Abstract
Purpose
To confirm the safety and tolerability, evaluate the pharmacokinetics (PK), and investigate the antitumor activity of abemaciclib in Japanese patients with advanced cancer.
Methods
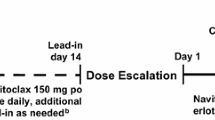
We conducted a non-randomized, single-arm, open-label, dose-escalation phase 1 study of abemaciclib administered orally every 12 h (Q12H) on a 28-day cycle at doses of 100 mg (Cohort 1, n = 3), 150 mg (Cohort 2, n = 3), or 200 mg [Cohort 3, n = 6, maximum tolerated dose (MTD)]. Dose escalation was based on the frequency of dose-limiting toxicity (DLT). MTD, as established in the previous phase 1 study in non-Japanese patients, was the highest dose level at which <33 % of patients experienced DLT.
Results
Eleven of the 12 patients who received treatment with abemaciclib discontinued: 10 patients due to progressive disease, and 1 due to a DLT (Cohort 3, grade 2 nausea). Diarrhea, the most common treatment-emergent adverse event (AE), was managed supportively and did not require study treatment discontinuation. There were no drug-related serious AEs and no patients with corrected QT (QTc) > 480 ms or QTc change of >60 ms from baseline. The abemaciclib PK profile was characterized by slow absorption and high PK variability after single or repeated doses. Two patients, one with breast cancer and one with neuroendocrine tumor, experienced >30 % decrease in tumor size from baseline.
Conclusions
In Japanese patients with advanced cancer, single-agent abemaciclib has an acceptable safety profile and demonstrates antitumor activity at a dose of 200 mg Q12H. These findings support ongoing development of abemaciclib for diverse populations with advanced cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cyclin-dependent kinases, CDK4 and CDK6, play an integral role in regulating the cell cycle by initiating the transition of cells through the G1 restriction point [1]. Aberrant activation of these kinases promotes cell cycle progression, which is a common mechanistic feature in human cancers [2, 3]. Therefore, pharmacologic inhibition of CDK4 and CDK6 may arrest tumor growth by preventing progression of tumor cells through the G1 restriction point. Various preclinical and clinical studies support CDK4 and CDK6 as potential tumor targets [4, 5]. Inhibitors of CDK4 and CDK6 have been investigated in clinical studies for the treatment of various cancers with evidence of anticancer effects in multiple tumor types, including hormone receptor-positive (HR+) breast cancer [6].
In clinical studies of breast cancer, several CDK4/6 inhibitors have been evaluated for efficacy, including palbociclib, ribociclib, and abemaciclib. PALOMA-3 is a randomized phase 3 study of palbociclib plus fulvestrant compared with placebo plus fulvestrant as second-line therapy for metastatic estrogen receptor positive (ER+), human epidermal growth factor receptor-2 negative (HER2−) breast cancer which demonstrated that progression-free survival was significantly longer in the experimental arm [7]. MONALEESA-2 is an ongoing phase 3 study that randomizes patients with metastatic HR+/HER2− disease to letrozole with or without ribociclib in the first-line setting [8]. MONARCH-2 is a randomized phase 3 study of abemaciclib plus fulvestrant compared to placebo plus fulvestrant as a second-line therapy for women with HR+, HER2− advanced breast cancer [9]. MONARCH-3 is a randomized phase 3 study of abemaciclib plus nonsteroidal aromatase inhibitor (NSAI; anastrazole or letrozole) compared to placebo plus NSAI in the first-line setting for women with HR+, HER2− advanced breast cancer [10].
Abemaciclib (LY2835219) is an oral, small molecule inhibitor with selectivity for CDK4 and CDK6 [11], which is distinguished from other drugs in its class by a clinical safety profile that enables dosing on a continuous schedule to achieve sustained target inhibition. A multicenter phase 1 study for patients with advanced cancer established the maximum tolerated dose (MTD) for abemaciclib as a single agent at 200 mg every 12 h (Q12H) (NCT01394016) [12]. Collectively, phase 1 and 2 studies for patients with advanced solid tumors and hematologic malignancies have shown evidence that single-agent abemaciclib has acceptable safety and clinical activity against multiple human tumors, including breast cancer, lung cancer, melanoma, and mantle cell lymphoma [12–15].
The aim of this phase 1 study in Japanese patients with advanced cancer was to evaluate abemaciclib at doses up to the MTD established in the previous phase 1 study in non-Japanese patients. The primary objective was to confirm the safety and tolerability of abemaciclib in Japanese patients with advanced cancer. Secondary objectives were to evaluate the pharmacokinetic (PK) parameters and antitumor activity of abemaciclib in this population.
Materials and methods
Study design
This clinical study was a non-randomized, single-arm, open-label, dose-escalation phase 1 study of oral abemaciclib (LY2835219), Eli Lilly and Company (Indianapolis, USA) in Japanese patients with advanced cancer up to the MTD of 200 mg Q12H. The study was conducted at a single clinical site in Japan from 21 December 2013 (first patient enrolled) to data cutoff on April 1, 2015; one patient is still on study treatment. Written approval was provided by the institutional review board, and the study was conducted in accordance with international ethics guidelines, including the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practices Guideline [E6]. Informed consent was obtained from each patient before any protocol procedures or administration of study drug. This study was registered at ClinicalTrials.gov (NCT02014129).
Study population
To be eligible, patients had histologically or cytologically confirmed advanced and/or metastatic cancer (solid tumor or lymphoma) with measurable or non-measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) or the Revised Response Criteria for Malignant Lymphoma [16, 17]. Patients were required to be appropriate candidates for experimental therapy in the judgment of the investigator after prior standard therapies had failed, to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤1 [18], to have adequate hepatic, hematologic, and renal function, and to have discontinued all previous therapies (including chemotherapy, radiotherapy, immunotherapy, and investigations therapy) ≥21 days prior to first dose of abemaciclib and recovered from acute toxicities.
Patients were specifically excluded if they met any of the following exclusion criteria: medical history of presyncope or syncope of either unexplained or cardiovascular etiology, ventricular arrhythmia, or sudden cardiac arrest; evidence at baseline of ventricular tachycardia, ventricular fibrillation, abnormally prolonged corrected QT by Bazett’s formula (QTcB) interval, or acute myocardial ischemia determined by electrocardiogram (ECG); presence of serious preexisting medical conditions that, in the judgment of the investigator, would preclude participation in this study; or symptomatic central nervous system malignancy or metastasis.
Treatment plan
Abemaciclib was administered Q12H without food for 1 h before or after the dose on a 28-day cycle. In Cycle 1, the initial dose of abemaciclib was taken on day 3 to enable PK sampling over 72 h following a single dose; the remaining doses were taken every 12 h on days 1–28. Abemaciclib was administered at one of 3 dose levels (100, 150, and 200 mg). Dose escalation proceeded, in cohorts of 3–6 patients, based on the frequency of dose-limiting toxicities (DLTs) observed in Cycle 1 until either ≥33 % of patients in one cohort experienced a DLT or the MTD was reached.
Adherence with study drug was assessed at each visit by direct questioning, reviewing the patient diary, and counting returned capsules. Patients were required to take ≥75 % of the intended dose to be deemed adherent with study drug administration.
Baseline and treatment assessments
Safety
In this study, adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03). A DLT was defined as an AE between day 3 and day 29 of Cycle 1 that was possibly related to abemaciclib and fulfilled any of the following criteria: grade 3 non-hematological toxicity, except for nausea, vomiting, diarrhea, electrolyte disturbance, or tumor lysis syndrome that could be controlled with treatment; grade 3 nausea, vomiting, diarrhea, or electrolyte disturbance that persisted >2 days despite maximal supportive intervention; grade 4 hematological toxicity that persisted >5 days; grade 3 thrombocytopenia with bleeding; febrile neutropenia; toxicity that required >25 % omissions of planned dose during Cycle 1 and considered a DLT by the investigator. Laboratory tests, including chemistry, hematology, and urinalysis panels, as well as observation of vital signs (including systolic and diastolic blood pressure, pulse rate, weight), were conducted at regular, prespecified time points. Time-matched ECGs were performed in triplicate at the same time as PK sampling (‘PK-matched ECGs’). QT values were corrected using QTcB and Fridericia’s (QTcF) formulae.
Pharmacokinetic evaluation
The following PK parameter estimates were included for abemaciclib: maximum observed plasma concentration (C max); area under the plasma concentration versus time curve (AUC) from time zero to last time point with a measurable plasma concentration [AUC(0–tlast)]; AUC from time zero to infinity [AUC(0–∞)]; AUC during 1 dosing interval at steady state (AUCτ,ss); time to reach maximal observed plasma concentration (t max); elimination half-life (t ½); apparent volume of distribution at steady state (V ss/F); and apparent total body clearance (CL/F). PK parameter estimates were calculated by standard non-compartmental methods (Phoenix® WinNonlin version 6.3, Certara, New Jersey, USA).
Efficacy
Efficacy was primarily assessed by determining the extent of antitumor activity, including the best overall response and time to event variables according to tumor measurement by RECIST 1.1 for patients with solid tumors or Revised Response Criteria for Malignant Lymphoma for patients with malignant lymphoma [16, 17]. Best overall response for solid tumors was defined as complete response, partial response, stable disease, progression/relapsed disease, or no evaluable response according to the evaluation criteria. Efficacy was also assessed by evaluation of tumor markers, when possible, and PS using the ECOG scale [18].
Statistical analysis
Sample size was based on the requirement for 3 or 6 patients at each dose level to assess the incidence of DLTs. MTD was defined as the highest dose level at which <33 % of patients experienced DLT. This study included at least three patients in each cohort with six patients enrolled at the MTD (200 mg Q12H) to collect additional data on safety, PK, and efficacy.
Details of patient disposition, demographics, and baseline disease characteristics were summarized for each cohort. Safety data (including vital signs, AEs, and PK-matched ECGs) were summarized by cohort in frequency tables or by summary statistics. A linear regression model was used to investigate the relationship between QTc changes from baseline and plasma abemaciclib concentration. PK parameters were summarized using descriptive statistics by dose. Efficacy data were listed and summarized by cohort using frequency tables or summary statistics. All efficacy analyses were performed on the full analysis set of patients, which was defined as patients who received at least 1 dose of any study drug. A waterfall plot of the maximal percent change in tumor size from baseline was determined for patients with solid tumors.
Results
Patient disposition and baseline characteristics
A total of 12 patients were enrolled in the 3 study cohorts as follows: Cohort 1, abemaciclib 100 mg Q12H (n = 3); Cohort 2, abemaciclib 150 mg Q12H (n = 3); and Cohort 3, abemaciclib 200 mg Q12H (n = 6). All enrolled patients received at least 1 dose of study drug (full analysis set) and were evaluable for DLTs. Of the 12 patients enrolled in the study, 11 patients (91.7 %) discontinued from treatment because of physician decision related to progressive disease (n = 10, 83.3 %) or an AE (n = 1, 8.3 %). One patient in Cohort 3 had reached Cycle 10 and was receiving ongoing treatment as of April 1, 2015 (day 297).
Overall, patients had a mean age of 59.1 years (range 37–73 years), a mean weight of 55.6 kg (range 37.2 to 74.7 kg) and a mean duration of cancer of 2.6 years (Table 1). All patients had metastatic solid tumors and had received at least 1 prior systemic therapy at study entry (Table 1). At baseline, 11 patients (91.7 %) had ECOG PS = 0 and 1 patient (8.3 %) had ECOG PS = 1. Finally, all patients received ≥75 % of planned doses during Cycle 1 except for 1 patient who experienced DLT.
Safety
Eight patients (66.7 %) completed 1 cycle and 1 patient (8.3 %) each completed <1 cycle, 2 cycles, 3 cycles, and 10 cycles, respectively. Overall, relative dose intensity (mean) was greater in Cohort 2 (98.2 %) than Cohort 1 (88.3 %) or Cohort 3 (74.7 %).
Diarrhea was the most common treatment-emergent AE (TEAE) possibly related to study drug and was experienced by 1 patient in Cohort 1, 2 patients in Cohort 2, and 6 patients in Cohort 3 (Table 2). However, these events were manageable and no patients discontinued study treatment because of diarrhea. TEAEs (regardless of causality) that were at least Grade 3 occurred in no patients in Cohort 1, 2 patients in Cohort 2, and 3 patients in Cohort 3. One AE leading to discontinuation on day 15 of treatment, which was considered possibly related to study drug, occurred in the patient in Cohort 3 who experienced DLT (Grade 2 nausea). Although serious AEs were reported by 2 patients in Cohort 2 (biliary tract infection in 1 patient; gastric fistula and lung infection in 1 patient) and 2 patients in Cohort 3 (cancer pain and decreased appetite in 1 patient; deep vein thrombosis in 1 patient), none of these events were considered related to abemaciclib.
Grade 3–4 toxicities related to study drug were seen among patients in Cohort 2 and Cohort 3, but not among patients in Cohort 1 (Table 2). Hematological toxicities seen in Cohort 2 and Cohort 3 were asymptomatic and manageable.
There were no clinically relevant changes in vital signs; moreover, laboratory abnormalities were predominantly CTCAE grade 1 or 2. Consistent with the observation that abemaciclib inhibits renal efflux transporters [multidrug and toxin extrusion (MATE) 1 and 2-K] that mediate active secretion of creatinine from the proximal tubule, grade 1 and 2 increases in serum creatinine were seen in 50.0 and 25.0 % of patients, respectively. Importantly, these increases in creatinine occurred during treatment but generally returned to within normal range following cessation of treatment. There were no patients with QTcB or QTcF of >480 ms or QTcB/QTcF change of >60 ms from baseline. One patient in Cohort 2 was observed with a change in QTcB/QTcF of >30 ms from baseline, and 1 patient in Cohort 3 had a change in QTcB of >30 ms from baseline. However, no relationships were detected between change in QTcF and plasma concentrations of abemaciclib in the Japanese population (Fig. 1).
Pharmacokinetic evaluation
PK data for abemaciclib after a single oral administration from pre-dose (day 3 of Cycle 1) through 72 h post-dose (day 1 of Cycle 1) were available from all 12 patients. The PK profile of abemaciclib after a single oral administration was characterized by slow absorption (median t max range approximately 5–6 h post-dose) with t ½ 14.2–27.5 h (Table 3; Fig. 2a). High PK variability was observed with coefficients of variation (CVs) reaching maximum values of 87 and 94 % for C max and AUC(0–tlast), respectively. The PK of abemaciclib at steady state after repeated oral administration was studied using data available from a total of nine patients (two patients each in Cohort 1 and 2, and 5 patients in Cohort 3) on day 28 of Cycle 1 (from pre-dose through 24 h post-dose). The PK profile of abemaciclib at steady state after repeated oral administration was also characterized by slow absorption (with a median t max,ss of approximately 4 h post-dose) (Table 3). High PK variability at steady state was observed with CVs of 64 and 73 % for C max,ss and AUCτ,ss, respectively.
The mean concentrations in Cohort 3 were lower than those in Cohort 1 or Cohort 2 (Fig. 2b), most likely because 1 patient each in Cohort 1 and Cohort 2 had relatively high exposures to abemaciclib.
Efficacy
Maximal percent change in tumor size from baseline for all patients with measurable disease ranged approximately from 35 % decrease in tumor size to 25 % increase in tumor size (Fig. 3). In Cohort 3, decreases in tumor size from baseline greater than 30 % were noted in 2 patients: One patient had breast adenocarcinoma that was estrogen receptor negative (ER−), progesterone receptor negative (PR−), and human epidermal growth factor receptor-2 positive (HER2+) and one patient (who completed Cycle 10 as of the reporting cutoff) had neuroendocrine carcinoma of the small intestine. Although no radiologically confirmed responses were observed in the study, durable disease control (>10 cycles) was achieved for 1 patient in Cohort 3. ECOG PS worsened from baseline to follow-up or final cycle in six patients.
Discussion
In this phase 1 study in Japanese patients with advanced cancer, abemaciclib demonstrated a safety profile that was manageable up to the previously established MTD (200 mg Q12H). Only 1 patient experienced DLT (Grade 2 nausea), which required dose omission. Diarrhea was manageable with supportive measures and hematological toxicity was acceptable. The PK profile was characterized by slow absorption and high PK variability. The elimination half-life of abemaciclib showed lower variability compared with other PK parameters, which suggests underlying variability in absorption rather than clearance. Regarding efficacy, 1 of 6 patients at the 200 mg Q12H dose achieved durable disease control for >10 cycles and continued to receive ongoing abemaciclib treatment at the reporting cutoff and at Cycle 22 as of February 26, 2016.
The overall safety and PK results of this phase 1 study in Japanese patients were similar to those observed in non-Japanese patients [12]. The most common DLT noted among non-Japanese patients was grade 3 fatigue, whereas one case of grade 2 nausea was noted among Japanese patients in this study. In both Japanese and non-Japanese patients, TEAEs were most commonly related to the gastrointestinal, renal, and hematopoietic systems. PK profiles of abemaciclib were similar in Japanese and non-Japanese patients. No remarkable differences in the PK of abemaciclib were observed between non-Japanese patients and the Japanese patients enrolled in this study. Although responses were not observed in this study, antitumor activity was evident in the Japanese population, with 2 patients experiencing decreases in tumor size greater than 30 %.
Abemaciclib inhibits renal efflux transporters [MATE 1 and 2-K] that mediate active secretion of creatinine from the proximal tubule. As such, increases in serum creatinine during abemaciclib therapy may not accurately reflect renal function. Instead, measures of glomerular filtration rate that are independent of renal efflux transporters (such as cystatin C) may yield a more accurate estimate of renal function than serum creatinine for patients receiving abemaciclib. In this study, mild increases in serum creatinine occurred during treatment but generally returned to within normal range following cessation of treatment. Based on these results, alternative methods to serum creatinine should be used if needed to evaluate renal function for patients receiving abemaciclib.
Two patients in Cohort 3 had significant decrease in tumor size, suggesting that 200 mg Q12H is the dose recommended by efficacy. Interestingly, one patient with hormone receptor-negative and HER2+ breast cancer experienced an antitumor effect, indicating potential activity in HER2+ disease.
Because the current study focused specifically on Japanese patients, these results must be considered in the context of previously reported studies [12–15]. Importantly, the overall design of this study is consistent with recommendations for phase 1 clinical studies, which principally aim to establish the recommended dose and schedule of an investigational drug for further efficacy testing in phase 2 and 3 studies [19].
In conclusion, this phase 1 study for Japanese patients with advanced solid tumors demonstrated that single-agent abemaciclib has an acceptable safety profile with evidence of antitumor activity. The safety profile observed at doses up to the MTD establishes 200 mg Q12H as the recommended dose for abemaciclib as a single agent in this population. These findings support ongoing development of abemaciclib for diverse populations with advanced cancer.
References
Lundberg AS, Weinberg RA (1998) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 18(2):753–761
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15(6):122
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ et al (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32(5):825–837
Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaria D, Barbacid M (2010) A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18(1):63–73
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Vidula N, Rugo HS (2016) Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer 16(1):8–17
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H et al (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219
Munster PN, Hamilton EP, Estevez LG, De Boer RH, Mayer IA, Campone M et al (2014) Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. J Clin Oncol 32, 2014 (suppl 26; abstr 143). http://meetinglibrary.asco.org/content/127461-144. Accessed 28 Feb 2016
Llombart A, Toi M, Klise SR, Frenzel M, Chan EM, Sledge GW (2014) A phase III study of abemaciclib (LY2835219) combined with fulvestrant in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) breast cancer (MONARCH 2) Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2014 Dec 9–13; San Antonio, TX Philadelphia (PA): AACR; Cancer Res 2015;75(9 Suppl):Abstract nr OT1-1-07 Accessed 15 March 2016
Goetz P, Toi M, Klise S, Frenzel M, Bourayou N (2015) MONARCH 3: A randomized phase III study of anastrozole or letrozole plus abemaciclib, a CDK4/6 inhibitor, or placebo in first-line treatment of women with HR, HER2-locoregionally recurrent or metastatic breast cancer (MBC). J Clin Oncol 33(15S, part 1):51; Abstract TPS624
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, del Prado M, Lallena MJ et al (2011) Abstract B233: identification and characterization of LY2835219: A potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) with broad in vivo antitumor activity. Mol Cancer Ther 10(11 Supplement):B233
Shapiro G, Rosen LS, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP et al (2013) A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol 31(15s):2500
Morschhauser F, Bouabdallah K, Stilgenbauer S, Thieblemont C, Wolf M, de Guibert S et al (2014) Clinical activity of abemaciclib (LY2835219), a cell cycle inhibitor selective for CDK4 and CDK6, in patients with relapsed or refractory mantle cell lymphoma. Blood 124(21):3067
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L et al (2014) LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol 32(15s):534
Tolaney SM, Rosen LS, Beeram M, Goldman JW, Gandhi L, Tolcher AW et al (2015) Abstract P5-19-13: clinical activity of abemaciclib, an oral cell cycle inhibitor, in metastatic breast cancer. Cancer Res 75(9 Supplement):P5-19-13
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101(10):708–720
Funding support
This study was sponsored by Eli Lilly Japan K.K, manufacturer/licensee of abemaciclib (LY2835219). Medical writing assistance was provided by Mark Snape, MB BS, CMPP and Tania Dickson, PhD of ProScribe—Envision Pharma Group—and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Other contributors/acknowledgments
The authors would like to thank study participants and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kitano, Tanabe, Tamura, and Shimomura have no conflicts of interest to declare. Kondo has received research funding from AstraZeneca, Eli Lilly Japan K.K., and Bayer Yakuhin, Ltd. Yamamoto has received research funding for clinical trials from Chugai Pharmaceutical Co., Ltd, Taiho Pharma, Eisai Co., Ltd, Quintiles, Astellas, Bristol-Myers Squibb, Kyowa-Hakko Kirin, Novartis Pharmaceuticals Japan, Daiichi-Sankyo, Pfizer, and Boehringer Ingelheim. Iwasa has received research funding for clinical trials from Eli Lilly Japan K.K. Fujiwara has served on advisory boards for Novartis Pharmaceuticals Japan and ONO Pharmaceuticals Japan, and received research funding for clinical trials from AstraZeneca, Eli Lilly Japan K.K, Chugai, Eisai Co., Daiichi-Sankyo, and MerckSerono. Ogasawara, Mori, and Asou are current employees of Eli Lilly Japan K.K. Turner and Chan are current employees of and own stock in Eli Lilly and Company.
Role of the sponsor
Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Role of contributors
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. KO, PKT, HA, and EMC were involved in the study design and data analyses. S Kitano, S Kondo, NY, KT, YT, SI, AS, and YF were investigators in the study and were involved in data collection. KO and JM conducted the statistical analysis.
Rights and permissions
About this article
Cite this article
Fujiwara, Y., Tamura, K., Kondo, S. et al. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol 78, 281–288 (2016). https://doi.org/10.1007/s00280-016-3085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3085-8