Abstract
Talquetamab (talquetamab-tgvs; TALVEY®), a humanized, bispecific G-protein coupled receptor family C group 5 member D (GPRC5D)-directed CD3 T-cell engager, is being developed by Janssen for the treatment of multiple myeloma (MM). In early August 2023, talquetamab was granted accelerated approval in the USA for the treatment of adults with relapsed or refractory MM (RRMM) and in late August 2023, talquetamab was granted conditional marketing authorisation in the EU for the treatment of adult patients with RRMM. This article summarizes the milestones in the development of talquetamab leading to this first approval for RRMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.24104082. |
A humanized, bispecific GPRC5D-directed CD3 T-cell engager being developed by Janssen for the treatment of MM |
Received its first approval on 9 August 2023 in the USA under accelerated approval |
Approved in the USA for the treatment of adult patients with RRMM who have received ≥ 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody |
1 Introduction
The development of B cell maturation antigen (BCMA)-specific T-cell redirecting therapies (bispecific T-cell antibodies that simultaneously bind to a protein on the surface of T-cells and a target on the surface of cancer cells) has significantly changed the outlook for patients with multiple myeloma (MM) who have relapsed or refractory disease (RRMM) after treatment with multiple therapies, including proteosome inhibitors, immunomodulatory agents and CD38-targeting antibodies [1,2,3,4]. This has included the anti-BCMA bispecific T-cell antibodies teclistamab and elranatamab. However, the response to BMCA-targeted treatment is not always durable and most patients experience further disease relapse. Thus, there is a need for additional targets for T-cell redirection, ideally proteins that are preferentially expressed on tumour cells compared with critical normal cells [1, 2, 5]. G-protein coupled receptor family C group 5 member D (GPRC5D), an orphan G-coupled protein receptor, has been identified as a potential target for immunotherapy in RRMM because it is highly and selectively expressed on the cell surface of MM cells independently of BCMA, with minimal or no expression on normal B-cells or B-cell precursors, and is only expressed on the surface of epithelial cells in keratinized tissues of the skin and tongue in normal tissues [1, 2, 6, 7].

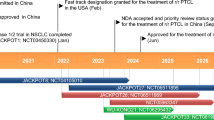
Key milestones in the development of talquetamab in multiple myeloma. BLA biologics license application, MAA marketing authorisation application, (RR)MM (relapsed/refractory) multiple myeloma
Talquetamab (talquetamab-tgvs; TALVEY®), a bispecific T-cell engaging antibody that binds to both the CD3 receptor expressed on the surface of T-cells and to GPRC5D-expressing cells [6, 7] is being developed by Janssen for the treatment of MM [8]. On 9 August 2023, talquetamab was granted accelerated approval in the USA for the treatment of adult patients with RRMM who have received ≥ 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody [8, 9]. On 22 August 2023, talquetamab was granted conditional marketing authorisation in the EU as monotherapy for the treatment of adult patients with RRMM who have received ≥ 3 prior therapies, including an immunomodulatory agent, a proteasome inhibitor and an anti-CD38 antibody and have demonstrated disease progression on the last therapy [7, 10].
Talquetamab is administered as a subcutaneous (SC) injection according to step-up weekly or bi-weekly (every 2 weeks) dose regimens to reduce the incidence and severity of cytokine release syndrome (CRS) [6, 7]. Pretreatment medications [corticosteroid (oral or IV dexamethasone 16 mg or equivalent), antihistamines (oral or IV diphenhydramine 50 mg or equivalent), and antipyretics (oral or IV paracetamol 650–1000 mg or equivalent)] should be administered 1–3 h prior to each dose of talquetamab in the step-up dose regimen. Patients should be monitored for 48 h after all doses within the step-up dose regimen due to the risk of CRS and neurological toxicity [including immune effector cell-associated neurotoxicity syndrome (ICANS)] [6, 7]. For patients administered talquetamab using the weekly dose regimen, step-up dose 1 (0.01 mg/kg) is administered on day 1, step-up dose 2 (0.06 mg/kg) is administered on day 4 and the first treatment dose (0.4 mg/kg) is administered on day 7. Subsequent treatment doses (0.4 mg/kg once weekly) are administered 1 week after the first treatment dose and weekly thereafter. For patients receiving talquetamab using the biweekly dose regimen, step-up dose 1 (0.01 mg/kg) is administered on day 1, step-up dose 2 (0.06 mg/kg) is administered on day 4, step-up dose 3 (0.4 mg/kg) is administered on day 7 and the first treatment dose (0.8 mg/kg) is administered on day 10. Subsequent treatment doses (0.8 mg/kg every 2 weeks) are administered 2 weeks after the first treatment dose and every 2 weeks thereafter. Treatment should be continued until disease progression or unacceptable toxicity [6, 7]. There is a warning for CRS, including life-threatening or fatal reactions, and for neurological toxic reactions, including ICANS and serious and life-threatening or fatal reactions. Patients should be monitored for signs and symptoms of these adverse reactions for 48 h after all talquetamab doses during the step-up dosing schedule [6, 7]. Because of the risk of CRS and neurological toxicity, in the USA, talquetamab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy [6].
1.1 Company Agreements
In July 2012, Janssen Biotech and Genmab entered into a research and development collaboration to create and develop bispecific antibodies using Genmab's DuoBody® technology for a panel of up to 10 DuoBody® programmes for multiple disease targets [11]. In December 2013, the companies amended the agreement to allow Janssen Biotech to develop up to 10 additional programmes [12].
2 Scientific Summary
2.1 Pharmacodynamics
In vitro, talquetamab administration was associated with dose-dependent lysis of GPRC5D+ MM cell lines and primary MM cells obtained from patients with newly diagnosed MM and RRMM, but had no effect on a GPRC5D- lymphoma cell line [2, 13]. Coadministration of daratumumab with talquetamab had an additive effect on talquetamab-mediated primary MM cell lysis [2]. Talquetamab treatment led to a dose-dependent activation and degranulation of CD4+ and CD8+ T cells in vitro. T-cell activation was associated with increased secretion of interferon (IFN)-γ, TNF-α, interleukin-2 (IL-2), IL-4, IL-6, IL-10, IL17A and granzyme B [2, 13]. Talquetamab showed anti-tumour activity in human MM xenograft mouse models, preventing tumour growth and causing regression of established tumours [13].
In the phase 1/2 MonumenTAL-1 trial (NCT03399799/NCT04634552) in patients with heavily pretreated RRMM, the SC talquetamab 0.4 mg/kg weekly and 0.8 mg/kg biweekly dose regimens achieved similar levels of increased T cell activation and cytokine induction [14]. Increased serum concentrations of IL-6, IL-10, and IL-2R were observed in both the step-up and treatment periods with both dose regimens [6].
Although higher talquetamab exposures are associated with a higher incidence of adverse reactions such as oral toxicity, nail toxicity and skin reactions, exposure-response relationships for efficacy and the time course of the pharmacodynamic response of talquetamab have not been fully characterized [6]. Cytokine release during talquetamab treatment may suppress activity of CYP enzymes, increasing exposure to drugs that are CYP substrates [6].
2.2 Pharmacokinetics
The pharmacokinetics of SC talquetamab (Cmax, AUCτ) increased dose proportionally over dose ranges of 0.005–0.8 mg/kg weekly and 0.8–1.2 mg/kg biweekly in the MonumenTAL-1 trial [6, 14, 15]. The mean bioavailability of talquetamab after SC administration relative to IV dosing was 59% [6]. 90% of steady-state exposure was achieved at 16 weeks after the first treatment dose with both the 0.4 mg/kg weekly (17th treatment dose) and 0.8 mg/kg biweekly (9th treatment dose) dose regimens [6, 15] and was maintained at or above the concentration associated with the 90% maximal effective concentration identified in an ex vivo cytotoxicity assay [13, 15]. Exposure (Cmax, Ctrough, Cavg) at 16 weeks with both dose regimens was comparable (mean Cmax 2940 vs 3410 ng/mL; mean Ctrough 2410 vs 1930 ng/mL; mean Cavg 2730 vs 2770 ng/mL); mean accumulation ratios for Cmax, Ctrough and Cavg were 4.4, 4.6 and 5.1 with the weekly dose regimen and 1.8, 2.3 and 2.0 for the biweekly dose regimen. The median Tmax of talquetamab after the 1st and 17th treatment doses of talquetamab 0.4 mg/kg weekly was 3.7 days and 2.5 days, respectively, and after the 1st and 9th treatment doses of talquetamab 0.8 mg/kg biweekly was 3.4 days and 3.6 days, respectively. The mean Vd of talquetamab was 10.1 L [6].
Talquetamab is expected to be metabolized into small peptides via catabolic pathways. Talquetamab clearance decreases over time, with a mean maximal reduction of 40% from the first treatment dose to 16 weeks after the first treatment dose. Mean CL at 16 weeks after the first treatment dose was 0.90 L/day. The mean t1/2 was 8.41 days after the first treatment dose and 12.2 days at 16 weeks after the first treatment dose [6].
Features and properties of talquetamab
Alternative names | Talquetamab-tgvs; TALVEY; GPRC5D/CD3-duobody-antibody-JNJ-64407564; JNJ 64407564; JNJ-7564 |
Class | Antineoplastics; Bispecific antibodies; Immunotherapies |
Mechanism of action | Antibody-dependent cell cytotoxicity; Cytotoxic T lymphocyte stimulants |
Route of administration | SC |
Pharmacodynamics | Bispecific antibody that binds to both the CD3 receptor expressed on the surface of T-cells and to GPRC5D-expressing cells, including MM cells. Activates T-cells, causing release of proinflammatory cytokines resulting in lysis of MM cells. Anti-tumour activity demonstrated in mouse models of MM |
Pharmacokinetics | Mean bioavailability 59%; 90% of steady state exposure achieved at 16 wks after 1st treatment dose; similar exposure at steady state with 0.4 mg/kg weekly (17th treatment dose) and 0.8 mg/kg biweekly (9th treatment dose) dose regimens; Vd 10.1 L, mean CLss 0.9 L/day, mean t1/2ss 12.2 days |
Adverse events | |
Most frequent (any grade) | Pyrexia, CRS, dysgeusia, nail disorder, musculoskeletal pain, skin disorder, rash, fatigue, ↓ weight, dry mouth, xerosis, dysphagia, URTI, diarrhoea, hypotension, headache |
Occasional | ICANS |
ATC codes | |
WHO ATC code | L01F-X29 (Talquetamab) |
EphMRA ATC code | L1 (Antineoplastics) |
2.3 Therapeutic Trials
2.3.1 MonumenTAL-1 Trial
Treatment with the SC talquetamab 0.4 mg/kg weekly or 0.8 mg/kg biweekly dose regimens achieved a meaningful and durable response in patients with RRMM who had received ≥ 4 prior therapy lines in the MonumenTAL-1 trial (NCT03399799/ NCT04634552) [6, 7, 16]. In patients who had no prior exposure to T-cell redirecting therapies, the overall response rate (ORR) was 74.1% [very good partial response or better (≥ VGPR) 59.5%; complete response or better (≥ CR) 33.6%] in those receiving the 0.4 mg/kg weekly regimen (n = 143; median follow-up 18.8 months) and 71.7% (≥ VGPR 60.8%; ≥ CR 38.7%) in those receiving the 0.8 mg/kg biweekly regimen (n = 145; median follow-up 12.7 months) [7, 16]. Median progression-free survival (PFS) was 7.5 months in the weekly group and 11.9 months in the biweekly group. The median duration of response (DOR) was 9.5 months in the weekly group and not estimable in the biweekly group; 51.5% of patients in the weekly group and 76.3% of those in the biweekly group maintained a response for ≥ 9 months and the respective 12-month overall survival (OS) rates were 76.4% and 77.4% [7, 16]. In the cohort of patients with prior exposure to T-cell redirection therapy (including CAR-T cell therapy and/or a bispecific antibody) who were treated with either dose regimen (n = 51; median follow-up 14.8 months), the ORR was 64.7% (≥ VGPR 54.9%; ≥ CR 35.3%). Median PFS was 5.1 months, median DOR was 11.9 months and the 12-month OS rate was 62.9% [16].
In the phase 1 portion of the MonumenTAL-1 trial (NCT03399799) [14], the ORR was 70% in patients receiving SC talquetamab 0.405 mg/kg weekly (n = 30), 64% in patients receiving SC talquetamab 0.8 mg/kg weekly or biweekly (n = 44) and 72% in patients receiving the most active IV talquetamab doses (0.02–0.18 mg/kg) [n = 18] [14].
The phase 1 portion of MonumenTAL-1 enrolled 232 heavily pretreated patients with RRMM that had progressed with established therapies or who could not tolerate established therapies. 102 patients received IV talquetamab 0.0005–0.18 mg/kg weekly or biweekly with or without step-up doses, and 130 patients received SC talquetamab (0.005–0.405 mg/kg weekly, 0.8 mg/kg weekly or biweekly, 1.2 mg/kg biweekly or 1.6 mg/kg monthly) with step-up doses [14]. In the phase 2 portion of MonumenTAL-1 (NCT04634552), eligible patients had previously received ≥ 4 prior therapy lines including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 antibody. SC talquetamab was administered weekly or biweekly. Those treated with the weekly dose regimen received step-up doses of 0.01 mg/kg and 0.06 mg/kg followed by weekly doses of 0.4 mg/kg and those treated with the biweekly dose regimen received step-up doses of 0.01 mg/kg, 0.06 mg/kg, and 0.3 mg/kg followed by biweekly doses of 0.8 mg/kg [6, 7, 16]. In patients who were treated with SC talquetamab 0.4 mg/kg weekly or 0.8 mg/kg biweekly in either phase of MonumenTAL-1, median age at entry was 67 years, median number of prior therapy lines was 5 and median time since diagnosis was 6.7 years [6, 7, 16].
2.3.2 Other Trials
Treatment with the combination of SC talquetamab 0.4 mg/kg weekly or 0.8 mg/kg biweekly (with step up dosing) and SC daratumumab achieved a durable response in patients with heavily pretreated RRMM (n = 65) in the phase 1 TRIMM-2 trial (NCT04108195) [17, 18]. The ORR was 71.4% (≥ VGPR 57.1%; ≥ CR 42.9%) in patients in the talquetamab 0.4 mg/kg weekly arm (n = 14; median follow-up 16.8 months) and 84.0% (≥ VGPR 74.0%; ≥ CR 52.0%) in patients in the talquetamab 0.8 mg/kg biweekly arm (n = 50; median follow-up 15.0 months). At data cut-off, 65.4% of responders remained on treatment. Median DOR and median PFS had not been reached in the talquetamab 0.4 mg/kg weekly arm, and were 20.3 months and 19.4 months, respectively, in the talquetamab 0.8 mg/kg biweekly arm. The 12-month PFS rates were 77.4 and 67.4% in the talquetamab 0.4 mg/kg weekly and 0.8 mg/kg biweekly arms and 12-month OS rates were 92.3 and 91.5%, respectively. Median OS was not reached in either treatment arm. Eligible patients had received ≥ 3 prior therapy lines (including a proteasome inhibitor and an immunomodulatory agent) or were double refractory to a proteasome inhibitor and an immunomodulatory agent. At baseline, median age was 63 years and median number of prior therapy lines was 5 [17, 18].
Key clinical trials of talquetamab (Janssen)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Talquetamab, daratumumab, pomalidomide, dexamethasone | RRMM | 3 | Ongoing | Global | NCT05455320; MonumenTAL-3; EudraCT 2021-000202-22 |
Talquetamab, daratumumab, teclistamab, | RRMM | 1/2 | Ongoing | Canada, Israel, Republic of Korea, Spain | NCT04586426; RedirecTT-1; EudraCT 2019-004124-38 |
Talquetamab | RRMM | 1/2 | Ongoing | Global | NCT03399799/NCT04634552; MonumenTAL-1; EudraCT 2017-002400-26 |
Talquetamab | RRMM | 1 | Ongoing | Japan | NCT04773522 |
Talquetamab, teclistamab, PD-1 inhibitor | RRMM | 1 | Ongoing | USA, France, Germany, Spain | NCT05338775; TRIMM-3; EudraCT 2021-005073-22 |
Talquetamab, daratumumab, teclistamab, pomalidomide | RRMM | 1 | Ongoing | USA, Canada, Germany, Spain | NCT04108195; TRIMM-2 EudraCT2019-000330-19 |
Talquetamab, daratumumab, lenalidomide, teclistamab, dexamethasone | Newly diagnosed MM | 3 | Ongoing | Global | NCT05552222; MajesTEC-7; EudraCT 2022-000909-28 |
Talquetamab, teclistamab, daratumumab, bortezomib, lenalidomide, dexamethasone | Newly diagnosed MM | 2 | Ongoing | Spain | NCT05849610; GEM-TECTAL; EudraCT2022-000598-15 |
Talquetamab, carfilzomib, daratumumab, lenalidomide, pomalidomide | Newly diagnosed MM | 1 | Ongoing | Global | NCT05050097; MonumenTAL-2; EudraCT 2020-004502-55 |
The combination of SC teclistamab (a BMCA-directed bispecific antibody) and SC talquetamab achieved a good response in patients with heavily pretreated RRMM, including those with extramedullary disease, in the dose escalating phase 1b portion of the phase 1/2 RedirecTT-1 trial (NCT04586426) [19]. The ORR was 86.6% (≥ CR 40.2%) across all dose levels (n = 93; median follow-up 13.4 months) and 96.3% (≥ CR 40.7%) in patients who received the recommended phase 2 dose regimen of SC teclistamab 3.0 mg/kg biweekly plus SC talquetamab 0.8 mg/kg biweekly (n = 34; median follow-up 8.1 months). The median DOR was not reached in either treatment group; median PFS was 20.9 months across all dose levels and not reached in the recommended phase 2 regimen group. In the subgroup of patients with extramedullary disease, the ORR was 71.4% (≥ CR 21.2%) across all dose levels (n = 35) and 85.7% (≥ CR 28.6%) with the recommended phase 2 regimen (n = 11; median follow-up 7.2 months). The median DOR was 12.9 months in the all dose levels group and not reached in the recommended phase 2 regimen group; median PFS in the respective groups was 6.1 months and 9.9 months [19]. Eligible patients were refractory or intolerant to established treatments for MM and had previously been exposed to a proteasome inhibitor, an immunomodulatory agent and a CD38-targeting antibody. At baseline, median age was 65 years, median number of prior therapy lines was 4, median time since diagnosis was 5.9 years and 38% of patients had extramedullary disease [19].
2.4 Adverse Events
The most frequent adverse reactions (incidence ≥ 20%) reported in SC talquetamab recipients (n = 339) in the MonumenTAL-1 trial (NCT03399799/NCT04634552) were pyrexia (83.0% any grade; 4.7% grade 3 or 4), CRS (76.0%; 1.5%), dysgeusia (70.0%; 0%), nail disorder (50.0%; 0%), musculoskeletal pain (43.0%; 3.2%), skin disorder (41.0%; 0.3%), rash (38.0%; 3.5%), fatigue (37.0%; 3.5%), decreased weight (35.0%; 1.5%), dry mouth (34.0%; 0%), xerosis (30.0%; 0%), dysphagia (23.0%; 0.9%), upper respiratory tract infection (URTI) [22.0%; 2.7%], diarrhoea (21.0%; 0.9%), hypotension (21.0%; 2.9%) and headache (21.0%; 0.6%) [6]. The most common Grade 3 or 4 laboratory abnormalities (incidence ≥ 30%) were decreased lymphocyte count (80%), decreased neutrophil count (35%), decreased white blood cell count (35%), decreased haemoglobin (30%) and decreased platelet count (22%) [6].
Serious adverse reactions were reported in 47% of talquetamab recipients, the most frequent of which were CRS (13%), bacterial infection including sepsis (8%), pyrexia (4.7%), ICANS (3.8%), COVID-19 infection (2.7%), neutropenia (2.1%), and URTI (2.1%). Adverse reactions were generally manageable; dosage interruptions due to an adverse reaction occurred in 56% of talquetamab recipients and permanent discontinuation due to an adverse event occurred in 9% [6]. Fatal adverse reactions occurred in 3.2% of SC talquetamab recipients [6]; however, no deaths were considered to be talquetamab related [14, 16].
Most of these CRS events in the MonumenTAL-1 trial occurred after step-up dose 1 (29%), step-up dose 2 (44%) or step-up dose 3 (33%; biweekly regimen). The median time to CRS was 27 h from the last dose and median duration was 17 h [6]. Neurological toxicity (all grades) occurred in 55% of patients who received the recommended talquetamab weekly or biweekly dose regimen; 6% of patients experienced grade 3 or 4 neurological toxicity. The most common toxicities were headache (20%), encephalopathy (15%), sensory neuropathy (14%) and motor dysfunction (10%). ICANS was reported in 9% of evaluable patients (n = 265), mostly following step-up doses and recurrent ICANS was reported in 3%. The median time to onset of ICANS was 2.5 days after the most recent dose and median duration was 2 days [6].
Although anti-talquetamab antibodies developed in 25% of patients treated with the recommended weekly dose regimen and 18% of those treated with the biweekly dose regimen during up to 25 months’ treatment in the MonumenTAL-1 trial, there was no clinically significant effect on talquetamab pharmacokinetics, pharmacodynamics, safety or efficacy in these patients [6].
2.5 Ongoing Clinical Trials
In addition to MonumenTAL-1, TRIMM-2 and RedirecTT-1, three other trials of talquetamab in RRMM are ongoing: the phase 3 MonumenTAL-3 combination therapy trial (NCT05455320), the phase 1 TRIMM-3 combination therapy trial (NCT05338775) and a phase 1 monotherapy Japanese trial (NCT04773522). Talquetamab is also being investigated in combination with other cancer therapies in patients with newly diagnosed MM in the phase 3 MajesTEC-7 trial (NCT05552222), the phase 2 GEM-TECAL trial (NCT05849610) and the phase 1 MonumenTAL-2 trial (NCT05050097).
3 Current Status
Talquetamab received its first approval in the USA under accelerated approval on 9 August 2023 for the treatment of adult patients with RRMM who have received ≥ 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody [6, 9]. On 22 August 2023, talquetamab was granted conditional marketing authorisation in the EU for use as monotherapy for the treatment of adult patients with RRMM who have received ≥ 3 prior therapies, including an immunomodulatory agent, a proteasome inhibitor and an anti-CD38 antibody and have demonstrated disease progression on the last therapy [7, 10].
References
Nath K, Costa BA, Mailankody S. GPRC5D as a novel immunotherapeutic target in multiple myeloma. Nat Rev Clin Oncol. 2023;20(5):281–2.
Verkleij CPM, Broekmans MEC, van Duin M, et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5(8):2196–215.
Swan D, Murphy P, Glavey S, et al. Bispecific antibodies in multiple myeloma: opportunities to enhance efficacy and improve safety. Cancers. 2023;15(6). https://doi.org/10.3390/cancers15061819.
Ravi G, Costa LJ. Bispecific T-cell engagers for treatment of multiple myeloma. Am J Hematol. 2023;98(S2):S13–21.
Harousseau JL, Mohty M. Sequencing anti-BCMA therapies in myeloma. Blood. 2023;141(3):211–2.
Janssen Biotech Inc. Talquetamab (talquetamab-tgvs): US prescribing information. 2023. https://www.talvey.com/. Accessed 11 Aug 2023.
European Medicines Agency. Talquetamab: EU prescribing information. 2023. https://ec.europa.eu/health/documents/community-register/2023/20230821160195/anx_160195_en.pdf. Accessed 28 Aug 2023.
Janssen. U.S. FDA approves TALVEY™ (talquetamab-tgvs), a first-in-class bispecific therapy for the treatment of patients with heavily pretreated multiple myeloma [media release]. 10 Aug 2023. https://www.jnj.com/.
US Food & Drug Administration. FDA grants accelerated approval to talquetamab-tgvs for relapsed or refractory multiple myeloma [media release]. 8 Aug 2023. https://www.fda.gov/.
Janssen. European Commission approves TALVEY® (talquetamab), Janssen’s novel bispecific therapy for the treatment of patients with relapsed and refractory multiple myeloma [media release]. 22 Aug 2023. https://www.janssen.com/.
Genmab A/S. Genmab enters broad collaboration with Janssen Biotech, Inc. for DuoBody platform [media release]. 12 Jul 2012. https://www.genmab.com.
Genmab A/S. Genmab announces expansion of DuoBody platform collaboration with Janssen Biotech, Inc. [media release]. 4 Dec 2013. https://www.genmab.com.
Pillarisetti K, Edavettal S, Mendonça M, et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135(15):1232–43.
Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232–44.
Ma X, Gong J, Zhou J, et al. Efficacy, safety, pharmacokinetic (PK), and pharmacodynamic (PD) support for talquetamab (tal) QW and Q2W dosing in patients (pts) with relapsed/refractory multiple myeloma (RRMM): analyses from MonumenTAL-1 [abstract no. 8041]. J Clin Oncol. 2023;41(16 Suppl):8041.
Schinke CD, Touzeau C, Minnema MC, et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM) [abstract no. 8036 plus presentation]. J Clin Oncol. 2023;41(16 Suppl):8036.
Dholaria BR, Weisel K, Mateos MV, et al. Talquetamab (tal) + daratumumab (dara) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): updated TRIMM-2 results [abstract no. 8003]. J Clin Oncol. 2023;41(16 Suppl):8003.
Janssen. Janssen presents longer-term talquetamab follow-up data showing overall response rates of more than 70 percent in heavily pretreated patients with multiple myeloma [media release]. 3 June 2023. https://www.janssen.com.
Cohen YC, Morillo D, Gatt ME, et al. First results from the RedirecTT-1 study with teclistamab (tec) + talquetamab (tal) simultaneously targeting BCMA and GPRC5D in patients (pts) with relapsed/refractory multiple myeloma (RRMM) [abstract no. 8002 plus presentation]. J Clin Oncol. 2023;41(16 Suppl):8002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Talquetamab: First Approval. Drugs 83, 1439–1445 (2023). https://doi.org/10.1007/s40265-023-01945-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01945-x