Abstract
A gradual rise in drug-resistant trends among Gram-negative organisms, especially carbapenem-resistant (CR) Enterobacteriaceae (CRE), CR-Pseudomonas aeruginosa, and extensively-drug-resistant (XDR) Acinetobacter baumannii, poses an enormous threat to healthcare systems worldwide. In the last decade, many pharmaceutical companies have devoted enormous resources to the development of new potent antibiotics against XDR Gram-negative pathogens, particularly CRE. Some of these novel antibiotics against CRE strains are β-lactam/β-lactamase-inhibitor combination agents, while others belong to the non-β-lactam class. Most of these antibiotics display good in vitro activity against the producers of Ambler class A, C, and D β-lactamase, although avibactam and vaborbactam are not active in vitro against metallo-β-lactamase (MβL) enzymes. Nevertheless, in vitro efficacy against the producers of some or all class B enzymes (New Delhi MβL, Verona integron-encoded MβL, etc) has been shown with cefepime-zidebactam, aztreonam-avibactam, VNRX-5133, cefiderocol, plazomicin, and eravacycline. As of Feburary 2019, drugs approved for treatment of some CRE-related infections by the US Food and Drug Administration included ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, and eravacycline. Although active against extended-spectrum and AmpC β-lactamase-producing Enterobacteriaceae, delafloxacin does not show in vitro activity against CRE. Murepavadin is shown to be specifically active against CR- and colistin-resistant P. aeruginosa strains. Despite successful development of novel antibiotics, strict implementation of an antibiotic stewardship policy in combination with the use of well-established phenotypic tests and novel multiplex PCR methods for detection of the most commonly encountered β-lactamases/carbapenemases in hospitals is important for prescribing effective antibiotics against CRE and decreasing the resistance burden due to CRE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Because of the rise of multidrug-resistance over the last decade, an antibiotic pipeline for Gram-negative bacteria (GNB) has emerged, especially for carbapenem-resistant Enterobacteriaceae (CRE) and Acinetobacter baumannii complex isolates. |
Novel antibiotics against extensively drug-resistant GNB are grossly categorized into β-lactam combination agents and non-β-lactam agents according to their structural differences. The US Food and Drug Administration has approved the β-lactam combination agents ceftazidime-avibactam and meropenem-vaborbactam and the non-β-lactam agents eravacycline and plazomicin for clinical treatment. Many novel antibiotics are under clinical investigation. |
In order to reduce unnecessary antibiotic consumption and the clinical CRE burden, strict implementation of an antibiotic stewardship policy in combination with use of well-established phenotypic tests and novel multiplex polymerase chain reaction methods for the detection of the most commonly encountered β-lactamases/carbapenemases in hospitals is important for prescribing effective antibiotics against CRE and decreasing the resistance burden caused by CRE. |
1 Introduction
For the last two decades, the spread of drug-resistant Gram-negative bacteria (GNB), especially third-generation cephalosporin- and carbapenem-resistant (CR) Enterobacteriaceae (CRE), CR-Pseudomonas aeruginosa and CR-Acinetobacter baumannii, has substantially increased morbidity and mortality rates worldwide [1,2,3,4,5]. The global spread of CRE (particularly the producers of Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-β-lactamase (MβL) (NDM), and oxacillinase (OXA)-48-like enzymes among K. pneumoniae and other Enterobacteriaceae isolates) is an important issue because CRE infections are usually associated with delayed administration of appropriate antibiotics and high case-fatality rates, while CRE colonization is a risk associated with high clinical severity [1, 6,7,8,9,10]. In 2018, the World Health Organization ranked the three above-mentioned resistant organisms as top critical-priority with Gram-negative bacteria (GNB) far outweighing methicillin-resistant Staphylococcus aureus (MRSA) [11, 12].
Clinical trials of antibiotics against difficult-to-treat multidrug-resistant (MDR)-GNB are now underway. These novel agents are quite distinct from conventional β-lactamase inhibitor-(sulbactam, clavulanate, and tazobactam) containing antibiotics. Those showing potent in vitro activity against MDR pathogens are classified into two structurally different categories, β-lactam/β-lactamase inhibitor antibiotics and non-β-lactam antibiotics [13]. The former group includes ceftolozane-tazobactam, ceftazidime-avibactam, ceftaroline-avibactam, aztreonam-avibactam, imipenem/cilastatin-relebactam, meropenem-vaborbactam, meropenem-nacubactam, cefepime-zidebactam, and cefiderocol, whereas the latter group includes murepavadin, plazomicin, eravacycline, omadacycline, finafloxacin, and delafloxacin [13]. In addition, the development of arylomycin derivative and antibiotic hybrids has demonstrated new directions in the design of antimicrobials against the clinical extensively drug resistant (XDR) pathogens [14, 15]. A few of these novel antibiotics (including ceftazidime-avibactam, delafloxacin, eravacycline, omadacycline, and meropenem-vaborbactam) have been approved by the US Food and Drug Administration (FDA). Most are presently being evaluated for efficacy and safety in treating CR-GNB infections. As more novel antibiotics become available for prescription in clinical settings, overdependence on tigecycline and colistin against XDR-GNB could be alleviated. Ceftolozane-tazobactam has demonstrated in vitro potency against most CR-P. aeruginosa strains, and has been approved for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI). It is, however, inactive against the KPC-producing Enterobacteriaceae isolates and many extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae isolates [16,17,18]. Therefore, it is not discussed in this article.
This article mainly focuses on in vitro and clinical study data on novel anti-CRE antibiotics, and also introduces other novel antibiotics that have shown in vitro activity against other important clinical MDR bacterial pathogens.
2 β-Lactams and β-Lactamase Inhibitor Combinations
Avibactam (formerly NXL104) is a diazabicyclooctane (DBO, non-lactam class) derivative antibiotic. It has very good potency in reversibly inhibiting serine β-lactamase enzymes including Ambler class A (mainly ESBL, KPC), class C, and partial class D (including OXA-1, OXA-10, and OXA-48-like), while sparing the class B enzymes. It has undoubtedly been a key breakthrough in treating CRE infections [19]. Sader et al. reported that the addition of avibactam significantly reduced the minimum inhibitory concentrations (MICs) of ceftazidime and meropenem against most MDR-Enterobacteriaceae species [20, 21]. The overall treatment success rates using ceftazidime-avibactam against several CRE infections ranged between 45 and 76% [18]. With a recommended dose of 2.5 g intravenously every 8 h, ceftazidime-avibactam (4:1 fixed ratio) has an efficacy comparable to carbapenem. One phase III trial comparing the therapeutic efficacy of ceftazidime-avibactam and meropenem (against implicated etiologies mainly including K. pneumoniae, P. aeruginosa, REPROVE trial, NCT01808092) showed clinical non-inferiority in treating patients with hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) [22]. In addition, Bassetti et al. have suggested that ceftazidime-avibactam can be combined with gentamicin, fosfomycin, colistin, or plazomicin as a potential alternative treatment regimen in settings with high (> 20%) CRE prevalence rates [18]. The FDA and the European Medicines Agency (EMA) have approved ceftazidime-avibactam in treating cIAI (combined with metronidazole), cUTI, and nosocomial pneumonia [18, 19, 23].
Ceftaroline [a fifth-generation anti-MRSA cephalosporin approved for treatment of community-acquired pneumonia (CAP)] combined with avibactam has also been shown to be active against ESBL, KPC, and AmpC-producing Enterobacteriaceae isolates [24]. Furthermore, ceftaroline-avibactam showed efficacy comparable to doripenem in treating cUTI in a phase II study (NCT01281462).
Aztreonam is active against MβL-producing GNB, while it is readily hydrolyzed by most class A and class C enzymes [1]. A combination of avibactam and aztreonam has exhibited good in vitro potency against most of the ESBL-, KPC-, MβL-, and AmpC-producing Enterobacteriaceae isolates. Nevertheless, aztreonam-avibactam is inactive against the MDR-P. aeruginosa and A. baumannii strains [18]. A phase III trial comparing the clinical efficacy of aztreonam-avibactam (plus metronidazole in cIAI) and meropenem (plus colistin, if necessary) in various infections (cIAI, HAP/VAP) related to MDR-GNB was initiated in 2018 (NCT03329092) and will end in 2021.
Relebactam (formerly MK-7655A) is another DBO class drug. When combined with imipenem/cilastatin, this β-lactam combination agent displays in vitro activity similar to that of ceftazidime-avibactam [25, 26]. In a phase II trial involving patients with cUTI (including acute pyelonephritis (APN)), imipenem/cilastatin-relebactam at a prescription dose of 500 mg/250 mg, i.e., 125 mg or 250 mg every 6 h, showed non-inferiority compared with imipenem/cilastatin (500 mg every 6 h) for clinical and microbiological endpoints (NCT01506271) [27]. Another phase II multicenter clinical trial involving imipenem/cilastatin-relebactam at a dose of 500 mg, i.e., 125 mg or 250 mg every 6 h, for treatment of patients with cIAI also showed favorable outcomes [25]. The other phase III trial investigating the difference in clinical efficacy between imipenem/cilastatin-relebactam and imipenem/cilastatin plus colistin in patients with serious infections (HAP, VAP, cIAI, and cUTI) due to imipenem-resistant bacterial strains ended in 2017 (NCT02452047) [18], and the results are pending. In addition, it is noteworthy that ceftazidime-avibactam and imipenem/cilastatin-relebactam also have good pulmonary penetration. A phase III study comparing clinical efficacy, tolerability, and safety between imipenem/cilastatin-relebactam and piperacillin-tazobactam for treating patients with HAP or VAP is ongoing (NCT02493764) [18].
Another DBO-type β-lactamase inhibitor zidebactam (formerly WCK 5222; Wockhardt Ltd., Mumbai, India), with a unique high binding affinity to penicillin-binding protein (PBP)-2, remarkably increases the efficacy of cefepime against serine β-lactamase (ESBL, KPC) producers, probably the Ambler class D producers, and CR-P. aeruginosa isolates [13, 28, 29]. Nevertheless, reduced susceptibility of cefepime-zidebactam is reported against some MβL-producing organisms [13, 28, 29]. Moreover, the addition of zidebactam did not significantly potentiate the in vitro efficacy of cefepime against MDR-A. baumannii [29]. The safety and tolerability of zidebactam (1–2 g intravenously every 8 h), and zidebactam combined with cefepime (2 g intravenously every 8 h) were evaluated among healthy volunteers beginning in 2016 (MAD study NCT02674347, and phase I MED study NCT02707107, respectively), but the formal reports are still pending.
Nacubactam (formerly OP0595; F. Hoffmann-La Roche Ltd., Basel, Switzerland), a new DBO-type compound, has been reported to be a β-lactam (biapenem, cefepime, piperacillin, etc.) enhancer due to good PBP-2 affinity (although weaker than zidebactam). Its antibacterial spectrum is similar to cefepime-zidebactam [13, 30]. The safety and tolerability of nacubactam alone and nacubactam plus meropenem among healthy volunteers showed favorable results [31], and further clinical study about its efficacy in pneumonia is ongoing.
Vaborbactam, a cyclic boronate non-β-lactam agent (structurally distinct from avibactam and relebactam), does not have intrinsic antibacterial activity. However, it potentiates the in vitro activity of meropenem against KPC-, ESBL-, and AmpC-producing Enterobacteriaceae isolates [26]. Meropenem-vaborbactam has also been approved by the FDA in treating cUTI at a recommended dose of 2 g meropenem plus 2 g vaborbactam every 8 h in patients with estimated glomerular filtration rates ≥ 50 ml/min/1.73 m2 [32]. Although meropenem-vaborbactam lacks in vitro activity against the class B and class D enzymes [26, 33], it demonstrated non-inferior outcomes in the treatment of patients with HAP/VAP/bacteremia and cIAI compared with the currently best available antibiotics in the TANGO II trial (clinical cure rate, 57.1% vs. 33.3%, P = 0.04, NCT02168946 [34]). The TANGO III trial (phase III study, NCT03006679) comparing the efficacy of meropenem-vaborbactam with piperacillin-tazobactam in HAP/VAP is ongoing.
Another promising boronate-based β-lactamase inhibitor, VNRX-5133 (VenatoRx Pharmaceuticals, Inc., Malvern, PA, USA), also reportedly possesses broad-spectrum activity against Ambler class A, C, and D enzymes and some MβL-producing organisms (including CRE, and possibly some CR-P. aeruginosa, CR-A. baumannii isolates) [35]. Its clinical safety among 84 healthy volunteers was established in a phase I trial (NCT02955459) [13, 28, 36].
Cefepime/AAI101 (Allerca Therapeutics, Weil am Rhein, Germany), an antimicrobial agent containing the β-lactamase inhibitor AAI101 (with a β-lactam scaffold) was reported to possess acceptable in vitro activity against cefepime-non-susceptible, ESBL- and KPC-producing Enterobacteriaceae strains [13, 28, 37]. A phase II evaluation was conducted in 2017 to compare the clinical efficacy between cefepime/AAI101 (500 or 750 mg) and cefepime (1–2 g) in the treatment of adult patients with cUTI (NCT03680612); the data are pending [38].
Cefiderocol (formerly S-649266; Shionogi & Co. Ltd., Osaka, Japan) is a siderophore (catechol moiety)-containing cephalosporin agent preferentially binding to the PBP-3 in GNB. By taking advantage of the bacterial iron transport system, its entry into the periplasmic space is significantly facilitated (a “Trojan Horse” strategy) [13, 39, 40]. Cefiderocol shows excellent in vitro activity against most class A, B, C, and D β-lactamase-producing GNB (Enterobacteriaceae, non-fermenters). Reduced in vitro activity, however, was noted among a few P. aeruginosa isolates with a deficiency in the iron-regulated outer membrane proteins [39]. As seen in the data of Dobias et al., the MIC inhibiting the in vitro growth of 90% of target organisms (MIC90) for cefiderocol ranged from 2 to 4 μg/ml against KPC producers, or class B enzyme variants (including NDM)-producing Enterobacteriaceae isolates. Among the various antibiotics compared, only colistin and tigecycline displayed in vitro activities equivalent to cefiderocol against overall MDR-GNB [40]. The MIC90 values for cefiderocol were 1–2, 4–8, and 0.25 μg/ml against CR-P. aeruginosa, A. baumannii, and Stenotrophomonas maltophilia isolates, respectively [40, 41]. In a population pharmacokinetic study, a high serum concentration of cefiderocol (> 75 μg/ml) was achieved when subjects were administered doses ranging from 2 g every 8 h to 0.75 g every 12 h (with 3-h infusions) depending upon creatinine clearance rates. Approximately two-thirds of administered cefiderocol is excreted by the kidney as unchanged parent drug. Excellent probabilities (> 90%) of target attainment in blood and urine were observed against GNB with a cefiderocol MIC ≤ 4 μg/ml [42]. In the APEKS-cUTI trial, cefiderocol showed composite clinical and microbiological non-inferiority to high-dose imipenem/cilastatin (1 g/1 g with a 1-h intravenous drip every 8 h) in treating patients at high risk of acquiring MDR-GNB [18, 43]. It is also notable that cefiderocol has an acceptable ratio (0.239) for the epithelial lung fluid (ELF)-to-plasma concentration [44]. Two phase III trials [APEKS-NP trial (NCT03032380); and CREDIBLE-CR trial (NCT02714595), respectively] comparing the treatment efficacy of cefiderocol with meropenem (2 g with a 3-h intravenous drip duration every 8 h for both drugs), colistin, and other best available antibiotics in patients with various serious infections (including healthcare-associated pneumonia, HAP, VAP, cUTI, bloodstream infections) are ongoing [15, 18]. Table 1 illustrates the spectra of novel β-lactam combination antibiotics against the Enterobacteriaceae isolates that produce important β-lactamases (including carbapenemases), CR-P. aeruginosa and CR-A. baumannii.
3 Non-β-Lactam Antibiotics
Plazomicin (formerly ACHN-490; Achaogen Inc., South San Francisco, CA, USA) is a sisomycin derivative that is unaffected by aminoglycoside-modifying enzymes, although it is vulnerable to other aminoglycoside resistance mechanisms. It displays potent in vitro activity against the majority of β-lactamase-producing bacteria (except those containing NDM enzymes) as well as MRSA [20, 45,46,47]. Prescribed at a dosage of 15 mg/kg intravenously once daily, plazomicin demonstrated efficacy comparable to levofloxacin (phase II trial, NCT01096849) [48] and meropenem (EPIC study, phase III trial) [49] in treating cUTI. A combination of plazomicin with tigecycline or meropenem demonstrated improved efficacy (survival) and a better safety profile than colistin in treating HAP/VAP/cUTI/bacteremia caused by CRE (CARE trial; mortality rate, 23.5% vs. 50%, NCT01970371) [18]. This agent was approved for treatment of cUTI by the FDA in June 2018. It was suggested that plazomicin be combined with ceftazidime-avibactam to provide better therapeutic efficacy in treating serious infections caused by KPC producers [28, 50].
Eravacycline (Tetraphase Pharmaceuticals, Inc., Watertown, MA, USA) is a fluorocycline belonging to the tetracycline class (with structural similarity to tigecycline, but with more chemical modifications). It is available in both intravenous and oral formulations (oral bioavailability in healthy volunteers, > 90%) [18]. Eravacycline has an antibacterial spectrum equivalent to tigecycline but has been shown to be more potent against MDR-Enterobacteriaceae and A. baumannii isolates [28, 39, 51]. Eravacycline has shown efficacy comparable to ertapenem (86.8% vs. 87.6%) in treating cIAI in a phase III trial (IGNITE 1 study, NCT01844856) [52]. In August 2018, eravacycline was approved for treating cIAI by the FDA. It is noteworthy that eravacycline has better pulmonary penetration for both ELF and alveolar macrophages than tigecycline (6- and 50-fold of serum concentrations, respectively) [39, 53]. Therefore, it is an attractive treatment option in HAP/VAP as well. By contrast, eravacycline (1.5 mg/kg intravenously once daily) failed to show clinical superiority to ertapenem (1 g intravenously once daily) in the treatment of cUTI (IGNITE3 phase III trial) [54]. As of February 2019, no clinical trials had evaluated the role of eravacycline in HAP.
Omadacycline (Paratek Pharmaceuticals, Inc., Boston, MA, USA) is a modified minocycline (aminomethylcycline) antibiotic with a lower plasma protein-binding affinity than tigecycline (20–30% vs. 60%) [28, 55]. It has less potential for inducing nausea and vomiting compared with the glycylcyclines. Although omadacycline has excellent efficacy against MRSA, MDR-Streptococcus pneumoniae, and ESBL-producing Escherichia coli isolates (but is not active against ESBL-producing K. pneumoniae, ceftazidime-non-susceptible Enterobacter spp.), it displays limited in vitro activity against CRE and CR-A. baumannii [28, 56]. Initial proof regarding omadacycline in treating urinary tract infections (UTIs) found that it was active in vitro against ESBL-producing Enterobacteriaceae isolates and CR-E. coli (but not K. pneumoniae) [13, 57]. Omadacycline (100 mg intravenously once daily, followed by 300 mg orally once daily) was shown to have the clinical efficacy comparable to linezolid (600 mg orally every 12 h) in treating complicated skin and skin structure infections (SSSIs) caused by Gram-positive bacteria (OASIS-1 phase III trial; NCT02378480) [58]. The other phase III study (OASIS-2; NCT02877827) involving a new regimen of omadacycline (450 mg on days 1 and 2 orally, then 300 mg orally once daily) for treating adult subjects with acute bacterial SSSI also showed clinical non-inferiority as compared with linezolid (600 mg orally every 12 h) [59]. It also showed non-inferior clinical efficacy to moxifloxacin in the treatment of community-acquired pneumonia (CAP) (EudraCT #2013-004071-13, NCT02531438) [13, 60]. In October 2018, omadacycline was approved for the treatment of CAP and acute bacterial SSSI by the FDA [61].
Ciprofloxacin and levofloxacin lose treatment efficacy in an acidic medium. Finafloxacin (Novartis, Basel, Switzerland) is a fluoroquinolone antibiotic (of the 8-cyano subclass) that is being developed in both an intravenous and an oral formulation [13]. It has higher potency (lower MICs) against ESBL-producing E. coli and K. pneumoniae isolates than the conventional fluoroquinolones owing to a higher binding affinity to DNA gyrase and topoisomerase IV in an acidic environment (pH 5.0–6.5). Thus, it is suitable for infections of the skin, urinary tract, and vagina [13, 39], where the environment is mostly acidic. In a phase II clinical study of cUTI (including APN), finafloxacin (800 mg intravenously or orally once daily) exhibited clinical and microbiological efficacy equivalent to ciprofloxacin (NCT01928433) [62]. Currently, finafloxacin is only available as a topical formulation for treatment of otitis media (ear drops, approved by the FDA).
Another new fluoroquinolone antibiotic, delafloxacin, has additional in vitro activity against levofloxacin-resistant S. pneumoniae and MRSA [28, 63, 64]. Compared with vancomycin plus aztreonam, delafloxacin monotherapy showed clinical non-inferiority as well as similar tolerability in the treatment of acute bacterial SSSI (NCT01811732) [65], and was approved by the FDA for this indication [28]. However, these two new fluoroquinolones were not demonstrated to have in vitro activities against CRE.
Murepavadin (formerly POL7080; Polyphor Ltd., Allschwil, Switzerland) is a 14-amino-acid synthetic peptidomimetic that acts on the outer membrane protein involved in the transport of the lipopolysaccharide component [13, 28]. It was proven to be a potent antibiotic highly specific to P. aeruginosa, including carbapenemase producers and colistin-resistant strains [66]. Two clinical trials evaluating the efficacy and safety of murepavadin in treating lower respiratory tract infections caused by P. aeruginosa (suspected or confirmed) among patients with VAP or bronchiectasis unrelated to cystic fibrosis have been completed (NCT02096315, and NCT02096328, respectively), and formal results are pending.
Table 2 illustrates the spectra of novel non-β-lactam antibiotics against Enterobacteriaceae producers of various β-lactamases (including carbapenemases), isolates of CR-P. aeruginosa, CR-A. baumannii, and S. maltophilia. Table 3 illustrates the clinical indications (or potential indications) for these novel antibiotics in treatment of various infections caused by XDR or MDR Gram-negative pathogens.
4 Arylomycin and Antibiotic Hybrids
There has been a greater degree of difficulty in designing new antibiotics with unique antibacterial mechanisms in the present decade than in previous decades. Arylomycin A-C16 exerts antibacterial effects by inhibition of type I signal peptidase, which leads to an insufficient flux of proteins through the secretion pathway. In target bacteria (including nutrient-depleted S. aureus isolates, and rapidly growing E. coli), arylomycin causes mislocalization of the essential proteins. Although the MIC breakpoints of arylomycin are not established, its unique antibacterial mechanism is significantly different from that of existing antibiotics. Thus, it has great potential [14, 67].
Some antimicrobial peptides to treat XDR-GNB infections are also under development [68]. Research on antibiotic hybrids, defined as synthetic constructs of two or more pharmacophores belonging to an established agent known to produce a desired antimicrobial effect (over the outer membrane, and/or the constitutively over-expressed efflux pumps in bacteria), has opened a new direction in designing powerful antibiotics [15]. An example is a tobramycin-ciprofloxacin hybrid antibiotic that was found to significantly optimize the in vitro efficacy of ciprofloxacin against ciprofloxacin-resistant and MDR-P. aeruginosa strains [69].
5 Rapid Phenotypic Diagnostics and Molecular Methods for Delineating Carbapenemase Production in Carbapenem-Resistant Enterobacteriaceae
Based on the aforementioned analyses, many new antibiotics (with the exception of omadacycline, finafloxacin, delafloxacin, and murepavadin) are active against CRE related to Ambler class A, C, and/or D enzyme production, while fewer exhibit in vitro activity against class B enzyme-(especially NDM variants) producing CRE [13, 18, 20]. It is alarming that since 2011, NDM-producing Enterobacteriaceae isolates have been detected in patients with cIAI or cUTI in southeastern Asia [2]. To prescribe these novel antibiotics judiciously and expeditiously, we urgently need rapid phenotypic (see below) and molecular diagnostic tools to detect MβL-producing CRE strains early in countries where the MβL prevalence is ≥ 10% [47].
6 Time for Stewardship
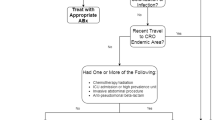
In order to achieve precision medicine that avoids erroneous prescription of these novel antibiotics [46], a strict antibiotic stewardship policy should be considered after resistance information on the implicated pathogens is obtained [70]. Effective interventions are of paramount importance in infection control. These include leadership commitment by infection experts, implementation of educational objectives, collaboration between antimicrobial stewardship teams and primary-care physicians, optimization of appropriate doses as well as de-escalation of antibiotics according to culture data and patient condition, and monitoring of in-hospital resistance trends [71]. Despite the arrival of new antibiotics, the consumption of carbapenem class agents has shown a notably positive relationship, with the incidence of Enterobacteriaceae showing non-susceptibility to carbapenems [72]. Consequently, judicious prescription of carbapenem agents is absolutely indicated in hospitals. It is noteworthy that utilization of the modified carbapenem inactivation method (CIM) in combination with the EDTA-modified CIM test could reliably differentiate MβL-producing CRE strains (those displaying a negative result on only the EDTA-modified CIM test) from serine-class carbapenemase producers (showing positive results for both tests) [73, 74]. In addition, polymerase chain reaction tests are of great help in understanding resistance epidemiology and delineating the carbapenemase-encoding alleles in XDR-GNB. An algorithm for antibiotic choices in the treatment of CRE infections is illustrated in Fig. 1.
7 Conclusions
Currently, the global burden of XDR-GNB is remarkably high. This resistance burden boosts the development of new potent antibiotics to combat difficult-to-treat pathogens. Robust clinical trials are warranted to evaluate the spectrum of activity, efficacy, and safety of these novel drugs before they are available clinically. In addition, despite the development of some rapid phenotypic tests to detect carbapenemase-producing GNB strains, rapid genotypic diagnostics are still required to accurately delineate the resistance mechanisms. Finally, from a pharmacoeconomic aspect, further studies of these new antibiotics are needed to evaluate the benefits of combination therapy compared with monotherapy in further studies.
References
Jean SS, Lee WS, Lam C, Hsu CW, Chen RJ, Hsueh PR. Carbapenemase-producing Gram-negative bacteria: current epidemics, antimicrobial susceptibility and treatment options. Future Microbiol. 2015;10(3):407–25.
Jean SS, Hsueh PR, SMART Asia-Pacific Group. Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008–14: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother. 2017;72(1):166–71.
Lee CH, Su TY, Ye JJ, Hsu PC, Kuo AJ, Chia JH, et al. Risk factors and clinical significance of bacteremia caused by Pseudomonas aeruginosa resistant only to carbapenems. J Microbiol Immunol Infect. 2017;50(5):677–83.
Tseng SP, Wang SF, Ma L, Wang TY, Yang TY, Siu LK, et al. The plasmid-mediated fosfomycin resistance determinants and synergy of fosfomycin and meropenem in carbapenem-resistant Klebsiella pneumoniae isolates in Taiwan. J Microbiol Immunol Infect. 2017;50(5):653–61.
Ku YH, Chen CC, Lee MF, Chuang YC, Tang HJ, Yu WL. Comparison of synergism between colistin, fosfomycin and tigecycline against extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates or with carbapenem resistance. J Microbiol Immunol Infect. 2017;50(6):931–9.
Lee CM, Lai CC, Chiang HT, Lu MC, Wang LF, Tsai TL, et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2017;50(2):133–44.
Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895.
De la Calle C, Rodríguez O, Morata L, Marco F, Cardozo C, García-Vidal C, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2018. https://doi.org/10.1016/j.ijantimicag.2018.11.015 (Epub ahead of print).
Mitgang EA, Hartley DM, Malchione MD, Koch M, Goodman JL. Review and mapping of carbapenem-resistant Enterobacteriaceae in Africa: using diverse data to inform surveillance gaps. Int J Antimicrob Agents. 2018;52(3):372–84.
Syue LS, Chen YH, Ko WC, Hsueh PR. New drugs for the treatment of complicated intra-abdominal infections in the era of increasing antimicrobial resistance. Int J Antimicrob Agents. 2016;47(4):250–8.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27.
Liu CY, Lai CC, Chiang HT, Lu MC, Wang LF, Tsai TL, et al. Predominance of methicillin-resistant Staphylococcus aureus in the residents and environments of long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2019;52(1):62–74.
Avery LM, Nicolau DP. Investigational drugs for the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Expert Opin Investig Drugs. 2018;27(4):325–38.
Smith PA, Koehler MFT, Girgis HS, Yan D, Chen Y, Chen Y, et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature. 2018;561(7722):189–94.
Domalaon R, Idowu T, Zhanel GG, Schweizer F. Antibiotic hybrids: the next generation of agents and adjuvants against Gram-negative pathogens? Clin Microbiol Rev. 2018;31(2):e00077-17.
Jean SS, Lu MC, Shi ZY, Tseng SH, Wu TS, Lu PL, et al. In vitro activity of ceftazidime-avibactam, ceftolozane-tazobactam, and other comparable agents against clinically important Gram-negative bacilli: results from the 2017 Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect Drug Resist. 2018;11:1983–92.
Tato M, García-Castillo M, Bofarull AM, Cantón R. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centers: results of the CENIT study. Int J Antimicrob Agents. 2015;46(5):502–10.
Bassetti M, Vena A, Castaldo N, Righi E, Peghin M. New antibiotics for ventilator-associated pneumonia. Curr Opin Infect Dis. 2018;31(2):177–86.
Falcone M, Paterson D. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother. 2016;71(10):2713–22.
Kidd JM, Kuti JL, Nicolau DP. Novel pharmacotherapy for the treatment of hospital-acquired and ventilator-associated pneumonia caused by resistant gram-negative bacteria. Expert Opin Pharmacother. 2018;19(4):397–408.
Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014;58(3):1684–92.
Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Sharma R, Park TE, Moy S. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination for the treatment of resistant Gram-negative organisms. Clin Ther. 2016;38(3):431–44.
Castanheira M, Sader HS, Farrell DJ, Mendes RE, Jones RN. Activity of ceftaroline-avibactam tested against Gram-negative organism populations, including strains expressing one or more β-lactamases and methicillin-resistant Staphylococcus aureus carrying various staphylococcal cassette chromosome mec types. Antimicrob Agents Chemother. 2012;56(9):4779–85.
Lucasti C, Vasile L, Sandesc D, Venskutonis D, McLeroth P, Lala M, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother. 2016;60(10):6234–43.
Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78(1):65–98 (26).
Sims M, Mariyanovski V, McLeroth P, Akers W, Lee YC, Brown ML, et al. Prospective, randomized, double-blind, phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72(9):2616–26.
World Health Organization. Antibacterial agents in clinical development. An analysis of the antibacterial clinical development pipeline, including tuberculosis. 2017. http://www.who.int/medicines/news/2017/IAU_AntibacterialAgentsClinicalDevelopment_webfinal_2017_09_19.pdf. Accessed 12 Nov 2018.
Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother. 2017;72(5):1373–85.
Livermore DM, Mushtaq S, Warner M, Woodford N. Activity of OP0595/β-lactam combinations against Gram-negative bacteria with extended-spectrum, AmpC and carbapenem-hydrolysing β-lactamases. J Antimicrob Chemother. 2015;70(11):3032–41.
Bentley D, Fettner S, Patel K, Zwanziger E, Attley G, Rodriguez I, et al. (P2214) Safety, tolerability, and pharmacokinetics of nacubactam, a novel β-lactamase inhibitor, administered alone and with meropenem in healthy volunteers. In: 28th European congress of clinical microbiology and infectious diseases, Madrid, Spain, 21–24 April, 2018.
Dhillon S. Meropenem/vaborbactam: a review in complicated urinary tract infections. Drugs. 2018;78(12):1259–70.
Wong D, van Duin D. Novel β-lactamase inhibitors: unlocking their potential in therapy. Drugs. 2017;77(6):615–28.
Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7(4):439–55.
Daigle D, Hamrick J, Chatwin C, Kurepina N, Kreiswirth BN, Shields RK, et al. Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug-resistant K. pneumoniae and P. aeruginosa. Open Forum Infect Dis. 2018;5(Suppl 1):S419–20.
Paul Taylor N. VenatoRx raises $42 M to take drug for breaking bacterial resistance to approval [Internet]. FierceBiotech. 2017. https://www.fiercebiotech.com/biotech/venatorx-raises-42m-to-take-drug-for-breaking-bacterial-resistance-to-approval. Accessed 5 Nov 2018.
Crandon JL, Nicolau DP. In vitro activity of cefepime/AAI101 and comparators against cefepime non-susceptible Enterobacteriaceae. Pathogens. 2015;4(3):620–5.
Allerca announces initiation of a phase 2 clinical trial for its novel antibiotics to treat resistant bacterial infections [Internet]. Weil am Rhein (Germany): Allerca Therapeutics. 2017. http://allecra.com/2017/09/07/allecra-announces-initiation-of-a-phase-2-clinical-trial-for-its-novel-antibiotic-to-treat-resistant-bacterial-infections/. Accessed 16 Nov 2018.
Falagas ME, Mavroudis AD, Vardakas KZ. The antibiotic pipeline for multi-drug resistant gram negative bacteria: what can we expect? Expert Rev Anti Infect Ther. 2016;14(8):747–63.
Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis. 2017;36(12):2319–27.
Hsueh SC, Lee YJ, Huang YT, Liao CH, Tsuji M, Hsueh PR. In vitro activities of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J Antimicrob Chemother. 2019;74(2):380–6.
Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother. 2016;61(1):e01381-16.
Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–28.
Matsunaga Y, Echols R, Katsube T, Yamano Y, Ariyasu M, Nagata TD. Cefiderocol (S-649266) for nosocomial pneumonia caused by Gram-negative pathogens: study design of APEKS-NP, a phase 3 double-blind parallel-group randomized clinical trial. Am J Respir Crit Care Med. 2018;197:A3290.
López Díaz MC, Ríos E, Rodríguez-Avial I, Simaluiza RJ, Picazo JJ, Culebras E. In-vitro activity of several antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA) isolates expressing aminoglycoside-modifying enzymes: potency of plazomicin alone and in combination with other agents. Int J Antimicrob Agents. 2017;50(2):191–6.
Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2011;66(1):48–53.
Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17(6):761–81.
Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother. 2018;62(4):e01989-17.
Cloutier DJ, Komirenko AS, Cebrik DS, Keepers TR, Krause KM, Connolly LE, et al. Plazomicin vs. meropenem for complicated urinary tract infection (cUTI) and acute pyelonephritis (AP): diagnosis-specific results from the phase 3 EPIC Study. Open Forum Infect Dis. 2017;4(Suppl 1):S532.
Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect. 2017;23(10):704–12.
Seifert H, Stefanik D, Sutcliffe JA, Higgins PG. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(1):62–4.
Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating Gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 2017;152(3):224–32.
Connors KP, Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, et al. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother. 2014;58(4):2113–8.
Evaracycline vs. levofloxacin or ertapenem in treatment of complicated urinary tract infections [Internet]. https://ir.tphase.com/news-releases/news-release-details/tetraphase-pharmaceuticals-completes-enrollment-ignite3-phase-3. Accessed 15 Feb 2019.
Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2005;49(1):220–9.
Pfaller MA, Huband MD, Rhomberg PR, Flamm RK. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia–Western Pacific), 2010–2011. Antimicrob Agents Chemother. 2017;61(5):e00018-17.
Omadacycline activity vs. CDC urgent antibiotic resistance threats [Internet]. https://paratekpharma.com/media/1332/omadacycline-coverage-for-cdc-pathogen-threats-dk-11-23-16.pdf. Accessed 24 Nov 2018.
O’Riordan W, Green S, Overcash JS, Puljiz I, Metallidis S, Gardovskis J, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Eng J Med. 2019;380(6):528–38.
O’Riordan W, Cardenas C, Sirbu A, Garrity-Ryan L, Das A, Eckburg P, et al. A phase-3 randomized, double-blind, multicentre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study). Poster abstract O0425. ECCMID April 2018. Madrid, Spain. https://paratekpharma.com/media/1542/eccmid-2018-o0425-oasis2-top-line-efficacy-safety.pdf. Accessed 25 Mar 2019.
Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380(6):517–27.
Markham A, Keam SJ. Omadacycline: first global approval. Drugs. 2018;78(18):1931–7.
Wagenlehner F, Nowicki M, Bentley C, Lückermann M, Wohlert S, Fischer C, et al. Explorative randomized phase II clinical study of the efficacy and safety of finafloxacin versus ciprofloxacin for treatment of complicated urinary tract infections. Antimicrob Agents Chemother. 2018;62(4):e02317-17.
Remy JM, Tow-Keogh CA, McConnell TS, Dalton JM, Devito JA. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: resistance selection and characterization. J Antimicrob Chemother. 2012;67(12):2814–20.
Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. In vitro activity of delafloxacin tested against isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2016;60(10):6381–5.
Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, et al. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72(12):3471–80.
Sader HS, Flamm RK, Dale GE, Rhomberg PR, Castanheira M. Murepavadin activity tested against contemporary (2016–17) clinical isolates of XDR Pseudomonas aeruginosa. J Antimicrob Chemother. 2018;73(9):2400–4.
Smith PA, Romesberg FE. Mechanism of action of the arylomycin antibiotics and effects of signal peptidase I inhibition. Antimicrob Agents Chemother. 2012;56(10):5054–60.
Chung PY, Khanum R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J Microbiol Immunol Infect. 2017;50(4):405–10.
Gorityala BK, Guchhait G, Fernando DM, Deo S, McKenna SA, Zhanel GG, et al. Adjuvants based on hybrid antibiotics overcome resistance in Pseudomonas aeruginosa and enhance fluoroquinolone efficacy. Angew Chem Int Ed Engl. 2016;55(2):555–9.
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–77.
Wong D, Spellberg B. Leveraging antimicrobial stewardship into improving rates of carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):383–90.
McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother. 2013;57(10):5131–3.
Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321–33.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-eighth informational supplement M100-S28. Wayne: CLSI; 2018.
Acknowledgements
The content of this manuscript is partly based on content presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Madrid, Spain in April 2018 during the session ‘Therapy of MDR/XDR Gram-Negative Bacteria: Dealing with the Devil’ (SY026).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
No specific grant from any funding agency in the public, commercial, or not-for-profit sector was obtained for this article.
Conflict of interest
Authors SSJ, IMG, WSL, and PRH have no conflicts of interest to declare. Author IMG has served as a speaker on antibiotic resistance and stewardship for Merck, Bayer, Norma Hellas, Pfizer, Basilea, Xelia, and Sanofi and has received consulting honoraria from Merck, Bayer, Basilea, Pfizer, Achaogen, and AstraZeneca.
Rights and permissions
About this article
Cite this article
Jean, SS., Gould, I.M., Lee, WS. et al. New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs 79, 705–714 (2019). https://doi.org/10.1007/s40265-019-01112-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01112-1