence (NICE). Mental health and behavioural disorders. London: NICE; 2006.
Komossa K, Rummel-Kluge C, Schmid F, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2009; (4): CD006569.
Mir A, Shivakumar K, Williamson RJ, et al. Change in sexual dysfunction on aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22(3):244–53.
Aitchison KJ, Mir A, Shivakumar K, et al. Costs and outcomes associated with an aripiprazole add-on or switching open-label study in psychosis. J Psychopharmacol. 2011;25(5):675–84. doi:10.1177/0269881109358198.
Acknowledgments
I.C.K.W., F.M.C.B., K.J.A., M.L.M., and N.B.A. conceived the idea of the study. All authors were involved in the study design. N.B.A. and Y.L. analyzed the data; N.B.A., F.M.C.B., M.L.M., and I.C.K.W. interpreted the data. All authors had full access to the study data and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors drafted, revised, and approved the final manuscript. I.C.K.W. and F.M.C.B. supervised the study.
Conflicts of interest and funding
F.M.C.B. has received lecture fees, consultancy fees, research grants, and equipment grants from, and has been sponsored to attend conferences by, various pharmaceutical companies. He was previously editor-in-chief of a journal sponsored by GlaxoSmithKline. He has been asked to organize conferences supported by an unrestricted educational grant from Janssen-Cilag, a company marketing risperidone. None of these monies have been paid directly to F.M.C.B.; all monies since 2001 have been paid to his NHS Trust. F.M.C.B. has recently been sponsored to attend international epilepsy conferences by Eisai. No monies are currently being received from pharmaceutical companies, or from any source other than his employer, the NHS in the UK. K.J.A. has been on the Advisory Board for the Bristol-Myers Squibb and Otsuka Pharmaceuticals Ltd, and in addition, has received consultancy fees including payment for lectures and educational presentations from the same company. She was previously a member of various advisory boards, receiving consultancy fees and honoraria, and has received research grants from various companies, including Lundbeck and GlaxoSmithKline. She currently holds an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta. I.C.K.W. has received research funding and honoraria from various pharmaceutical companies, including Janssen-Cilag and Bristol-Myers Squibb (manufacturers of antipsychotic medicines). I.C.K.W. is currently receiving funding from the EU Commission to investigate the safety of risperidone in children. M.L.M. has received funding from pharmaceutical companies (Shire and Pfizer), but none of the funding is related to this study. The authors N.B.A. and Y.L. declare that they have no conflict of interest. N.B.A. is supported by a scholarship from the Ministry of Higher Education in the Kingdom of Saudi Arabia. No additional sources of funding were used to prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almandil, N.B., Liu, Y., Murray, M.L. et al. Weight Gain and Other Metabolic Adverse Effects Associated with Atypical Antipsychotic Treatment of Children and Adolescents: A Systematic Review and Meta-analysis. Pediatr Drugs 15, 139–150 (2013). https://doi.org/10.1007/s40272-013-0016-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-013-0016-6