Abstract
Abemaciclib (Verzenio™) is an orally administered inhibitor of cyclin-dependent kinases 4 and 6 that is being developed by Eli Lilly and Company. Abemaciclib has been approved in the USA for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, in combination with fulvestrant in women with disease progression following endocrine therapy, and as monotherapy in adult patients with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. In addition, abemaciclib is in various stages of development internationally for a variety of cancers. This article summarizes the milestones in the development of abemaciclib leading to its first approval for the treatment of patients with HR-positive, HER2-negative advanced or metastatic breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Abemaciclib (Verzenio™), an orally administered inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), is being developed by Eli Lilly and Company for the treatment of various cancers [1]. CDK4/6, in combination with cyclin D, are key drivers of cell proliferation, making them effective targets for cancer treatment [2]. Abemaciclib received its first global approval in the USA on 28 September 2017 for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, in combination with fulvestrant in women with disease progression following endocrine therapy, and as monotherapy in adult patients with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting [3, 4]. The recommended starting dosage of oral abemaciclib is 150 mg twice daily (in combination with fulvestrant) or 200 mg twice daily (as monotherapy) until disease progression or unacceptable toxicity [3]. Abemaciclib is the third in a line of highly selective oral CDK4/6 inhibitors that have been developed, and is the first among them with a safety profile that allows for continuous dosing [5, 6].
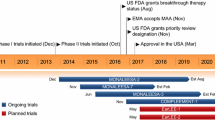
In the third quarter of 2017, Eli Lilly and Company completed regulatory submissions for abemaciclib in the EU and in Japan [7]. In October 2017, the US FDA accepted for priority review a new drug application (NDA) for abemaciclib in combination with an aromatase inhibitor (AI) as initial endocrine-based therapy in the treatment of women with HR-positive, HER2-negative advanced or metastatic breast cancer [7]. In various countries worldwide, abemaciclib is undergoing phase 1–3 development for the treatment of breast cancer and non-small cell lung cancer (NSCLC), phase 2 development for the treatment of brain tumours, liposarcoma, mantle-cell lymphoma and pancreatic ductal adenocarcinoma, and preclinical development for several other solid tumours [1].

1.1 Company Agreements
In October 2015, Eli Lilly and Company and AstraZeneca announced that they would be expanding their existing immuno-oncology research collaboration in the exploration of novel combination therapies for the treatment of solid tumours [8]. In the expanded agreement, the two companies agreed to evaluate additional combinations across the companies’ portfolios (including the combination of Lilly’s abemaciclib with AstraZeneca’s fulvestrant), with Lilly leading the studies and both companies contributing resources [8].
In December 2015, Eli Lilly and Company and Merck (known as MSD outside of Canada and the USA) expanded their immuno-oncology collaboration by planning a phase 1 study to examine Lilly’s abemaciclib and Merck’s pembrolizumab across multiple tumour types [9]. Pursuant to the terms of the agreement, Lilly sponsors the phase 1 study and any subsequent phase 2 studies [9].
2 Scientific Summary
2.1 Pharmacodynamics
Abemaciclib is a CDK4/6 inhibitor that causes cell cycle arrest in retinoblastoma protein (Rb)-competent cells [5, 6]. CDK4/6 and cyclin D1 form activating complexes that promote Rb phosphorylation, cell cycle progression and cell proliferation [3, 10]. In estrogen receptor-positive breast cancer cell lines, continuous exposure to abemaciclib inhibited Rb phosphorylation, blocked the G1- to S-phase progression of the cell cycle and caused senescence and apoptosis in vitro [3]. In breast cancer xenograft models, daily uninterrupted doses of abemaciclib (as a single agent or in combination with antiestrogens) resulted in tumour size reduction [3]. In postmenopausal women with early-stage, invasive, HR-positive, HER2-negative breast cancer, oral abemaciclib (alone or in combination with oral anastrozole) resulted in significantly (p < 0.001) greater suppression of Ki67 (a marker of proliferative activity [11]) than anastrozole alone after 14 days of treatment (n = 64 evaluable) [neoMONARCH; NCT02441946] [12, 13].

In addition, oral abemaciclib (alone or in combination with oral anastrozole) showed anticancer effects on the immune system, as evidenced at the end of 4 months by cytotoxic T cell tumour infiltration (without regulatory T cell infiltration) [14]. In patients and healthy volunteers, abemaciclib did not cause large (i.e. 20 ms) mean increases in the corrected QT interval [3].
2.2 Pharmacokinetics
The pharmacokinetic profile of abemaciclib is based on data from healthy subjects as well as patients with solid tumours, including those with metastatic breast cancer [3].
Following a single oral dose of abemaciclib 200 mg, the absolute bioavailability was 45%, and the median time to peak plasma concentration (Cmax) was 8.0 h (range 4.1–24.0 h) [3]. Following single as well as repeated twice daily doses of abemaciclib 50–200 mg, increases in the Cmax and area under the concentration time curve (AUC; plasma exposure) were approximately dose-proportional. Steady state occurred within 5 days of repeated twice daily dosing, and the estimated geometric mean accumulation ratio was 2.3 based on Cmax and 3.2 based on AUC. Abemaciclib was highly (≈ 96.3%) protein bound, and had a systemic volume of distribution of ≈ 690.3 L (geometric mean) [3].
Hepatic metabolism is the main route of abemaciclib clearance, and abemaciclib is metabolized primarily by CYP3A4 to several metabolites, including N-desethylabemaciclib (M2; the major metabolite), hydroxyl-N-desethylabemaciclib (M18) and hydroxyabemaciclib (M20) [these are all equipotent to abemaciclib] [3]. M2, M18 and M20 were also highly (> 90%) protein bound, and had AUCs that accounted for 25, 13 and 26% of total circulating plasma analytes, respectively. The geometric mean hepatic clearance rate was 26.0 L/h, and the mean plasma elimination half-life of abemaciclib was 18.3 h. Following the administration of a single dose of radiolabelled oral abemaciclib 150 mg, the majority (≈ 81%) of the dose was recovered in the faeces (mostly as metabolites), and ≈ 3% was recovered in the urine [3].
Age (24–91 years), bodyweight (36–175 kg), gender and mild to moderate renal impairment (creatinine clearance of 30 to < 90 mL/min) do not have an effect on abemaciclib exposure, according to a population pharmacokinetic analysis in cancer patients (n = 990) [3]. The effect of severe renal impairment on abemaciclib pharmacokinetics is unknown [3]. Of note, the pharmacokinetic profile of abemaciclib was similar between Japanese and non-Japanese patients in phase 1 studies [6, 15]. The dosing frequency of abemaciclib should be reduced to once daily in patients with severe hepatic impairment; the mean plasma elimination half-life of abemaciclib is 55 h in subjects with severe hepatic impairment compared with 24 h in subjects with normal hepatic function [3].
Features and properties of abemaciclib
Alternative names | Abemaciclib mesylate; bemaciclib; bemaciclib mesylate; LY-2835219; Verzenio™ |
Class | Aminopyridines, antineoplastics, benzimidazoles, piperazines, pyrimidines, small molecules |
Mechanism of Action | CDK4/6 inhibitor |
Route of Administration | Oral |
Pharmacodynamics | Causes cell cycle arrest in retinoblastoma protein-competent cells |
Pharmacokinetics | Median time to Cmax of 8.0 h; mean plasma elimination t1/2 of 18.3 h |
Adverse events | |
Most frequent (≥ 20%) | Diarrhoea, fatigue, nausea, decreased appetite, abdominal pain, neutropenia, vomiting, infections, anaemia, headache, thrombocytopenia, leucopenia |
Occasional | Constipation, dry mouth, stomatitis, pyrexia, dehydration, cough, arthralgia, dysgeusia, dizziness, alopecia, increased creatinine, decreased weight |
ATC codes | |
WHO ATC code | L01X-E (protein kinase inhibitors) |
EphMRA ATC code | L1H (protein kinase inhibitor antineoplastics) |
Chemical name | N-(5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-amine |
2.3 Therapeutic Trials
Oral abemaciclib first showed antitumour activity in patients with advanced cancers in the multicentre, phase 1, JPBA study (NCT01394016) [6]. The study enrolled patients who were no longer benefiting from available standard therapies. Results from the dose-escalation cohort established abemaciclib as safe for use in a continuous schedule, and led to the selection of 150 mg every 12 h and 200 mg every 12 h as dosages for further study in tumour-specific cohorts (advanced breast cancer, colorectal cancer, glioblastoma, melanoma and NSCLC). As single-agent therapy, abemaciclib elicited a best overall response of partial response (PR) or stable disease (SD), respectively, in 31 and 50% of patients with HR-positive advanced breast cancer (n = 36; four of the patients who achieved a PR had been allowed to continue prior endocrine therapy), 3 and 52% of patients with KRAS-mutated NSCLC (n = 29), 0 and 18% of patients with glioblastoma (n = 17), 0 and 13% of patients with colorectal cancer (n = 15), and 4 and 23% of patients with melanoma (n = 26). As combination therapy with fulvestrant, abemaciclib elicited a best overall response of PR or SD, respectively, in 21 and 58% of patients with HR-positive advanced breast cancer (n = 19; all of these patients had received prior endocrine therapy) [6]. Based on data from this trial [6], a number of phase 2 and 3 trials have been initiated.
2.3.1 HR-Positive, HER2-Negative, Advanced or Metastatic Breast Cancer
2.3.1.1 As Subsequent Endocrine-Based Therapy
Abemaciclib plus fulvestrant (an estrogen receptor antagonist [16]) significantly prolonged median progression-free survival (PFS) [by ≈ 7 months] compared with placebo plus fulvestrant in women with previously treated HR-positive, HER2-negative advanced breast cancer (i.e. inoperable locally advanced or metastatic breast cancer) in the randomized, double-blind, placebo-controlled, multinational, phase 3, MONARCH 2 trial (n = 669) [NCT02107703] [17]. All of the patients in this trial had experienced disease progression on or after prior endocrine therapy, and had not received chemotherapy or more than one line of endocrine therapy for metastatic disease [3, 17]. Patients received oral abemaciclib or matching placebo, plus intramuscular fulvestrant, until disease progression, patient withdrawal or death [17]. The dosage of abemaciclib was 150 mg twice daily on a continuous schedule (some patients initially received 200 mg twice daily until the protocol was amended after a safety review), and the dosage of fulvestrant was 500 mg on days 1 and 15 of the first 28-day cycle and on day 1 of subsequent cycles. At a median follow-up of 19.5 months, the investigator-assessed median PFS (the primary endpoint) was 16.4 months in abemaciclib plus fulvestrant recipients and 9.3 months in placebo plus fulvestrant recipients [hazard ratio (HR) 0.553; 95% CI 0.449–0.681; p < 0.001]. These results were consistent with those of a blinded central analysis, and an improvement in PFS was seen across all patient subgroups. The objective response rate [ORR; the proportion of patients with a complete response (CR) or PR] was also significantly greater with abemaciclib plus fulvestrant than with placebo plus fulvestrant (35.2 vs. 16.1% of patients; p < 0.001). Responses were durable, with 67.8% of abemaciclib plus fulvestrant recipients and 66.9% of placebo plus fulvestrant recipients reaching a duration of response of 12 months [17]. Overall survival (OS) data were not mature at the time of the primary analysis of PFS [3].
Abemaciclib as single-agent therapy demonstrated antitumour activity in women with previously treated HR-positive, HER2-negative metastatic breast cancer in the open-label, multinational, phase 2, MONARCH 1 trial (n = 132) [NCT02102490] [18]. All of the patients in this trial had experienced disease progression on or after prior endocrine therapy, had received one or two chemotherapy regimens for metastatic disease and had received a taxane in any setting [3, 18]. Patients received oral abemaciclib 200 mg every 12 h continuously until disease progression and/or unacceptable toxicity [18]. At the 12-month analysis, the investigator-assessed ORR (the primary objective) was 19.7% (95% CI 13.3–27.5) [all PRs], with a median time to response of 3.7 months, and a median duration of response of 8.6 months. Of note, the 95% CI for the ORR did not exclude 15% (the null hypothesis ORR), but was consistent with what can be expected from approved cytotoxic chemotherapies in taxane-pretreated metastatic breast cancer patients based on historical data (an ORR of ≈ 10–20%) [18]. The ORR was generally consistent across subgroups analysed (regardless of disease burden or prior treatment) [19]. The clinical benefit rate (i.e. proportion of patients with a CR, PR or stable disease for ≥ 6 months) was 42.4%, the median PFS was 6.0 months (95% CI 4.2–7.5), and the median OS was 17.7 months [95% CI 16.0–not reported (NR)] [18]. These results were generally consistent with those of an independent review. At a final OS analysis (18 months after the last patient was enrolled), the median OS was 22.3 months (95% CI 17.7–NR), and other endpoints were consistent with those of the 12-month analysis [18].
Key clinical trials of abemaciclib
Drug(s) | Indication | Phase | Status | Location(s) | Identifier(s) | Sponsor(s) |
|---|---|---|---|---|---|---|
Advanced cancer | ||||||
Abemaciclib (+fulvestrant) | Advanced cancer | 1 | Ongoing | USA | NCT01394016; I3Y-MC-JPBA | Eli Lilly and Company |
Abemaciclib | Advanced cancer | 1 | Ongoing | Japan | NCT02014129; I3Y-JE-JPBC | Eli Lilly and Company |
Advanced breast cancer | ||||||
NSAI ± abemaciclib vs. fulvestrant ± abemaciclib | HR-positive, HER2-negative locoregionally recurrent or metastatic breast cancer in postmenopausal women | 3 | Recruiting | Multinational | MONARCH plus; NCT02763566 | Eli Lilly and Company |
NSAI ± abemaciclib | HR-positive, HER2-negative locoregionally recurrent or metastatic breast cancer in postmenopausal women | 3 | Ongoing | Multinational | MONARCH 3; NCT02246621 | Eli Lilly and Company |
Fulvestrant ± abemaciclib | HR-positive, HER2-negative locally advanced or metastatic breast cancer in postmenopausal women | 3 | Ongoing | Multinational | MONARCH 2; NCT02107703 | Eli Lilly and Company |
Abemaciclib | HR-positive, HER2-negative recurrent, locally advanced, unresectable or metastatic breast cancer (with disease progression following antiestrogen therapy) | 2 | Ongoing | Multinational | MONARCH 1; NCT02102490 | Eli Lilly and Company |
Abemaciclib ± tamoxifen or loperamide | HR-positive, HER2-negative relapsed or metastatic breast cancer (with disease progression following endocrine therapy) | 2 | Recruiting | Multinational | nextMONARCH 1; NCT02747004 | Eli Lilly and Company |
Abemaciclib + trastuzumab ± fulvestrant vs. trastuzumab + SOC chemotherapy | HR-positive, HER2-positive locally advanced recurrent or metastatic breast cancer (previously treated) | 2 | Recruiting | Multinational | monarcHER; NCT02675231 | Eli Lilly and Company |
Early-stage breast cancer | ||||||
Standard adjuvant endocrine therapy ± abemaciclib | High-risk, node-positive, early-stage, resected, HR-positive, HER2-negative breast cancer (regardless of menopausal status) | 3 | Recruiting | Multinational | monarchE; NCT03155997 | Eli Lilly and Company, NSABP Foundation Inc. |
Abemaciclib + anastrozole (combination and separately) | Previously untreated, early-stage, HR-positive, HER2-negative breast cancer in postmenopausal women | 2 | Completed | Multinational | neoMONARCH; NCT02441946 | Eli Lilly and Company |
NSCLC | ||||||
Abemaciclib or erlotinib, plus BSC | KRAS-mutated stage 4 NSCLC (with disease progression following platinum-based chemotherapy) | 3 | Ongoing | Multinational | JUNIPER; NCT02152631 | Eli Lilly and Company |
Abemaciclib, docetaxel | Stage 4 NSCLC (with disease progression following platinum-based chemotherapy for advanced disease) | 2 | Ongoing | Multinational | NCT02450539; I3Y-MC-JPBX | Eli Lilly and Company |
Brain tumours | ||||||
Abemaciclib | Brain metastases secondary to melanoma, NSCLC or HR-positive breast cancer | 2 | Recruiting | Multinational | NCT02308020; I3Y-MC-JPBO | Eli Lilly and Company |
Abemaciclib ± surgery | Recurrent glioblastoma | 2 | Recruiting | USA | NCT02981940 | Dana-Farber Cancer Institute, Eli Lilly and Company |
Temozolomide ± abemaciclib or neratinib, CC-115 | Intracranial glioblastoma or gliosarcoma after maximum surgical resection | 2 | Recruiting | USA | NCT02977780; INSIGhT | Dana-Farber Cancer Institute, Eli Lilly and Company, Celgene, Puma Biotechnology, Accelerate Brain Cancer Cure |
Abemaciclib | Recurrent brain tumours | 2 | Recruiting | USA | NCT03220646 | Memorial Sloan Kettering Cancer Center, Eli Lilly and Company |
Liposarcoma | ||||||
Abemaciclib | Locally advanced, locally recurrent or metastatic dedifferentiated liposarcoma (any number of prior therapies) | 2 | Recruiting | USA | NCT02846987 | Memorial Sloan Kettering Cancer Center, Eli Lilly and Company |
Mantle cell lymphoma | ||||||
Abemaciclib | Relapsed or refractory mantle cell lymphoma | 2 | Ongoing | France, Germany | NCT01739309 | Eli Lilly and Company |
Pancreatic cancer | ||||||
Abemaciclib + LY3023414 or galunisertib vs. SOC (gemcitabine or capecitabine) | Metastatic pancreatic ductal adenocarcinoma (previously treated) | 2 | Recruiting | Multinational | NCT02981342 | Eli Lilly and Company |
2.3.1.2 As Initial Endocrine-Based Therapy
Abemaciclib plus a nonsteroidal AI (anastrozole or letrozole) as initial therapy significantly improved PFS and ORR compared with a nonsteroidal AI alone in postmenopausal women with HR-positive, HER2-negative advanced [i.e. locoregionally recurrent (not amenable to radiotherapy or surgical resection) or metastatic] breast cancer in the randomized, double-blind, placebo-controlled, multinational, phase 3, MONARCH 3 trial (n = 493) [NCT02246621] [20]. All of the patients in the trial had not received systemic treatment for advanced disease. Patients received either oral abemaciclib 150 mg twice daily or matching placebo, plus an oral nonsteroidal AI daily (anastrozole 1 mg or letrozole 2.5 mg, per physician’s choice) until disease progression, unacceptable toxicity, patient withdrawal for any reason, or death. At the prespecified interim analysis (at a median follow-up of 17.8 months), the investigator-assessed median PFS was significantly longer with abemaciclib plus an AI than with an AI alone (not reached in abemaciclib plus AI recipients vs. 14.7 months in placebo plus AI recipients; HR 0.54; 95% CI 0.41–0.72; p < 0.0001). These results were consistent with those of an independent central review, and a PFS benefit was generally seen across all prespecified subgroups. In patients overall, the ORR was also significantly greater in abemaciclib plus AI recipients than in placebo plus AI recipients (48.2 vs. 34.5%; p = 0.002). Results were consistent in patients with measurable disease (59.2 vs. 43.8%; p = 0.004). OS data were not mature at the time of this analysis [20].
2.3.2 Secondary Brain Metastases in Breast Cancer
Abemaciclib showed preliminary evidence of antitumour activity in patients with brain metastases secondary to HR-positive, HER2-negative breast cancer in the open-label, phase 2, JPBO study (NCT02308020) [21]. The study enrolled patients with brain metastases secondary to melanoma, NSCLC or HR-positive breast cancer and evaluated the efficacy of oral abemaciclib 200 mg every 12 h [22]. Among patients with HR-positive, HER2-negative metastatic breast cancer with at least one measurable brain lesion (n = 23 analysed) in stage 1, 8.7% of patients had a confirmed PR; this met the predefined threshold for advancement to stage 2 (results not yet available) [21].
2.3.3 Metastatic Non-small Cell Lung Cancer
Abemaciclib did not meet its primary endpoint of OS in patients with KRAS-mutated, advanced NSCLC according to topline results [23] from the randomized, open-label, multinational, phase 3, JUNIPER trial (n = 453) [NCT02152631] [24]. The trial enrolled adults with confirmed stage 4 NSCLC and a codon 12 or 13 mutation in the KRAS oncogene who had disease progression following platinum-based chemotherapy and one other prior chemotherapy and were not eligible for further chemotherapy [24]. Patients received oral abemaciclib 200 mg every 12 h with best supportive care (BSC) or oral erlotinib 150 mg every 24 h with BSC until disease progression or unacceptable toxicity [23, 24]. An analysis of secondary endpoints such as PFS and ORR showed evidence of activity with abemaciclib monotherapy (details are not yet available and expected in 2018) [23].
2.4 Adverse Events
In MONARCH 1 and MONARCH 2, the most common adverse events (AEs) were gastrointestinal AEs, haematological AEs, fatigue, infections and headache, and mostly grade 1 or 2 in severity [3]. Diarrhoea, which was the most frequently occurring AE with abemaciclib (in ≥ 85% of patients) in these trials, typically occurred soon after treatment initiation (generally in the first treatment cycle) and was effectively managed in most cases with dose adjustments or antidiarrhoeal medications [17, 18]. Increased serum creatinine, which was a laboratory abnormality seen in patients receiving abemaciclib, was shown to stem from inhibition of renal tubular secretion transporters, did not affect glomerular function and was reversible [3].
In patients receiving abemaciclib plus fulvestrant in MONARCH 2 (n = 441 analysed, with a median duration of treatment of 12 months), the most common (≥ 20%) AEs of any grade were diarrhoea (86% of patients), fatigue (46%), neutropenia (46%), nausea (45%), infection (43%), abdominal pain (35%), anaemia (29%), leucopenia (28%), decreased appetite (27%), vomiting (26%) and headache (20%) [all occurring in numerically more abemaciclib plus fulvestrant than placebo plus fulvestrant recipients] [3]. AEs led to dose reductions in 43% of patients [most frequently (in ≥ 5% of patients) because of diarrhoea or neutropenia] and permanent treatment discontinuation in 9% of patients [because of diarrhoea in 1% and fatigue in 0.7%]. Deaths were reported in 4% of patients [3].
In patients receiving abemaciclib as single-agent therapy in MONARCH 1 (n = 132 analysed, with a median duration of treatment of 4.5 months), the most common (≥ 20%) AEs of any grade were diarrhoea (90% of patients), fatigue (65%), nausea (64%), decreased appetite (45%), abdominal pain (39%), neutropenia (37%), vomiting (35%), infections (31%), anaemia (25%), headache (20%) and thrombocytopenia (20%) [3]. AEs led to dose reductions in 49% of patients [most frequently because of diarrhea (20%), neutropenia (11%) or fatigue (9%)] and treatment discontinuation in 8% of patients (only one patient discontinued because of diarrhoea). Deaths were reported in 2% of patients [3].
2.5 Ongoing Clinical Trials
MONARCH 1 and MONARCH 2 (Sect. 2.3.1.1), the phase 2 and 3 trials on which the approval of abemaciclib in advanced breast cancer in the USA are based, have estimated completion dates of April 2018 and February 2020 [22]. MONARCH 3 (Sect. 2.3.1.2), the phase 3 trial on which the new NDA in the USA is based, has an estimated completion date of July 2021 [22].
Additional trials that are underway to examine abemaciclib (as a single agent or in combination with other agents) in breast cancer include phase 3 trials in HR-positive, HER2-negative advanced or metastatic breast cancer (MONARCH plus; NCT02763566) and HR-positive, HER2-negative early-stage breast cancer (monarchE; NCT03155997), and phase 2 trials in HR-positive, HER2-negative metastatic breast cancer (nextMONARCH 1; NCT02747004), HR-positive, HER2-negative advanced or metastatic breast cancer (NCT02779751), early-stage, invasive, HR-positive, HER2-negative breast cancer (neoMONARCH; NCT02441946), HR-positive, HER2-positive advanced or metastatic breast cancer (monarcHER; NCT02675231), Rb-positive, triple-negative metastatic breast cancer (NCT03130439) and HR-positive early breast cancer in patients who are candidates for initial breast surgery (NCT02831530).
Additional trials that are underway to examine abemaciclib (as a single agent or in combination with other agents) in other cancer indications include a phase 3 trial in KRAS-mutated stage 4 NSCLC (JUNIPER; NCT02152631), and phase 2 trials in stage 4 squamous cell lung cancer (NCT02450539), stage 4 squamous or KRAS-mutated NSCLC (NCT02779751) and metastatic pancreatic ductal adenocarcinoma (NCT02981342).
3 Current Status
Abemaciclib received its first global approval on 28 September 2017 in the USA for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer, in combination with fulvestrant in women with disease progression following endocrine therapy, and as monotherapy in adult patients with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting [3, 4].
References
Adis Insight. Drug profile: abemaciclib. 2017. http://www.adisinsight.springer.com. Accessed 13 Oct 2017.
Xu H, Yu S, Liu Q, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol. 2017. https://doi.org/10.1186/s13045-017-0467-2.
Eli Lilly and Company. VERZENIO™ (abemaciclib): US prescribing information. 2017. https://www.fda.gov. Accessed 17 Oct 2017.
US FDA. FDA approves new treatment for certain advanced or metastatic breast cancers [media release]. 28 Sep 2017. https://www.fda.gov.
Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017. https://doi.org/10.1007/s10549-017-4385-3.
Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–53.
Eli Lilly and Company. FDA grants priority review for potential new indication for Lilly’s Verzenio™ (abemaciclib) as initial treatment of advanced breast cancer [media release]. 12 Oct 2017. https://www.lilly.com.
Eli Lilly. Lilly and AstraZeneca expand immuno-oncology research collaboration with new combinations [media release]. 22 Oct 2015. https://www.lilly.com/.
Eli Lilly. Lilly and Merck expand immuno-oncology collaboration adding abemaciclib and KEYTRUDA® combination trial [media release]. 10 Dec 2015. https://www.lilly.com/.
Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016. https://doi.org/10.1186/s13058-015-0661-5.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23(28):7212–20.
Hurvitz S, Martin M, Fernandez Abad M, et al. Biological effects of abemaciclib in a phase 2 neoadjuvant study for post-menopausal patients with HR+, HER2-breast cancer [abstract no. S4-06]. Cancer Res. 2017;77(4 Suppl).
Hurvitz S, Abad MF, Rostorfer R, et al. Interim results from neoMONARCH: a neoadjuvant phase II study of abemaciclib in postmenopausal women with HR +/HER2-breast cancer (BC) [abstract no. LBA13]. Ann Oncol. 2016;27(Suppl 6).
Goodman A. Abemaciclib plus anastrozole: promising signals reported in phase II study of early breast cancer. 2017. http://www.ascopost.com Accessed 2 Nov 2017.
Fujiwara Y, Tamura K, Kondo S, et al. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol. 2016;78(2):281–8.
AstraZeneca Pharmaceuticals. FASLODEX® (fulvestrant) injection: US prescribing information. 2017. https://www.fda.gov. Accessed 1 Nov 2017.
Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–84.
Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–24.
Rugo H, Nanda S, Koustenis A. Subgroup analysis by prior treatment and disease burden in MONARCH 1: a phase 2 study of monotherapy abemaciclib, a CDK4 & 6 inhibitor, in patients with HR+/HER2 metastatic breast cancer (MBC) following chemotherapy [abstract no. P6-11-12]. Cancer Res. 2017;77(4 Suppl).
Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017. https://doi.org/10.1200/jco.2017.75.6155.
Tolaney SM, Lin NU, Thornton D, et al. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer [abstract no. 1019]. J Clin Oncol. 2017;35(15 Suppl).
US National Library of Medicine. Clinicaltrials.gov. 10 Oct 2017. https://clinicaltrials.gov. Accessed 12 Oct 2017.
Eli Lilly and Company. Lilly reports topline results from phase 3 JUNIPER trial evaluating Verzenio™ (abemaciclib) in KRAS-mutated, advanced non-small cell lung cancer [media release]. 10 Oct 2017. https://www.lilly.com.
Goldman JW, Shi P, Reck M, et al. Treatment rationale and study design for the JUNIPER study: a randomized phase III study of abemaciclib with best supportive care versus erlotinib with best supportive care in patients with stage IV non-small-cell lung cancer with a detectable KRAS mutation whose disease has progressed after platinum-based chemotherapy. Clin Lung Cancer. 2016;17(1):80–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Esther Kim is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/3BCCF0602F7F3723.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Kim, E.S. Abemaciclib: First Global Approval. Drugs 77, 2063–2070 (2017). https://doi.org/10.1007/s40265-017-0840-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0840-z