Abstract
Tislelizumab (百泽安®;替雷利珠单抗; Tileilizhu Dankang Zhusheye) is an anti-human programmed death receptor-1 (PD-1) monoclonal IgG4 antibody that is being developed by BeiGene as an immunotherapeutic, anti-neoplastic drug. Tislelizumab has been investigated in haematological cancers and advanced solid tumours, leading to its approval in December 2019 in China for patients with relapsed or refractory classical Hodgkin’s lymphoma after at least second-line chemotherapy. This article summarises the major milestones in the development of tislelizumab for this first approval for classical Hodgkin’s lymphoma, and its potential upcoming approvals in other indications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A humanized IgG4 anti-PD-1 monoclonal antibody is being developed by BeiGene Ltd. for the treatment of haematological malignancies and advanced solid tumours |
Received its first approval on 26 December 2019 in China |
Approved for use in patients with relapsed or refractory classical Hodgkin’s lymphoma who have received at least two therapies |
1 Introduction
BeiGene Ltd. (BeiGene), are developing tislelizumab (百泽安®;替雷利珠单抗; Tileilizhu Dankang Zhusheye) as an anti-neoplastic agent. Tislelizumab is a human IgG4 monoclonal antibody that binds to and blocks the programmed cell death-1 (PD-1) receptor expressed on activated immune cells, including T lymphocytes. The interaction between PD-1 and its ligands, PD-L1 and PD-L2, results in the exhaustion of T cells, thus attenuating the anti-cancer immune response [1]. As tislelizumab prevents the binding of PD-1 to its ligands, it has an immunotherapeutic effect that results in increased anti-cancer immune activity. The low affinity of tislelizumab for the Fc receptor, Fc-γ receptor 1 (FcγRI), may result in improved anti-cancer efficacy [2]. FcγRI is expressed by macrophages in the tumour microenvironment, and the binding of PD-1 blocking antibodies to FcγRI results in crosslinking between PD-1 expressing effector T cells and FcγRI expressing macrophages. As a result of this crosslinking, the efficacy of other anti-PD-1 antibodies, such as nivolumab and pembrolizumab, may be diminished due to antibody-dependent cellular phagocytosis of T cells by macrophages [2].
Tislelizumab was conditionally approved in China in December 2019 for patients with relapsed or refractory classical Hodgkin’s lymphoma after at least second-line chemotherapy [3], and full approval will be granted based on confirmatory randomised clinical trials [4]. The recommended dosage of tislelizumab is 200 mg administered by intravenous infusion once every 3 weeks. Treatment with tislelizumab is recommended until disease progression or intolerable toxicity. Temporarily or permanently withholding tislelizumab is recommended for patients with immune-related adverse reactions, where necessary [4].

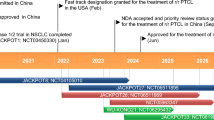
Key registration-enabling or potentially registration-enabling trials of tislelizumab for R/R cHL and UC. XL x line, FDA Food and Drugs Administration (USA), NMPA National Medical Products Administration (China), R/R cHL relapsed or refractory classical Hodgkin's lymphoma, NDA New drug application, UC urothelial carcinoma
1.1 Company Agreements
In July 2017, BeiGene entered into a development and marketing agreement with Celgene Corporation to develop and commercialise tislelizumab for solid tumours in the USA, Europe, Japan and rest of world outside Asia. Under the terms of the agreement, BeiGene retained exclusive rights for the development and commercialisation of tislelizumab for haematological malignancies globally and for solid tumours in Asia, except Japan. BeiGene received an aggregate of $US413 million from Celgene in upfront licensing fees and equity investment, and was eligible to receive up to $US980 million in development, regulatory and sales milestone payments and royalties on future sales of tislelizumab. The agreement was closed in August 2017 [5,6,7]. However, in June 2019, BeiGene entered into a mutual agreement with Celgene Corporation to terminate the parties’ global collaboration for tislelizumab [8]. BeiGene reacquired global rights to tislelizumab, and Celgene agreed to pay $US150 million to BeiGene due to the pending acquisition of Celgene by Bristol-Myers Squibb [9].
In January 2018, BeiGene entered into a commercial supply agreement with Boehringer Ingelheim Biopharmaceuticals (China) for tislelizumab. Under the terms of the agreement, Boehringer Ingelheim will manufacture tislelizumab as part of a Marketing Authorization Holder trial project initiated by BeiGene and Boehringer Ingelheim. Boehringer Ingelheim will continue to manufacture tislelizumab in China under an exclusive multi-year agreement, with option for contract extension. BeiGene obtained certain preferred rights for future capacity expansion by Boehringer Ingelheim in China [10].
In April 2019, BioAtla and BeiGene entered into a global collaboration agreement to co-develop, manufacture and commercialise BA3071, a conditionally active CTLA-4 antibody for the treatment of cancer that may be used in combination with tislelizumab [11].
1.2 Patent Information
As of February 2019, BeiGene owns three issued US patents and one issued patent in China directed to tislelizumab and its use in cancer. Furthermore, the company also owns the corresponding pending patent applications in other jurisdictions. The issued US patents are scheduled to expire in 2033, excluding any additional patent term extensions. The company plans to pursue marketing exclusivity periods that are available under regulatory provisions in certain countries [12].
2 Scientific Summary
2.1 Pharmacodynamics
Tislelizumab binds to human PD-1 with high specificity and affinity (disassociation constant, KD 0.15 nmol/L) [13], using the critical epitopes, Gln75, Thr76, Asp77 and Arg86 that are present on PD-1 [14]. This is in contrast to nivolumab and pembrolizumab that do not require these epitopes for binding; tislelizumab has a slower disassociation rate from PD-1 in comparison with nivolumab (50-fold slower) and pembrolizumab (100-fold slower) [14].
The S228P mutation in the IgG4 isotype of antibodies is known to bind to FcγRI, and is present in other PD-1 blocking antibodies (nivolumab, pembrolizumab, cemiplimab, spartalizumab, JS001, camrelizumab and sintilimab), but not tislelizumab [1]. The effect of the S228P mutation was studied using tislelizumab and a variant of tislelizumab with the S228P mutation. The binding of FcγRI to the tislelizumab S228P variant, but not tislelizumab, was confirmed using surface plasmon resonance [2]. In vitro studies with M2 macrophages and PD-1+ T cells resulted in antibody-dependent cellular phagocytosis of T cells by macrophages that were incubated with the tislelizumab S228P variant; in contrast, cells incubated with tislelizumab displayed comparable baseline antibody-dependent cellular phagocytosis activity to control antibodies (which did not bind to FcγRI) [2].
Immunodeficient mice who had been simultaneously injected with A431 cancer cells and peripheral blood monocytes showed significantly reduced tumour growth when tislelizumab was administered, whereas those treated with the tislelizumab S228P variant showed similar tumour progression to vehicle treated mice [2].
Tumour spheroids were treated with tislelizumab, nivolumab or pembrolizumab, and incubated with tumour-infiltrating lymphocytes isolated from human colorectal cancers or colorectal liver metastases in an ex vivo study. All three PD-1 blocking antibodies demonstrated significant increases in IFN-γ production and proliferation of tumour-infiltrating lymphocytes. However, spheroids treated with tislelizumab 0.1, 1 and 10 µg/mL yielded significantly higher quantities of IFN-γ compared with treatment with nivolumab or pembrolizumab. Incubation with tislelizumab resulted in better activation of tumour-infiltrating lymphocytes isolated from colorectal liver metastases, which is hypothesised to be due to the higher frequency of macrophages in this tumour type (leading to FcγRI binding with nivolumab or pembrolizumab) [15, 16].
Other FcR-mediated effects, such as antibody-dependent cell-mediated cytotoxicity or compliment-dependent cytotoxicity were not observed in activated T cells incubated with tislelizumab and primary immune cells, up to a tislelizumab concentration of 100 µg/mL [13].
2.2 Pharmacokinetics
The pharmacokinetics of intravenous (IV) tislelizumab 0.5, 2, 5 or 10 mg/kg once every 2 weeks, 2 or 5 mg/kg or 200 mg once every 3 weeks were investigated in a population pharmacokinetic analysis of 798 patients from three trials (NCT02407990, NCT04068519 and NCT03209973), and data from 112 patients in a non-compartmental pharmacokinetic model. After a single IV dose of tislelizumab, exposure (Cmax and AUC14d) was linear over the dose range 0.5–10 mg/kg [4].
Tislelizumab is completely bioavailable following intravenous infusion. The volume of distribution is 4.41 L following a single infusion of tislelizumab 200 mg, and 5.247 L at steady state. Following a single dose of tislelizumab 200 mg, the clearance of tislelizumab was 0.247 L/day and the half-life was 13.3 days, while after repeat administration in population pharmacokinetic analyses, the clearance was 0.171 L/day and the half-life was 26 days [4].
The effect of renal or hepatic impairment on tislelizumab pharmacokinetics has not been directly evaluated. Population pharmacokinetic analyses suggest that mild to moderate renal impairment and mild hepatic impairment had no effect on tislelizumab pharmacokinetics. There are insufficient pharmacokinetic data in patients with severe renal impairment or moderate or severe hepatic impairment [4].
Features and properties of tislelizumab
Alternative names | 百泽安®,替雷利珠单抗, Tileilizhu Dankang Zhusheye, BGB-A317, 0KVO411B3N |
Class | Antineoplastics, immunotherapies, monoclonal antibodies |
Mechanism of Action | Antibody-dependent cell cytotoxicity; Programmed cell death-1 receptor antagonists; T lymphocyte stimulants |
Route of Administration | Intravenous |
Pharmacodynamics | KD 0.15 nmol/L for human PD-1; low affinity for FcγRI (KD not detectable) |
Pharmacokinetics | Single dose: Vd 4.41 L, CL 0.247 L/day, t½ 13.3 days. Population analyses (repeat administration): Vss 5.247 L, CL 0.171 L/day, t½ 26 days |
Adverse events (in classical Hodgkin’s lymphoma) | |
Most frequent | Fever, weight gain, hypothyroidism |
Occasional | Pruritus, rash, upper respiratory tract infections, fatigue, cough |
Rare | Immune-related adverse reactions |
ATC codes | |
WHO ATC code | L01X-C |
EphMRA ATC code | L1G |
Chemical Name | Immunoglobulin G4, anti-(human programmed cell death protein 1) synthetic clone 317-4B6 heavy chain VH fragment fusion protein with human γ4 chain clone mut10 effector/constant domain fragment, disulfide with anti-(human programmed cell death protein 1) synthetic clone 317-4B6 light chain VL fragment fusion protein with human κ chain constant region fragment, dimer |
2.3 Therapeutic Trials
2.3.1 Hodgkin’s Lymphoma
The independently assessed overall response rate (ORR, defined as a complete or partial response) was 87.1% (p < 0.0001 vs predefined non-significance threshold of ORR = 35%) after a median follow-up of 9.79 months in an open-label, phase II trial (NCT03209973, CTR20170119). Complete (CR) and partial (PR) responses were observed in 44 patients (62.9%) and 17 patients (24.3%). The median progression-free (PFS) and overall survival (OS) were not yet reached, and the 9-month PFS and OS rates were 74.5% and 98.6%. This pivotal, single-arm trial enrolled 70 patients from China, where 85.7% of patients had advanced disease (Ann Arbor Stages IIB–IV), all patients were previously treated with chemotherapy and 52.3% of patients did not achieve at least partial response to their prior therapy. Patients were treated with intravenous tislelizumab 200 mg once every 3 weeks until disease progression, unacceptable toxicity or termination of the study [17].
2.3.2 Urothelial Carcinoma
In treatment-experienced patients with PD-L1 + urothelial carcinoma, tislelizumab achieved an independently assessed ORR of 23% after a median follow-up of 8 months (data cut-off date 28 February 2019). CR and PR were achieved in 8 and 16 patients (8% and 15%), and the median PFS and OS were 2.1 and 9.8 months. For this pivotal phase II trial, 113 patients who had previously been treated with ≥ 1 platinum-based therapy were recruited from China and Korea, (NCT04004221, CTR20170071), where a tumour response was evaluable in 104 patients after treatment with tislelizumab 200 mg once every 3 weeks [18].
A cohort of patients with urothelial carcinoma (median of 1 prior treatment), achieved an ORR of 14% (3/22 patients) after a median 4.2 months of follow-up (data cut-off date 1 December 2018). All responses were PR, and median OS was 4.3 months. All patients in this cohort were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks in the open label, phase I/II trial in solid tumours (n = 300) [NCT04068519, CTR20160872] [19].
An ORR of 29.4% (5/17 patients; data cut-off date 31 August 2018), with one CR (5.9%) and four PR (23.5%), was observed in the cohort of patients with previously-treated urothelial carcinoma treated with tislelizumab in an open-label phase Ia/Ib trial (NCT02407990). All patients were evaluable for tumour response in this cohort [20].
2.3.3 Gastric and Esophageal Cancer
Thirty Chinese patients were enrolled in an open-label phase II trial positioning tislelizumab in combination with chemotherapy for first-line therapy (NCT03469557, CTR20170515), half of the patients (15 patients) were enrolled in the gastric/gastroesophageal junction adenocarcinoma cohort, and the other half of patients were enrolled in the esophageal squamous cell carcinoma cohort. All patients were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks and concurrent chemotherapy [21].
Patients with gastric/gastroesophageal junction adenocarcinoma achieved an ORR of 46.7% (7/15 patients) after treatment with tislelizumab, oxaliplatin and capecitabine with a median follow up period of 15.4 months (data cut-off date 31 March 2019). All objective responses were partial responses. Median PFS was 6.1 months and median OS was not yet reached (62% OS rate at 12 months) [21].
The ORR for patients with esophageal squamous cell carcinoma was 46.7% (7/15 patients) after treatment with tislelizumab, cisplatin and 5-fluorouracil and a median follow up period of 13.0 months (data cut-off date 31 March 2019). No complete responses were achieved among responders. Median PFS was 10.4 months and median OS was not yet reached (OS rate of 50% at 12 months) [21].
During earlier trials, cohorts of generally previously-treated patients (median of 2 prior treatments) with esophageal squamous cell cancer and gastric cancer attained an ORR of 8% (2/26 patients) and 17% (4/24 patients) with tislelizumab, where CR were not observed after a median 5 months and 6 months of follow up, and median OS was 4.8 months and 4.7 months (data cut-off date 1 December 2018). All patients were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks during the open-label, phase I/II trial (NCT04068519, CTR20160872) [19].
An ORR of 11.1% (6/54 patients; 1 CR, 5 PR) and 13.0% (7/54 patients; 7 PR) were achieved in the cohorts of patients with previously-treated advanced esophageal cancer and previously-treated gastric cancer who received tislelizumab, after a median duration of follow up of 5.2 months and 4.9 months (data cut-off date 31 August 2018). In patients with advanced esophageal cancer, 1 CR (3.0%) and 3 PR (9.1%) were reached in patients with PD-L1+ tumours (n = 33), 1 PR (5.9%) in patients with PD-L1− tumours (n = 17) and 1 PR (25.0%) in patients with PD-L1 unknown tumours (n = 4). In patients with gastric cancer, 5 PR (22.7%) were observed in patients with PD-L1+ tumours (n = 22), 1 PR (4.3%) in patients with PD-L1− tumours (n = 23) and 1 PR (11.1%) in patients with PD-L1 unknown tumours (n = 9). All patients were evaluable for tumour response, and were treated with tislelizumab 5 mg/kg once every 3 weeks during the indication expansion phase in this open label, phase Ia/Ib trial (NCT02407990) [22].
2.3.4 Lung Cancer
The primary endpoint of independently reviewed PFS in patients (n = 360) with previously untreated stage IIIB or IV squamous non-small cell lung cancer who were randomised to receive chemotherapy only (carboplatin in addition to either paclitaxel or nab-paclitaxel) or chemotherapy and tislelizumab 200 mg once every 3 weeks was met in a pre-planned interim analysis of an open-label phase III trial in China (NCT03594747, CTR20180292) [23].
In an earlier open-label phase II trial in China (NCT03432598, CTR20170361), 36 patients with advanced lung cancer (n = 54) achieved an objective response (ORR 66.7%) when treated with concurrent doublet chemotherapy and tislelizumab. Four cohorts of patients were treated based on their indications, patients with non-squamous non-small cell lung cancer (16 patients), squamous non-small cell lung cancer (21 patients across two cohorts) and small cell lung cancer (17 patients). All treated patients were evaluable for tumour response [19].
The ORR of the patients in the non-squamous non-small cell lung cancer cohort was 43.8% (7/16 patients; data cut-off date 25 February 2019). At data cut-off, the median PFS was 9.0 months, and median OS was not yet reached in this cohort after a median 17.4 months of follow up [19, 24]. Of the two cohorts of patients with squamous non-small cell lung cancer, the ORR was 80.0% (12/15 patients) in cohort A and 66.7% (4/6 patients) in cohort B. The median PFS was 7.0 months in cohort A, but was not reached in cohort B. Median OS was not reached in both cohorts after a median follow up of 18.3 months in cohort A and 18.1 months in cohort B [19, 24]. In 17 patients with small cell lung cancer, 13 patients achieved an objective response (ORR of 76.5%). The median PFS was 6.9 months, while the median OS was 15.6 months after a median 15.3 months of follow up [19].
During an earlier phase I/II trial (NCT04068519, CTR20160872), the ORR with tislelizumab treatment was 18% (10/56 patients, all responses were PR) after a median 9 months of follow up in a cohort of generally previously-treated patients with non-small cell lung cancer (median of 2 prior therapies). Median OS was not yet reached, and median PFS was 4.0 months (data cut-off date 1 December 2018). All patients were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks [19].
The earliest phase Ia/Ib trial of tislelizumab (NCT02407990) enrolled a cohort of 49 treatment-experienced patients with non-small cell lung cancer, where an ORR of 13.0% (6/46 evaluable patients; all PR) was achieved after a median 11.2 months of follow up (data cut-off date 31 August 2018). 3 PR (18.8%) were achieved in patients with PD-L1+ tumours (n = 16), 2 PR (9.5%) in patients with PD-L1− tumours (n = 21) and 1 PR (8.3%) in patients with unknown PD-L1 (n = 12). Patients were treated with tislelizumab 5 mg/kg once every 3 weeks during the indication expansion phase (Ib) [22].
2.3.5 Ovarian, Fallopian Tube or Peritoneal Cancers
An ORR of 23.5% (4/17 evaluable patients; data cut-off date 17 July 2019), where all responses were PR, was achieved with tislelizumab in a cohort of patients with recurrent, platinum-resistant ovarian cancer (n = 20). Patients were treated with tislelizumab and sitravatnib (a small molecule drug that inhibits receptor tyrosine kinases) in this phase Ib trial (NCT03666143). The median PFS was 18 weeks [26].
The combination of tislelizumab and pamiparib (a small molecule, poly [ADP-ribose] polymerase inhibitor) in treatment-experienced patients resulted in an ORR of 20% after a median follow up of 8.3 months (data cut-off date 26 March 2018), in an open label phase Ia/Ib trial (NCT02660034). Two CR (4%) and eight PR (16%) were achieved across all cohorts (n = 49; all patients were evaluable for tumour response), where all objective responses (except for 1 PR in the breast cancer cohort) were observed in patients with ovarian, fallopian tube or peritoneal cancers (34/49 patients) [27].
Monotherapy with tislelizumab resulted in an ORR of 3.9% (2/51 patients achieved PR) in the cohort of patients with previously-treated ovarian cancer (data cut-off date 6 March 2017), during an open label, phase Ia/Ib trial (NCT02407990). All patients in this cohort were evaluable for tumour response [28].
2.3.6 Hepatocellular Carcinoma
An ORR of 17% (3/18 patients achieved a PR) was seen with tislelizumab in patients with generally previously-treated hepatocellular carcinoma (median of 1.5 prior therapies) after a median follow up period of 8 months. Median OS was yet to be reached at the data cut-off date of 1 December 2018. All patients were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks during this phase I/II trial (NCT04068519, CTR20160872) [19].
In an earlier open label, phase Ia/Ib trial (NCT02407990), the ORR in the cohort of 50 treatment-experienced patients with hepatocellular carcinoma treated with tislelizumab was 12.2% (6 PR from 49 evaluable patients) after a median follow up of 10.8 months (data cut-off date 31 August 2018). All 6 PR (23.1%) were observed in patients with PD-L1+ tumours (n = 26). Patients were treated with tislelizumab 5 mg/kg once every 3 weeks during the indication expansion phase (Ib) [22].
2.3.7 Other Phase I/II Trials
The ORR achieved with tislelizumab treatment was 15% (5/34 patients; median follow up 8 months), 10% (2/21 patients; median follow up 16 months) and 19% (3/16 patients; median follow up 11 months) in cohorts of generally treatment-experienced patients (median of 2 prior therapies) with melanoma, renal cell carcinoma and microsatellite instability-high/mismatch repair deficient solid tumours, respectively (data cut-off date 1 December 2018) in an open-label, phase I/II trial (NCT04068519, CTR20160872). All observed responses were PR, and median OS was 11.3 months in patients with melanoma, and not yet reached for patients with renal cell carcinoma or microsatellite instability-high/mismatch repair deficient solid tumours. All patients were evaluable for tumour response, and were treated with tislelizumab 200 mg once every 3 weeks [19].
Long-term exposure to tislelizumab (> 12 months) in an open-label, phase Ia/Ib trial (NCT02407990) resulted in 4 CR (6.3%) and 38 PR (60.3%) in 63 patients with generally previously-treated (70% of patients received 1 ≥ therapy) advanced solid tumours (data cut-off date 31 August 2018). 35 of these patients had PD-L1+ tumours (defined as ≥ 1% PD-L1 expression), 22 patients had PD-L1− tumours and 6 patients had tumours with unknown PD-L1 status. All 4 CR and 21 PR were observed in patients with PD-L1+ tumours, 13 PR were observed in patients with PD-L1− tumours and 4 PR were observed in patients with PD-L1 unknown tumours. All patients were evaluable for tumour response, and were treated with tislelizumab once every 2 or 3 weeks [29].
Preliminary results of an open-label phase Ib dose escalation and expansion trial (NCT02795182, CTR20180193) indicated that the combination of tislelizumab and zanubrutinib (a small molecule drug that inhibits Bruton’s tyrosine kinase), achieved an ORR of 38% (19/50 evaluable patients) in previously treated patients with relapsed or refractory non-Hodgkin’s lymphoma (n = 53) after a median 4.1 months of follow up (data cut-off date April 10 2019) [25].
Key clinical trials of tislelizumab (BeiGene). Only registration-enabling or potentially registration-enabling studies are represented in this table
Drug(s) | Indication | Phase | Status | Location(s) | Identifiers |
|---|---|---|---|---|---|
Tislelizumab | Relapsed/refractory classical Hodgkin’s lymphoma | II | Ongoing | China | NCT03209973, CTR20170119, BGB-A317-203 |
Tislelizumab | PD-L1+ advanced urothelial carcinoma | II | Ongoing | China, Korea | NCT04004221, CTR20170071, BGB-A317-204 |
Tislelizumab, cisplatin, carboplatin, gemcitabine, placebo | Advanced urothelial carcinoma | III | Recruiting | China | NCT03967977, CTR20190543, BGB-A317-310 |
Tislelizumab | Previously treated, unresectable hepatocellular carcinoma | II | Ongoing | Global | NCT03419897, CTR20171257, 2017-003983-10, RATIONALE-208, BGB-A317-208 |
Tislelizumab, sorafenib | Unresectable hepatocellular carcinoma | III | Ongoing | Global | NCT03412773, CTR20170882, 2017-002423-19, JapicCTI-194569, RATIONALE-301, BGB-A317-301 |
Tislelizumab, paclitaxel, docetaxel, irinotecan | Advanced esophageal squamous cell carcinoma | III | Recruiting | Global | NCT03430843, CTR20171026, 2017-003699-30, BGB-A317-302 |
Tislelizumab, cisplatin, oxaliplatin, 5-fluorocuracil, capeciatbine, paclitaxel, placebo | Advanced esophageal squamous cell carcinoma | III | Recruiting | Global | NCT03783442, CTR20181013, 2018-000587-28, JapicCTI-194741, BGB-A317-306 |
Tislelizumab, paclitaxel, cisplatin, radiotherapy, placebo | Localized esophageal squamous cell carcinoma | III | Recruiting | China | NCT03957590, CTR20190198, BGB-A317-311 |
Tislelizumab, oxaliplatin, capecitabine, 5-fluorouracil, cisplatin, placebo | Advanced gastric or gastroesophageal junction adenocarcinoma | III | Recruiting | Global | NCT03777657, CTR20181841, 2018-000312-24, JapicCTI-194799, BGB-A317-305 |
Tislelizumab, gemcitabine, cisplatin, placebo | Advanced nasopharyngeal cancer | III | Recruiting | China | NCT03924986, CTR20182534, BGB-A317-309 |
Tislelizumab, carboplatin, paclitaxel, nab-paclitaxel | Advanced squamous non-small cell lung cancer | III | Ongoing | China | NCT03594747, CTR20180292, BGB-A317-307 |
Tislelizumab, cisplatin, pemetrexed, carboplatin | Stage IIIB or IV non-squamous non-small cell lung cancer | III | Ongoing | China | NCT03663205, CTR20180032, BGB-A317-304 |
Tislelizumab, docetaxel | Stage IIIB or IV non-small cell lung cancer | III | Recruiting | Global | NCT03358875, CTR20190198, 2018-000245-39, BGB-A317-303 |
Tislelizumab, carboplatin, cisplatin, etoposide, placebo | Untreated extensive-stage small cell lung cancer | III | Recruiting | China | NCT04005716, CTR20190511, BGB-A317-312 |
Tislelizumab | Previously treated, advanced microsatellite-instability-high or mismatch repair deficient solid tumours | II | Recruiting | China | NCT03736889, CTR20180867, BGB-A317-209 |
2.4 Adverse Events
The safety population for tislelizumab was composed of 821 patients from three studies (NCT02407990, NCT04068519 and NCT03209973), where 383 patients received the recommended dosage of 200 mg once every 3 weeks. The median duration of treatment was 16 weeks and 20.0% of patients received tislelizumab for ≥ 12 months. The overall incidence of adverse reactions was 71.0% across the safety population. The most common adverse reactions (incidence ≥ 10%) were fatigue, rash, hypothyroidism and elevated alanine or aspartate transaminases. Grade ≥ 3 adverse reactions were reported in 18.4% of patients, where reactions with an incidence ≥ 1% were elevated gamma-glutamyltransferase, alanine or aspartate transaminases, pulmonary inflammation, severe skin reactions and anaemia [4].
Immune-related adverse reactions have been reported with treatment with tislelizumab, including hypothyroidism (7.4%), skin adverse reactions (6.6%), hyperthyroidism (3.4%), pulmonary inflammation (2.9%), hepatitis (1.8%), diarrhoea and colitis (1.1%), other thyroid disorders (0.6%), type 1 diabetes (0.4%), adrenal insufficiency (0.2%), myocarditis (0.2%), nephritis (0.2%) and pancreatitis (0.1%) [4].
Treatment with tislelizumab may be temporarily or permanently discontinued in response to immune-related adverse reactions, however, adjusting the dose is not recommended for adverse reactions [4].
2.5 Ongoing Clinical Trials
There are 27 ongoing trials for tislelizumab in various malignancies, either as a monotherapy or in combination with other therapies; of these, 15 are considered to be registration-enabling or potentially registration-enabling studies, such as those for non-Hodgkin’s lymphoma (NCT03209973 [phase II, monotherapy]), urothelial carcinoma (NCT04004221 [phase II, monotherapy] and NCT03967977 [phase III, with chemotherapy]), hepatocellular carcinoma (NCT03412773 [phase III, with sorafenib] and NCT03419897 [phase II, monotherapy]), esophageal squamous cell carcinoma (NCT03430843 [phase III, monotherapy versus chemotherapy], NCT03783442 [phase III, with chemotherapy] and NCT03957590 [phase III, with chemotherapy]), gastric or gastroesophageal junction adenocarcinoma (NCT03777657 [phase III, with chemotherapy]), nasopharyngeal cancer (NCT03924986 [phase III, with chemotherapy]), non-small cell lung cancer (NCT03594747 [phase III, with chemotherapy], NCT03663205 [phase III, with chemotherapy] and NCT03358875 [phase III, with docetaxel]), small cell lung cancer (NCT04005716 [phase III, with chemotherapy]) and microsatellite-instability-high or mismatch repair deficient solid tumours (NCT03736889 [phase II, monotherapy]).
Phase I or II trials are ongoing in patients with gastroesophageal junction adenocarcinoma or esophageal squamous cell carcinoma (NCT03469557 [phase II, with chemotherapy]), non-small cell and small cell lung cancer (NCT03432598 [phase II, with chemotherapy]), mature T- and natural killer-cell neoplasms (NCT03493451 [phase II, monotherapy]), B cell lymphoid malignancies (NCT02795182 [phase Ib, with zanubrutinib]) and advanced solid tumours (NCT03666143 [phase Ib, with sitravatinib], NCT04068519 [phase I/II, monotherapy], NCT02407990 [phase Ia/Ib, monotherapy] and NCT02660034 [phase Ia/Ib, with pamiparib]). Furthermore, there are early trials in advanced solid tumours to assess the combination of tislelizumab with investigational products from BeiGene, including BGB-A333 (NCT03379259 [phase Iab/IIab]), BGB-A445 (NCT04215978 [phase Ia/Ib]) and BGB-A425 (NCT03744468 [phase I/II]). The long term safety of patients who have received tislelizumab will be assessed in NCT04164199 (phase III).
3 Current Status
On the 26th of December 2019, the National Medical Products Administration (NMPA, formerly China Food and Drug Administration) approved tislelizumab for patients with classical Hodgkin’s lymphoma who have received at least two prior therapies. The approval was based on the results from a pivotal phase II trial (NCT03209973, CTR20170119) [3]. The New Drug Application (NDA) for tislelizumab was accepted by the NMPA in August 2018 [30], and was granted priority review status in November 2018 [31].
The registration of tislelizumab for other indications is currently underway. In July 2019, the supplementary NDA for tislelizumab in patients with previously treated locally-advanced or metastatic urothelial carcinoma was granted priority review status [32], and the supplementary NDA was accepted in May 2019 by the NMPA based on results from a pivotal phase II trial (NCT04004221, CTR20170071) [33]. In January 2020, BeiGene announced that the primary endpoint was met in a phase III trial of tislelizumab for the first-line treatment of patients with squamous non-small cell lung cancer (NCT03594747, CTR20180292), and discussions with the NMPA are planned [23].
References
Chen X, Song X, Li K, et al. FcγR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. 2019;10:292.
Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079–90.
BeiGene. China National Medical Products Administration approves BeiGene’s tislelizumab for patients with classical Hodgkin’s lymphoma who have received at least two prior therapies. [media release] 27 Dec 2019. http://www.beigene.com.
BeiGene. Tislelizumab; China prescribing information [Chinese]. 2019. http://drugs.medlive.cn/drugref/html/21697.shtml. Accessed 10 Mar 2020.
BeiGene. BeiGene announces closing of global strategic oncology collaboration with Celgene Corporation. [media release] 31 Aug 2017. http://www.beigene.com.
BeiGene. BeiGene reports second quarter 2017 financial results. [media release] 9 Aug 2019. http://www.beigene.com.
Celgene Corporation. Celgene Corporation enters into global strategic immuno-oncology collaboration with BeiGene to advance PD-1 inhibitor program for solid tumor cancers. [media release] 5 Jul 2017. http://www.beigene.com.
BeiGene. BeiGene regains full global rights to its investigational anti-PD-1 antibody tislelizumab. [media release] 17 Jun 2019. http://www.beigene.com.
BeiGene. BeiGene reports second quarter 2019 financial results. [media release] 8 Aug 2019. http://www.beigene.com.
BeiGene. BeiGene and Boehringer Ingelheim announce commercial supply agreement for anti-PD-1 antibody tislelizumab. [media release] 9 Jan 2018. http://www.beigene.com.
BioAtla, BeiGene. BioAtla and BeiGene form worldwide collaboration to develop and commercialize novel conditionally active biologic CTLA-4 therapy. [media release] 9 Apr 2019. http://www.beigene.com.
BeiGene. US Securities and Exchange Comission Form 10-K, BeiGene, LTD. Annual Filings. 2019.
Zhang T, Song J, Li Y, et al. Antihuman PD-1 antibody BGB-A317 exhibits potent immune cell activation [abstract no. 2226]. Cancer Research Conference: 107th AACR Annual Meeting 2016;76(Suppl 14).
Feng Y, Hong Y, Sun H, et al. The molecular binding mechanism of tislelizumab, an investigational anti-PD-1 antibody, is differentiated from pembrolizumab and nivolumab [abstract no. 2383]. Cancer Research Conference: AACR Annual Meeting. 2019;79(Suppl 13).
Wu X, Zhang T, Fu C, et al. Activation of tumor infiltrating lymphocytes from colorectal cancer and colorectal liver metastasis patients by anti-human PD-1 antibody BGB-A317 in a 3D spheroid system [abstract no. e14560]. JCO Conference. 2016;34(Suppl 15).
Luo L, Wu X, Zhang T, et al. Investigation of T cell activation by anti-human PD-1 antibodies nivolumab, pembrolizumab and BGB-A317 using tumor-infiltrating lymphocytes (TILs) from colorectal cancer and colorectal liver metastasis patients [abstract no. 5626]. AACR Annual Meeting. 2017;77(Suppl 13).
Song Y, Gao Q, Zhang H, et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia. 2019.
Ye D, Liu J, Zhou A, et al. First report of efficacy and safety from a phase II trial of tislelizumab, an anti-PD-1 antibody, for the treatment of PD-L1 + locally advanced or metastatic urothelial carcinoma (UC) in Asian patients [abstract no. 920P]. Ann Oncol. 2019;30(Suppl 5):v367.
BeiGene. BeiGene announces clinical results on tislelizumab presented at the 22nd Annual Meeting of the Chinese Society of Clinical Oncology (CSCO). [media release] 22 Sep 2019. http://beigene.com.
BeiGene. BeiGene announces updated phase 1A/1B data on tislelizumab presented at the European Society for Medical Oncology Immuno-Oncology Congress. [media release] 15 Dec 2018. http://beigene.com.
BeiGene. BeiGene announces clinical data on tislelizumab presented at European Society for Medical Oncology (ESMO) Asia 2019 Congress. [media release] 23 Nov 2019. http://www.beigene.com.
Deva S, Lee JS, Lin CC, et al. A phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors [abstract no. 70O]. Ann Oncol. 2018;29(Suppl 10):x33.
BeiGene. BeiGene announces that the phase 3 clinical trial of its anti-PD-1 antibody tislelizumab in patients with first- line squamous non-small cell lung cancer met the primary endpoint of progression-free survival at interim analysis. [media release] 21 Jan 2020. http://beigene.com.
BeiGene. Data on file. 2020.
Tam CS, Cull G, Opat S, et al. An update on safety and preliminary efficacy of highly specific bruton tyrosine kinase (BTK) Inhibitor zanubrutinib in combination with PD-1 inhibitor tislelizumab in patients with previously treated B-Cell lymphoid malignancies. 61st ASH Annual Meeting 2019;134(Suppl 1):1594.
BeiGene. BeiGene announces clinical data on investigational anti-PD-1 antibody tislelizumab in combination with sitravatinib at European Society for Medical Oncology Immuno-Oncology (ESMO I-O) Congress 2019. [media release] 13 Dec 2019. http://beigene.com.
Friedlander M, Meniawy T, Markman B, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(9):1306–15.
Meniawy T, Richardson G, Townsend A, et al. Preliminary results from a subset of patients (pts) with advanced ovarian cancer (OC) in a dose-escalation/expansion study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb) [abstract no. 389P]. Ann Oncol. 2017;28(Suppl 5):v130.
Desai J, Markman B, Friedlander M, et al. Long-term exposure (LTE) to tislelizumab, an investigational anti-PD-1 antibody, in a first-in-human phase I study [abstract no. CT084]. AACR Annual Meeting. 2019;79(Suppl 13).
BeiGene. BeiGene announces acceptance of New Drug Application for anti-PD-1 antibody tislelizumab in Hodgkins lymphoma in China. [media release] 31 Aug 2018. http://beigene.com.
BeiGene. Priority review granted to BeiGene's New Drug Applications for zanubrutinib and tislelizumab in China. [media release] 15 Nov 2018. http://beigene.com.
BeiGene. Priority review granted to BeiGenes supplemental New Drug Application in China for tislelizumab in urothelial carcinoma. [media release] 7 Jul 2019. http://beigene.com.
BeiGene. BeiGene announces acceptance of a supplemental New Drug Application in China for tislelizumab in urothelial carcinoma. [media release] 30 May 2019. http://www.beigene.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Arnold Lee and Susan Keam are salaried employees of Adis International Ltd/Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.11910855.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lee, A., Keam, S.J. Tislelizumab: First Approval. Drugs 80, 617–624 (2020). https://doi.org/10.1007/s40265-020-01286-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01286-z