Abstract
Sintilimab (Tyvyt®) is a fully human IgG4 monoclonal antibody that binds to programmed cell death receptor-1 (PD-1), thereby blocking the interaction of PD-1 with its ligands (PD-L1 and PL-L2) and consequently helping to restore the endogenous antitumour T-cell response. It has been co-developed by Innovent Biologics and Eli Lilly and Company, and was recently approved in China for the treatment of classical Hodgkin’s lymphoma in patients who have relapsed or are refractory after ≥ 2 lines of systemic chemotherapy. Sintilimab is undergoing phase I, II and III development for use in various solid tumours, including non-small cell lung cancer and oesophageal cancer, in China. Phase I/II development of sintilimab for use in solid tumours is underway in the USA, with the US FDA accepting an Investigational New Drug application for sintilimab in January 2018. This article summarizes the milestones in the development of sintilimab leading to this first approval for the treatment of classical Hodgkin’s lymphoma in patients who have relapsed or are refractory after ≥ 2 lines of systemic chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Immune checkpoint pathways are involved in regulating the immune response; they may be appropriated by tumours expressing immune checkpoint proteins, thereby dysregulating the host’s antitumour response, which favours tumour cell growth and expansion [1, 2]. Disrupting the reliance of tumours on these pathways is one of the approaches utilized to enhance antitumour immunity [1]. Indeed, blocking interactions between the immune checkpoint protein programmed cell death receptor-1 (PD-1) and its ligands (PD-L1 and PD-L2) with an anti-PD-1 antibody has resulted in promising overall response rates in patients with relapsed or refractory Hodgkin’s lymphoma [3]. Sintilimab (Tyvyt®) is a fully human IgG4 anti-PD-1 monoclonal antibody co-developed by Innovent Biologics and Eli Lilly and Company [4, 5]. In December 2018, it was approved by the National Medical Products Administration of China for the treatment of classical Hodgkin’s lymphoma in patients who have relapsed or are refractory after ≥ 2 lines of systemic chemotherapy [4]. The recommended dosage is 200 mg once every 3 weeks, administered as an intravenous infusion over 30–60 min, until disease progression or unacceptable toxicity [5]. Local prescribing information should be consulted for information regarding dose modifications for the management of adverse events [5].

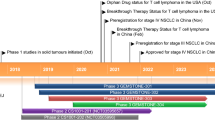
Key milestones in the development of sintilimab. See key clinical trials table for details on the phase III trials. cHL classical Hodgkin’s lymphoma, Est. estimated, IND Investigational New Drug, NDA New Drug Application, NSCLC non-small cell lung cancer
Sintilimab is undergoing phase I, II and III development for use in various solid tumours, including non-small cell lung cancer and oesophageal cancer, in China. Phase I/II development of sintilimab for use in solid tumours is underway in the USA, with the US FDA accepting an Investigational New Drug application for sintilimab in January 2018 [6].
1.1 Company Agreements
In March 2015, Innovent Biologics and Eli Lilly and Company entered into a development, manufacturing and commercialisation agreement to co-develop ≥ 3 cancer treatments [two from Innovent Biologics (with one being an immune-oncology molecule) and one from Eli Lilly and Company] over the next decade [7]. Under the terms of the agreement, Innovent Biologics received an upfront payment of $US56 million and will be eligible to receive future payments exceeding $US400 million if Innovent Biologics’ immune-oncology molecule achieves certain development, regulatory and commercial milestones [7]. In October 2015, the two companies expanded this collaboration to develop and commercialise up to three anti-PD-1-based bispecific antibodies for cancer therapy over the next decade within and outside of China [8]. Under the terms of the collaboration, Innovent Biologics will have the right to develop, manufacture and commercialize the antibodies within China (subject to the Eli Lilly and Company opt-in right for co-development and commercialisation), while Eli Lilly and Company will have the right to develop, manufacture and commercialize these antibodies outside of China. In addition, Innovate will be eligible to receive additional payments of over US$1 billion if the antibodies achieve certain development, regulatory and commercial milestones, both within and outside of China [8].
In November 2018, Innovent Biologics and Hutchison China MediTech entered into a global collaboration agreement to evaluate the safety and tolerability of sintilimab in combination with fruquintinib (a vascular endothelial growth factor receptor inhibitor) in patients with advanced solid tumours in China and the USA [9].
2 Scientific Summary
2.1 Pharmacodynamics
By binding to PD-1 (an inhibitory receptor expressed on activated T cells), sintilimab blocks its interaction with PD-L1 and PD-L2, thereby helping to restore the endogenous antitumour T-cell response [5, 9, 10]. In vitro, sintilimab binds with high affinity (dissociation constant of 0.25 nmol/L) and specificity to human PD-1, and efficiently inhibits the binding of human PD-1 to PD-L1 and PD-L2 (half maximal concentrations of 4.373 and 4.494 mg/L) [10]. According to an analysis of 13 patients with relapsed or refractory classical Hodgkin’s lymphoma participating in a multicentre, phase II study [NCT03114683 (ORIENT-1); see Sect. 2.3 for details], sintilimab (at a dose of 200 mg) rapidly (within 24 h) occupied PD-1 receptors on the surface of CD3-positive T cells in peripheral blood [11]. Mean PD-1 receptor occupancy was ≥ 95%, with high (> 90%) mean occupancy maintained with sintilimab 200 mg every 3 weeks for 18 cycles [11]. In vitro, sintilimab significantly (p value vs. control not reported) increased interleukin-2 and interferon-γ levels in a dose-dependent manner, with its potency comparable to that of nivolumab. Sintilimab did not, however, directly induce cytokine release [10].
Sintilimab demonstrated potent antitumour efficacy in a therapeutic human PD-1 knock-in mouse model of colon adenocarcinoma [10]. Compared with control, sintilimab 1 and 5 mg/kg significantly (p < 0.05) inhibited tumour growth (by 84 and 100%), with apparent maximal efficacy achieved with the 5 mg/kg dose. Moreover, in the same model, sintilimab significantly (p < 0.05) increased the ratios of tumour-infiltrating CD8/CD4 and CD8/Treg cells compared with control at day 14 [10].
2.2 Pharmacokinetics
Sintilimab exhibited linear pharmacokinetics over a 1–10 mg/kg dose range, according to a first-in-human, open-label, phase Ia, dose-escalation study (NCT02937116) [data from an abstract] in adults (aged ≥ 18 years) with advanced solid tumours [12].
Features and properties of sintilimab
Alternative names | IBI-308; PD-1 antibody—Eli Lilly/Innovent Biologics; sintilimab injection; Tyvyt |
Class | Antineoplastics; monoclonal antibodies |
Mechanism of action | Antibody-dependent cell cytotoxicity; programmed cell death-1 receptor antagonists; T lymphocyte stimulants |
Route of administration | Intravenous infusion |
Pharmacodynamics | Fully human IgG4 monoclonal antibody; by specifically binding to programmed cell death receptor-1 (PD-1) on activated T cells, sintilimab blocks PD-1 interacting with its ligands (PD-L1 and PL-L2) |
Exhibited rapid, high and sustained PD-1 receptor occupancy in patients with relapsed or refractory classical Hodgkin’s lymphoma; demonstrated potent antitumour efficacy in a mouse model of colon adenocarcinoma | |
Pharmacokinetics | Exhibited linear pharmacokinetics over a 1–10 mg/kg dose range |
Most frequent adverse event | Pyrexia |
ATC codes | |
WHO ATC code | L01X-C (monoclonal antibodies) |
EphMRA ATC code | L1G (monoclonal antibody antineoplastics) |
Chemical name | Immunoglobulin G4, anti-(human programmed cell death protein 1) (human monoclonal IBI308 gamma4-chain), disulphide with human monoclonal IBI308 kappa-chain, dimer |
2.3 Therapeutic Trials
2.3.1 Monotherapy
Sintilimab as monotherapy displayed preliminary activity in 12 adults with advanced solid tumours participating in a first-in-human, open-label, phase Ia, dose-escalation study (NCT02937116) [data from an abstract] [12]. The maximum tolerated dosage of sintilimab was not reached in any of the treatment arms (1, 3 or 10 mg/kg every 2 weeks, or 200 mg every 3 weeks), with the 200 mg once every 3 weeks dosage selected for further study [12].
Monotherapy with sintilimab was associated with promising activity in adults with classical Hodgkin’s lymphoma that was relapsed or refractory after receiving ≥ 2 lines of chemotherapy participating in a multicentre, phase II study [NCT03114683 (ORIENT-1)] [11]. At the data cut-off date of 16 April 2018 (median follow-up duration of 10.5 months), an independent radiological review committee (IRRC)-assessed objective response (OR; i.e. complete or partial remission) at 24 weeks (primary endpoint) was achieved by 80% of 92 patients. The beneficial effects of sintilimab on IRRC-assessed OR at 24 weeks were generally similar across various subgroups (e.g. number of previous lines of chemotherapy; history of radiotherapy; history of autologous hematopoietic stem cell transplantation; refractoriness to first-line chemotherapy, most recent chemotherapy or all prior therapies). IRRC-assessed complete remission, partial remission and stable disease at 24 weeks was achieved by 34, 47 and 17% of patients, respectively. These results were consistent with those of investigator analyses, with an OR achieved by 79% of patients, and complete remission, partial remission and stable disease occurring in 16, 63 and 21% of patients, respectively. IRRC- and investigator-assessed progressive disease at 24 weeks occurred in 2 and 0% of patients [11].
Among the 74 patients who achieved an IRRC-assessed OR, the median time to that response was 42 days, with 77% of patients achieving an IRRC-assessed OR by week 6 [11]. The median duration of response was not reached in either the 74 patients who achieved an IRRC-assessed OR or the 73 patients who achieved an investigator-assessed OR [11].
At the data cut-off date (full analysis population), IRRC-assessed median progression-free survival (PFS) was not reached and no deaths had occurred; IRRC-assessed PFS at 6 months was 77.6% [11].
Monotherapy with sintilimab was associated with numerical improvements in quality of life [as assessed by the 5-level EQ-5D (EQ-5D-5L) visual analogue score (VAS) and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 global health status/quality of life score] in ORIENT-1 [11]. Moreover, a significantly (p = 0.04) greater least-squares mean improvement from baseline in the EQ-5D-5L VAS was seen in responders (n = 64) compared with non-responders (n = 14) by the ninth cycle, according to a post hoc analysis [11].
ORIENT-1 is a noncomparative, open-label, multicentre, phase II study in which patients received sintilimab 200 mg, administered intravenously over 30–60 min, once every 3 weeks for a maximum of 24 months [11]. Patients were treated (median duration of exposure to sintilimab of 8.4 months) until disease progression, death, unacceptable toxicity or the withdrawal of consent. Analyses were conducted in the full-analysis population [11].
An encouraging response (disease control in 47.6% of patients, with 19.0% achieving a response) to sintilimab monotherapy (administered as 200 mg once every 3 weeks in most patients) was seen in a study in 21 patients with advanced neuroendocrine neoplasms who have failed standard therapy (data from an abstract) [13].
2.3.2 Combination Therapy
Preliminary activity with sintilimab (200 mg once every 3 weeks) in combination with gemcitabine and cisplatin was demonstrated in the treatment-naive, locally advanced (stage IIIb), recurrent or metastatic (stage IV) squamous non-small cell lung cancer cohort of a six-cohort, open-label, multicentre, phase Ib study (NCT02937116) [14]. Almost two-thirds (64.7%) of the 17 adults achieved an objective response; the remaining patients had a best response of stable disease. Median PFS and median duration of response were not reached. Patients in this cohort were treated until disease progression or unacceptable toxicity (median treatment duration of 21.1 weeks) [14].
Key clinical trials of sintilimab
Drug(s) | Indication | Phase | Status | Location | Identifier | Sponsor |
|---|---|---|---|---|---|---|
Sintilimab | Advanced/metastatic solid malignancies | I/II | Recruiting | USA | NCT03568539 | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab | Relapsed/refractory classical Hodgkin’s lymphoma | II | Active, not recruiting | NR | NCT03114683 (ORIENT-1) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab vs. paclitaxel/irinotecan | Advanced/metastatic esophageal squamous cell carcinoma | II | Not yet recruiting | NR | NCT03116152 | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab | Relapsed/refractory extranodal NK/T cell lymphoma | II | Not yet recruiting | NR | NCT03228836 (ORIENT-4) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab + gemcitabine + cisplatin + radiotherapy | Nasopharyngeal neoplasms | II | Not yet recruiting | China | NCT03619824 | Sun Yat-sen University |
Sintilimab | Thoracic cancer | II | Recruiting | China | NCT03732430 (CARTAI) | Taizhou Hospital |
Sintilimab + anlotinib | Carcinoma, NSCLC, lung neoplasm | II | Recruiting | China | NCT03765775 | First Hospital of Shijiazhuang City |
Sintilimab + docetaxel | NSCLC | II | Recruiting | China | NCT03798743 (SUCCESS) | Hunan Province Tumor Hospital |
Sintilimab + IBI305 vs. sorafenib | Hepatocellular carcinoma | II/III | Not yet recruiting | NR | NCT03794440 (ORIENT-32) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab vs. docetaxel | Squamous cell lung carcinoma | III | Recruiting | China | NCT03150875 (ORIENT-3) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab + pemetrexed + platinum vs. placebo + pemetrexed + platinum | Non-squamous NSCLC | III | Not yet recruiting | NR | NCT03607539 (ORIENT-11) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab + gemcitabine + platinum vs. placebo + gemcitabine + platinum | Squamous NSCLC | III | Not yet recruiting | NR | NCT03629925 (ORIENT-12) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab + gemcitabine + cisplatin + radiotherapy vs. gemcitabine + cisplatin + radiotherapy | Nasopharyngeal neoplasms | III | Not yet recruiting | China | NCT03700476 | Sun Yat-sen University |
Sintilimab + oxaliplatin + capecitabine vs. placebo + oxaliplatin + capecitabine | Gastric cancer | III | Recruiting | China | NCT03745170 (ORIENT-16) | Innovent Biologics (Suzhou) Co. Ltd. |
Sintilimab + paclitaxel + cisplatin vs. placebo + paclitaxel + cisplatin | Oesophageal squamous cell carcinoma | III | Not yet recruiting | NR | NCT03748134 (ORIENT-15) | Innovent Biologics (Suzhou) Co. Ltd. |
2.4 Adverse Events
Sintilimab as monotherapy had a manageable safety profile in adults with relapsed or refractory classical Hodgkin’s lymphoma participating in ORIENT-1 [11] that was similar to the published safety profiles of nivolumab and pembrolizumab. No unexpected or off-target safety signals were seen [11].
Although all of the patients in ORIENT-1 (n = 96) experienced a treatment-emergent adverse event (TEAE), few (n = 3) discontinued treatment because of a TEAE (grade 2 pneumonitis; grade 4 liver function abnormality; grade 3 pneumonitis and grade 4 decreased platelet count) [11]. Most TEAEs were grade 1 or 2 in severity; 25% of patients experienced a grade 3 or 4 TEAE, with no grade 5 TEAEs (i.e. TEAEs leading to death) reported after therapy commenced. The most frequently reported TEAE was pyrexia; 43% of patients experienced grade 1 or 2 pyrexia and 3% experienced grade 3 pyrexia. Of note, most of the pyrexia cases occurred within 24 h of the first infusion, resolved on the same day and did not reoccur with subsequent infusions [11].
Treatment-related adverse events (TRAEs; as assessed by the investigator) of any grade were reported in 93% of patients, with less than one-fifth (18%) experiencing grade 3 or 4 TRAEs [11]. Pyrexia was the most frequently reported TRAE, occurring as a grade 1 or 2 TRAE in 38% of patients and as a grade 3 TRAE in 3%. The incidence of each of the other grade 3 or 4 TRAEs was ≤ 2%. Serious TEAEs occurred in 15% of patients, with pneumonitis and lung infection (each occurring in 3% of patients) being the most frequently reported. Serious TRAEs were reported in 11% of patients; the reported serious TRAEs were pneumonitis and lung infection (each occurring in three patients); infusion reaction (occurring in two patients); and decreased platelet count, hyperthyroidism, liver function abnormality, peripheral neuropathy and upper respiratory tract infection (each occurring in one patient) [11].
Protocol-defined immune-related adverse events (all grades) occurred in 54% of patients, with hypothyroidism (occurring in 20% of patients), increased blood thyroid-stimulating hormone levels (17%) and decreased free thyroxine levels (11%) the most frequently reported [11]. Grade 3 or 4 immune-related adverse events (grade 3 increased alanine aminotransferase levels; grade 4 liver function abnormality; grade 3 pneumonitis and grade 4 decreased platelet count) were reported in three patients. Infusion reactions occurred in 13% of patients, with pyrexia (in 9% of patients) the most frequently reported [11].
Given the nature of cancer therapy, sintilimab in combination with gemcitabine and cisplatin was generally well tolerated in the treatment-naive, locally advanced, recurrent or metastatic squamous non-small cell lung cancer cohort of a multicentre, phase Ib study (NCT02937116) [14]. TEAEs occurred in all (n = 20) of the patients, with 70% being grade 3 or higher TEAEs, although none were considered to be related to sintilimab. The most frequently reported TEAEs were rash (occurring in three patients) and fever (occurring in two patients). Serious TEAEs were seen in eight patients, with all of the patients recovering or the TEAEs resolving following management apart from one case of dysphagia. No TEAEs resulting in death occurred. Immune-related adverse events (hyperthyroidism, pneumonitis and rash) occurred in three patients [14].
2.5 Ongoing Clinical Trials
There are several ongoing phase I/II (NCT03568539), II [NCT03114683 (ORIENT-1); NCT03116152; NCT03228836 (ORIENT-4); NCT03619824; NCT03732430 (CARTAI); NCT03765775; NCT03798743 (SUCCESS)] and II/III [NCT03794440 (ORIENT-32)] studies of sintilimab for the treatment of various solid tumours. In addition, a number of ongoing phase III studies are assessing sintilimab for the treatment of squamous cell lung carcinoma [NCT03150875 (ORIENT-3)]; non-squamous [NCT03607539 (ORIENT-11)] and squamous [NCT03629925 (ORIENT-12)] non-small cell lung cancer; nasopharyngeal neoplasms (NCT03700476); gastric cancer [NCT03745170 (ORIENT-16)] and oesophageal squamous cell carcinoma [NCT03748134 (ORIENT-15)].
3 Current Status
Sintilimab received its first global approval on 24 December 2018 for the treatment of classical Hodgkin’s lymphoma in patients who have relapsed or are refractory after ≥ 2 lines of systemic chemotherapy in China [4].
References
Merryman RW, Armand P, Wright KT, et al. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1(26):2643–54.
Marin-Acevedo JA, Dholaria B, Soyano AE, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39.
Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(5):704–15.
Innovent Biologics. China’s NMPA approves Innovent’s anti-PD-1 antibody Tyvyt® (sintilimab injection) for Hodgkin’s lymphoma [media release]. 26 Dec 2018. http://innoventbio.com/en/#/news/123.
Innovent Biologics. Tyvyt® (sintilimab): Chinese prescribing information. Suzhou: Innovent Biologics; 2019.
Innovent Biologics. Innovent receives an approval from the US FDA to initiate clinical trials for its anti-CD47 monoclonal antibody IBI-188 [media release]. 29 Sept 2018. http://innoventbio.com/en/#/news/111.
Eli Lilly and Company. Lilly and Innovent Biologics announce a strategic alliance to bring potential oncology therapies to patients in China and around the world [media release]. 20 Mar 2015. http://lilly.mediaroom.com/.
Eli Lilly and Company. Lilly and Innovent Biologics expand their strategic alliance to include immuno-oncology bispecific antibodies in China and globally [media release]. 11 Oct 2015. http://lilly.mediaroom.com/.
Innovent Biologics. Innovent announces global collaboration with Hutchison MediPharma to evaluate combination of sintilimab and fruquintinib in solid tumors [media release]. 29 Nov 2018. http://innoventbio.com/en/#/news/119.
Zhang S, Zhang M, Wu W, et al. Preclinical characterization of sintilimab, a fully human anti-PD-1 therapeutic monoclonal antibody for cancer. Antibody Ther. 2018;1(2):65–73.
Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6(1):e12–9.
Xu JM, Jia R, Wang Y, et al. A first-in-human phase 1a trial of sintilimab (IBI308), a monoclonal antibody targeting programmed death-1 (PD-1), in Chinese patients with advanced solid tumors [abstract no. e15125]. J Clin Oncol. 2018;36(15 Suppl).
Jia R, Li YL, Jin YS, et al. Efficacy and safety of anti-PD-1 antibody (IBI308) in treating advanced neuroendocrine neoplams [abstract no. 2143]. Neuroendocrinology. 2018;106(Suppl 1):230.
Ying K, Xu N, Jiang H, et al. Efficacy and safety of sintilimab combined with 1st line chemotherapy in advanced squamous cell non-small cell lung cancer [abstract no. OA08]. J Thorac Oncol. 2018;13 (12 Suppl):S1047.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan Hoy is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Sintilimab: First Global Approval. Drugs 79, 341–346 (2019). https://doi.org/10.1007/s40265-019-1066-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-1066-z