Abstract
Palbociclib (Ibrance®) is an oral, reversible, selective, small-molecule inhibitor of cyclin-dependent kinases (CDK) 4 and CDK6 developed by Pfizer for the treatment of cancer. CDKs are important modulators of cell cycle entry and progression in response to growth signals, and inhibition of these kinases with palbociclib could enhance the activity of other anticancer drugs in tolerable regimens. Palbociclib, in combination with letrozole, was recently approved in the US for the first-line treatment of advanced breast cancer. Phase III development is underway worldwide investigating its use as first-line treatment in advanced breast cancer, as well as treatment of recurrent or advanced breast cancer and high-risk, early-stage breast cancer. A phase II trial is underway in the USA for non-small cell lung cancer under a US National Cancer Institute-funded research collaboration, and several phase I and II investigations are being conducted for various other solid tumour types and haematological malignancies. This article summarizes the milestones in the development of palbociclib leading to this first approval for use in postmenopausal women with estrogen-positive, human epidermal growth factor receptor (HER) 2-negative advanced breast cancer as initial endocrine-based therapy for their metastatic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
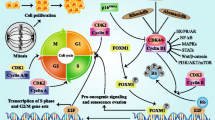
Cell proliferation and growth is tightly controlled by the cell-cycle regulatory machinery, dysregulation of which results in unrestrained growth, a hallmark of cancer [1–3]. Consequently, development of agents targeting pathways that regulate the cell-cycle has been of special interest. Cyclin-dependent kinases (CDKs) are key components of this machinery, which complex with their regulatory cyclin partner to control cell cycling [1–3].
Palbociclib is an oral, reversible, selective, small-molecule inhibitor of CDK4 and CDK6 developed by Pfizer for the treatment of cancer [4]. CDK4 and CDK6 along with their regulatory partner cyclin D1 play a key role in regulating the G1- to S-phase cell-cycle transition via regulation of phosphorylation of the retinoblastomaprotein (Rb) [1, 5].
Palbociclib (Ibrance®) received accelerated approval in the US in February 2015 [6] for the first-line systemic treatment of post-menopausal women with estrogen receptor (ER)-positive, human epidermal growth factor receptor (HER) 2-negative locally advanced or metastatic breast cancer [4]. Continued approval may be contingent upon verification and description of clinical benefit in a confirmatory trial [4, 6]. The recommended starting dosage of palbociclib is 125 mg once daily administered orally for 21 days followed by 7 days off treatment in a 28-day cycle; it should be administered in combination with letrozole 2.5 mg once daily given continuously throughout the 28-day cycle [4].
Features and properties of palbociclib
Alternative names | Ibrance™; PD 0332991; PD 332991; PD 991; PD-0332991; PD-332991; PD-991; PD0332991; PD332991; PF 332991; PF-332991; PF332991 |
Class | 2-ring-heterocyclic-compounds, cyclopentanes, ketones, piperazines, pyridines, pyrimidines, small-molecules |
Mechanism of action | Cyclin-dependent kinase 4 and 6 inhibitor |
Route of administration | Oral |
Pharmacodynamics | Reduces cellular proliferation of cancer cells by blocking progression of the cell from the G1- to S-phase of the cell-cycle by regulating the phosphorylation of Rb; combination treatment with antiestrogens (e.g. letrozole) increases the inhibition of Rb phosphorylation, downstream signalling and tumour growth compared to each drug alone |
Pharmacokinetics | Mean absolute bioavailability is 46 %; Cmax reached between 6 and 12 h and steady state reached within 8 days; mean plasma t½ is 29 h |
Adverse events (incidence >20 %) | Neutropenia, leukopenia, fatigue, anaemia, nausea, arthralgia, alopecia, diarrhoea, hot flush |
ATC codes | |
WHO ATC code | L01X-E (protein kinase inhibitors) |
EphMRA ATC code | L1X4 (antineoplastic protein kinase inhibitors) |
Chemical name | 6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2yl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one |
Palbociclib received Breakthrough Therapy designation from the US FDA in April 2013 and the New Drug Application (NDA), based on final data from the phase II PALOMA-1 trial, was accepted for priority review in October 2014 [7, 8]. A Prescription Drug User Fee Act date for this NDA was 13 April 2015 [7] and the rolling submission, which was initiated with the US FDA in June 2014, was completed in August 2014 [9]. Phase III development programmes are underway worldwide investigating its use as first-line treatment in advanced breast cancer, as well as treatment of recurrent or advanced breast cancer and high-risk, early-stage breast cancer. A phase II trial is underway in the US for non-small cell lung cancer (NSCLC) under a US National Cancer Institute-funded research collaboration and phase I and II investigations are being conducted for various other solid tumour types and haematological malignancies.
1.1 Company Agreements
Palbociclib emerged as a clinical candidate from a collaborative research programme conducted by Warner-Lambert (formerly Parke-Davis and now a subsidiary of Pfizer) and Onyx Pharmaceuticals (a subsidiary of Amgen). Onyx Pharmaceuticals was acquired by Amgen in October 2013 [10]. As of December 2010, Onyx had received $US1.5 million from Pfizer in milestone payments relating to palbociclib. The research phase of the programme ended in August 2001 and Pfizer continued the clinical development of palbociclib. Onyx is entitled to receive royalties on sales upon commercialisation of the product.
In November 2013, Pfizer entered into an agreement with GlaxoSmithKline to conduct a phase I/II trial of palbociclib in combination with GlaxoSmithKline’s trametinib for the treatment of advanced malignant melanoma [11]. Other inhibitors of this pathway are in clinical development by Lilly and by Novartis but their findings are mostly preliminary and will not be reviewed herein.
2 Scientific summary
2.1 Pharmacodynamics
Palbociclib reduced cellular proliferation of ER-positive breast cancer cell lines in vitro, by blocking Rb phosphorylation and resulting in G1 arrest in sensitive cells [12, 13]. Relative to treatment with palbociclib or an anti-estrogen (e.g. letrozole, fulvestrant) alone, combination treatment with these agents in breast cancer cell lines led to a greater decrease in Rb phosphorylation, resulting in reduced expression of E2F, FoxM1 and downstream target genes (e.g. polo-like kinase 1 and S-phase kinase-associated protein 2) and increased growth arrest [4, 14]. Increased cell senescence was seen with the combination of palbociclib and anti-estrogens in vitro [14], which was sustained for up to 6 days following drug removal [4]. Greater inhibition of Rb phosphorylation, downstream signalling and tumour growth with palbociclib plus the anti-estrogen letrozole (relative to either drug alone) was also seen in a patient-derived ER-positive breast cancer xenograft model [14].

Chemical structure of Palbociclib
The antitumour effects of palbociclib have also been demonstrated in other human tumour cell lines (e.g. lung and renal cell carcinoma, and mantle cell and B cell lymphoma) [13, 15–17], tumour xenografts (e.g. lung and prostate cancer, myeloma and brainstem glioma models) [13, 18–20] and primary tumours (prostate cancer and bone marrow myeloma cells) [18, 20].
In vitro studies have suggested Rb, cyclin D1, E2F transcription factor 1 and members of the inhibitors of CDK4 family (p16, p15) as potential biomarkers to identify response to palbociclib [12, 17, 21], and the genomic and expression markers of activation of the hedgehog/smoothened pathway as strong predictors of resistance to palbociclib [22].
No large change in QTc interval (>20 ms) was seen at the mean maximal steady-state concentration of palbociclib, following treatment with the approved dosage of palbociclib in 184 patients with advanced cancer [4].
2.2 Pharmacokinetics
The pharmacokinetic properties of palbociclib were assessed in patients with solid tumours, including advanced breast cancer [23–25], and in healthy subjects [26].
Following oral administration of palbociclib, peak drug concentrations (Cmax) were reached between 6 and 12 h. The mean absolute bioavailability of palbociclib was 46 % following administration of a 125 mg dose [4]. In general, palbociclib Cmax and area under the concentration-time curve increased dose proportionally when the drug was administered in the dose range of 25–225 mg [4, 23–25]. After repeated once daily administration, steady-state concentrations of palbociclib were achieved within 8 days [4] and the drug accumulated with a median accumulation ratio of 2.4 [4, 24].
In approximately 13 % of patients, palbociclib absorption and exposure was very low when it was administered under fasted conditions [4]. Food intake increased palbociclib exposure in these patients, but did not alter palbociclib exposure to a clinically relevant extent in the rest of the population [4, 26]. As food intake reduced intersubject variability of palbociclib, it is recommended that palbociclib be administered with food [4, 26].
Human plasma protein binding of palbociclib was ≈85 % and was not concentration dependent over the concentration range of 500–5000 ng/mL [4]. The geometric mean apparent distribution of palbociclib was 2583 L [4], suggesting that palbociclib penetrates extensively into peripheral tissues [24].
Palbociclib undergoes hepatic metabolism via the cytochrome P450 (CYP) 3A and sulfotransferase (SULT) A1 enzymes, according to in vitro and in vivo studies [4]. After oral administration of a 125 mg dose in humans, palbociclib was primarily metabolized by oxidation and sulfonation, with acylation and glucuronidation contributing as minor pathways. The major drug-derived entity in the plasma was palbociclib (23 % of a radiolabelled dose) and the major circulating metabolite was a glucuronide conjugate of palbociclib, although it accounted for only 1.5 % of the administered dose in the excreta. Unchanged drug accounted for 2.3 and 6.9 % of radioactivity in the faeces and urine, respectively, indicating that palbociclib was extensively metabolized. In the faeces, the major drug-related component was the sulfamic acid conjugate of palbociclib (26 % of the administered dose) [4].
The geometric mean apparent oral clearance of palbociclib was 63.1 L/h and its mean plasma elimination half-life was 29 h in patients with advanced breast cancer[4]. After a single radiolabelled oral dose of palbociclib in healthy subjects, a median of 91.6 % of the radioactivity was recovered in 15 days, with the majority of the drug excreted via the faeces (74.1 % of the dose) and 17.5 % of the dose was recovered in the urine; the majority of excreted material was metabolites [4].
Gender, age, bodyweight, mild or moderate renal impairment and mild hepatic impairment had no clinically important effects on the exposure to palbociclib, according to a population pharmacokinetic analysis in 183 patients with cancer [4, 27]. The pharmacokinetics of palbociclib have not been assessed in patients with severe renal impairment, or moderate or severe hepatic impairment [4].
2.2.1 Potential Drug Interactions
Palbociclib is metabolized primarily by CYP3A and SULT2A1 enzymes and is a time-dependent inhibitor of CYP3A [4]. Therefore, coadministration of palbociclib with a strong CYP3A inhibitor (e.g. itraconazole, clarithromycin, ritonavir) is likely to increase the exposure to palbociclib; therefore, its concomitant use with strong CYP3A inhibitors should be avoided. Grape fruit and grapefruit juice should also be avoided during palbociclib therapy. The dose of palbociclib should be reduced if coadministration with a strong CYP3A inhibitor cannot be avoided [4]. Coadministration of palbociclib with strong [e.g. phenytoin, rifampin (rifampicin), St John’s wort] or moderate (e.g. bosentan, efavirenz, modafinil) CYP3A inducers may decrease the plasma exposure to palbociclib; consequently, concomitant use of palbociclib with these agents should also be avoided [4].
Coadministration of palbociclib with midazolam (CYP3A4/5 substrate) increased the exposure to midazolam [28]; therefore, the dose of a sensitive CYP3A substrate with a narrow therapeutic index (e.g. alfentanil, everolimus, tacrolimus) may need to be reduced when coadministered with palbociclib [4].
There is no drug interaction between palbociclib and letrozole when these agents are administered concomitantly in breast cancer patients [4]. When coadministered under fed conditions, there is no relevant effect of proton pump inhibitors, H2 receptor antagonists or local antacids on the exposure to palbociclib, according to a study in healthy subjects [4].
In in vitro studies, palbociclib at clinically relevant concentrations has low potential to inhibit the activities of the drug transporters P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), organic anion transporters 1 and 3, organic cation transporter 2 and organic anion transporting polypeptide 1B1 and 1B3 [4]. In vitro studies also showed that P-gp and BCRP mediated transport are unlikely to affect the extent of oral absorption of palbociclib at therapeutic doses [4].
2.3 Therapeutic Trials
Palbociclib was administered orally at a dosage of 125 mg for 21 days in a 28-day cycle, unless indicated otherwise.
2.3.1 Breast Cancer
First-line treatment with palbociclib in combination with letrozole (at the approved dosages) significantly prolonged progression-free survival (PFS) relative to letrozole alone in postmenopausal women aged ≥18 years who had ER-positive, HER2-negative, advanced breast cancer in the randomized, phase II PALOMA-1/TRIO-18 trial (NCT00721409) [29]. At the time of final analysis of PFS, the median PFS in palbociclib plus letrozole recipients (n = 84) was 20.2 months relative to 10.2 months in letrozole recipients(n = 81), corresponding to a 51 % reduction in the risk of disease progression or death (HR 0.49; 95% CI 0.32–0.75; p = 0.0004); median follow-up was 29.6 and 27.9 months in the respective groups [29]. In addition, significantly more palbociclib plus letrozole than letrozole alone recipients experienced clinical benefit in the intent-to-treat population (68 vs. 47 %; p = 0.0009) and an objective response in patients with measurable disease at baseline (36 vs. 26 %; p < 0.047). The median duration of response in palbociclib plus letrozole recipients was 20.3 months compared with 11.1 months in letrozole alone recipients. Overall survival data at the time of final PFS analysis were not mature (median overall survival was 37.5 in the palbociclib plus letrozole vs. 33.3 months in the letrozole alone groups) [29].
In another phase II study in 37 patients with Rb-positive, metastatic breast cancer positive (NCT01037790; UPCC03909), palbociclib administered as monotherapy was associated with a median PFS of 3.7 months and a clinical benefit rate of 19 % [21 % in hormone receptor (HR)- and HER2-positive patients and 29 % in HR-positive/HER2-negative patients] [30].
2.3.2 Non-Small Cell Lung Cancer
A single-arm phase II study assessed the efficacy of palbociclib in patients with previously treated, advanced NSCLC (NCT01291017) [31]. No responses were seen in the 16 evaluable patients who received at least 1 month of treatment with palbociclib and the trial was closed to accrual. However, 8 patients who had progressive NSCLC had stable disease lasting 16–42 weeks following palbociclib therapy; the remaining 8 patients had progressive disease within 8 weeks. The median PFS was 12.5 weeks [31].
2.3.3 Other Cancers
An open-label, phase I, dose-escalation study (NCT00141297) in 41 patients with Rb-positive advanced solid tumours determined the maximum tolerated dose of palbociclib to be 125 mg administered once daily for 21 days in a 28-day cycle [23]. Another open-label, phase I study in 33 patients with Rb-positive advanced solid tumours or non-Hodgkin’s lymphoma refractory to standard therapy or for which no therapy was available determined the maximum tolerated dose of palbociclib to be 200 mg administered once daily for 14 days in a 21-day cycle [24].
Treatment with palbociclib 125 mg daily was associated with a favourable 24-week PFS rate of 28 % (90 % CI 15–44 %) in patients with incurable, refractory, Rb-positive germ cell tumours in an open-label, phase II study (n = 29 evaluable) [32]. A PFS rate of ≥15 % was considered promising and a rate of ≤5 % was not considered promising in this study [32].
Benefit with palbociclib (200 mg once daily for 14 days in a 21-day cycle) was also seen in another phase II study in patients with CDK4-amplified, Rb-expressing, well-differentiated or dedifferentiated liposarcoma (NCT01209598), as indicated by a favourable 12-week PFS rate of 66 % and a median PFS of 18 weeks (n = 29 evaluable) [33]. A PFS rate of ≥40 % was considered promising and a rate of ≤20 % was not considered promising in this study [33].
In December 2013, the University of California at San Francisco in collaboration with Pfizer completed a non-randomised, open-label phase II trial of palbociclib in patients with recurrent, Rb-positive glioblastoma, (NCT01227434). The primary outcome measure was the efficacy of the drug as assessed by progression free survival. The study was initiated in September 2010 and enrolled 74 patients in the US; no results are currently available.
In a dose-escalation arm of an open-label, phase I/II study in nine patients with Rb-positive, relapsed and refractory myeloma after at least 2 previous treatments (NCT00555906), treatment with oral palbociclib (100–125 mg once daily for 21 days in a 28-day cycle) plus intravenous bortezomib (1.0–1.3 mg/m2) and oral dexamethasone (20 mg) on days 8, 11, 15 and 18 was associated with an objective response rate of 25 %, with one patient each achieving a very good partial response, minimal response and stable disease [34].
Benefit with palbociclib was also seen in patients with mantle cell lymphomain a phase Ib study (NCT00420056; n = 16 evaluable), with 18 % of patients achieving an objective response (1 complete and 2 partial responses) following treatment with palbociclib [35]. In another phase I study in 19 patients with previously treated mantle cell lymphoma who received combination treatment with palbociclib (75–125 mg once daily for 12 days in a 21-day cycle) plus intravenous or subcutaneous bortezomib (1.0 or 1.3 mg/m2 on days 8, 11, 15 and 18), there appeared to be an association between the dose of palbociclib and response [36]. One patient each in the 75 and 100 mg group and 4 patients in the 125 mg group remained free from progression for 1 year (including one patient with complete response in the 125 mg group); only one responding patient progressed on therapy [36].
Key clinical trials of palbociclib
Drugs(s) | Indication | Phase | Status | Location(s) | Indentifier | Sponsor |
|---|---|---|---|---|---|---|
Palbociclib + letrozole, placebo + letrozole | Advanced breast cancer | Phase 3 | Planned | China | NCT02297438; PALOMA-4 | Pfizer |
Palbociclib + letrozole, placebo + letrozole | Advanced breast cancer | Phase 3 | Ongoing | Multinational | NCT01740427; PALOMA-2; Study 1008; EudraCT2012-004601-27 | Pfizer |
Palbociclib, letrozole | Advanced breast cancer | Phase 3 | No longer available | US | NCT02142868; A5481034 | Pfizer |
Palbociclib, palbociclib + ET | Advanced breast cancer | Phase 2 | Ongoing | Italy | TREnd_trial; EudraCT2011-005637-38 | Fondazione Sandro Pitigliani per la lotta contro i tumori - ONLUS |
Palbociclib, letrozole | Advanced breast cancer | Phase 1/2 | Ongoing | Multinational | NCT00721409; PALOMA-1/TRIO-18 | Pfizer |
Palbociclib + exemestane, Capecitabine | Metastatic breast cancer | Phase 3 | Recruiting | Spain | NCT02028507; PEARL; EudraCT2013-003170-27 | Spanish Breast Cancer Research Group |
Palbociclib + fulvestrant, placebo + fulvestrant | Metastatic breast cancer | Phase 3 | Ongoing | Multinational | NCT01942135; PALOMA-3; Study 1023; EudraCT2013-002580-26 | Pfizer |
Palbociclib, ET | Invasive breast cancer | Phase 2 | Recruiting | US | NCT02040857 | Dana-Farber Cancer Institute |
Palbociclib, placebo | Early-stage breast cancer | Phase 3 | Recruiting | Germany | NCT01864746; PENELOPE-B; EudraCT2013-001040-62 | German Breast Group |
Palbociclib, palbociclib + letrozole | Early-stage breast cancer | Phase 2 | Planned | NA | NCT02296801; PALLET; UKCRN17716; EudraCT2014-000887-16 | NSABP Foundation Inc. |
Palbociclib + anastrozole (and goserelin if premenopausal) | Early-stage breast cancer | Phase 2 | Recruiting | US | NCT01723774 | Washington University School of Medicine |
Palbociclib | Early-stage breast cancer | Phase 2 | Ongoing | France | NCT02008734 | Gustave Roussy, Cancer Campus, Grand Paris |
Palbociclib, docetaxel (S1400C sub-study) | Recurrent squamous NSCLC | Phase 2/3 | Recruiting | US | NCT02154490 | Southwest Oncology Group |
Palbociclib | Advanced NSCLC | Phase 2 | Recruiting | US | NCT01291017 | University of Florida |
Palbociclib | NSCLC and solid tumours | Phase 1/2 | Recruiting | US | NCT02022982 | Dana-Farber Cancer Institute |
Palbociclib + bortezomib + dexamethasone | Refractory multiple myeloma | Phase 1/2 | Completed | US, Germany, Czech Republic | NCT00555906 | Pfizer |
Palbociclib | Advanced or metastatic liposarcoma | Phase 2 | Active | US | NCT01209598 | Memorial Sloan Kettering Cancer Center |
Palbociclib | Advanced HCC | Phase 2 | Ongoing | US | NCT01356628 | Thomas Jefferson University |
Palbociclib | Metastatic melanoma | Phase 1/2 | Recruiting | France | NCT02202200; OPTIMUM | Assistance Publique - Hôpitaux de Paris |
Palbociclib | Recurrent glioblastoma | Phase 2 | Completed | US | NCT01227434 | University of California, San Francisco |
2.4 Adverse Events
The tolerability profile of palbociclib in combination with letrozole was predictable and generally manageable in patients with ER-positive, HER2-negative, advanced breast cancer in the randomized, phase II PALOMA-1 study [29]. At least one adverse event was reported in all palbociclib and letrozole recipients compared with 84 % of letrozole recipients (the safety population included 83 and 77 patients in the respective groups) [29].
The most common treatment-emergent adverse events with palbociclib plus letrozole relative to letrozole were neutropenia (75 vs. 5 % any-grade; 54 vs. 1 % grade 3/4), leukopenia (43 vs. 3 % any-grade; 19 vs. 0 % grade 3/4) and fatigue (41 vs. 23 % any-grade; 4 vs. 1 % grade 3/4) [29]. There were no cases of neutropenic fever in palbociclib plus letrozole recipients [29]. Moreover, neutropenia with palbociclib plus letrozole differed from that seen with chemotherapy in that it was not commonly associated with fever, is self-limited and is characterized by recovery after a brief dose interruption or cycle delay [37].
Other common (incidence >20 %) treatment emergent adverse events of any-grade severity with palbociclib plus letrozole relative to letrozole alone were anaemia (35 vs. 7 %; p < 0.0001), nausea (25 vs. 13 % any-grade), arthralgia (23 vs. 16 %), alopecia (22 vs. 3 %; p = 0.0002), diarrhoea (21 vs. 10 %) and hot flush (21 vs. 12 %), most of which were of grade 1 or 2 severity [29]. Serious treatment-emergent adverse events that occurred in more than one palbociclib plus letrozole recipients were pulmonary embolism (3 patients), back pain (2 patients) and diarrhoea (2 patients); no serious adverse events were reported in more than one letrozole recipient [29].
Dose interruptions because of adverse events were required in 33 % of palbociclib plus letrozole and 4 % of letrozole recipients [29]. In palbociclib plus letrozole recipients, a subsequent treatment cycle was delayed because of an adverse event in 45 % of patients and a dose reduction was required in 40 % of patients. Disease progression was the main reason for treatment discontinuation both in the palbociclib plus letrozole and the letrozole alone groups (50 vs. 70 %), and 13 and 2 % of patients in the respective groups discontinued treatment because of an adverse event. Treatment-related adverse events resulted in treatment discontinuation in 7 % of patients in the palbociclib plus letrozole group and 2 % of patients in the letrozole alone group; no treatment-related deaths were reported in either group. There was also no significant between-group difference in pain severity or on the effect of pain on daily activities [29].
The tolerability profile of palbociclib in phase I and II studies was generally similar to that discussed above, with neutropenia and leukopenia among the most common grade 3 or 4 toxicities in patients receiving palbociclib treatment [23, 24, 30, 32, 33].
2.5 Ongoing Clinical Trials
2.5.1 Breast Cancer
2.5.1.1 As First-Line Therapy
In February 2013, Pfizer initiated an international phase III trial of palbociclib in combination with letrozole for the first-line treatment of patients with advanced breast cancer (NCT01740427; PALOMA-2; Study 1008; EudraCT2012-004601-27). This randomised, double-blind trial is investigating the efficacy, tolerability, pharmacokinetics, pharmacodynamics and quality-of-life effects of palbociclib plus letrozole compared with letrozole alone. Enrolment of ≈650 postmenopausal women with ER-positive, HER2-negative advanced breast cancer has been completed; the study is being conducted in several countries, including the US, Canada, France, Germany, New Zealand, the UK and South Korea.
Pfizer has initiated an open-label phase III Expanded Access Programme for treatment with palbociclib, in combination with letrozole, in post-menopausal women with hormone-receptor positive, HER2-negative, advanced breast cancer for whom letrozole therapy is deemed appropriate (NCT02142868; A5481034). As at February 2015, Expanded Access to the treatment is not available in the USA.
Pfizer is also planning a phase III study to evaluate the efficacy of palbociclib plus letrozole, compared with placebo plus letrozole, for the first-line treatment of Asian women with ER-positive HER2-negative advanced breast cancer (NCT02297438; PALOMA-4). The randomised, double-blind study will enrol approximately 330 patients in China who have not received prior systemic anti-cancer therapy.
2.5.1.2 In Recurrent or Advanced Disease
In September 2013, Pfizer initiated a phase III trial to investigate palbociclib plus fulvestrant in patients with HR-positive, HER2-negative metastatic breast cancer (NCT01942135; PALOMA-3; Study 1023; EudraCT2013-002580-26). The randomised, double-blind, placebo-controlled trial plans to enrol approximately 417 patients with disease progression on or following prior adjuvant within 12 months or endocrine therapy in the metastatic setting [38]. The study aims to demonstrate the superiority of combination therapy over fulvestrant alone and is being conducted in the several countries worldwide, including the US, Australia, Canada, Germany, the UK, Japan, and South Korea.
The Spanish Breast Cancer Research Group in collaboration with Pfizer initiated a randomised, open-label phase III trial in March 2014 to assess the efficacy and safety of palbociclib plus exemestane compared with that of capecitabine in postmenopausal women with HR-positive/HER2-negative metastatic breast cancer refractory to prior non-steroidal aromatase inhibitor therapy (NCT02028507; PEARL; EudraCT2013-003170-27) [39]. The study is currently recruiting patients in Spain and four other European countries and will enrol 348 patients.
In addition, a phase II study is evaluating the efficacy of palbociclib as monotherapy or in combination with endocrine therapy in postmenopausal women with ER-positive, HER2-negative advanced breast cancer who have disease progression with previous therapy (TREnd_trial; EudraCT2011-005637-38) [40].
2.5.1.3 Early-Stage Disease
The German Breast Group in collaboration with Pfizer is conducting a phase III trial of palbociclib in addition to standard background endocrine therapy in women with ER-positive, HER2-normal, high-risk, early-stage breast cancer who have residual disease after neoadjuvant chemotherapy and surgery (NCT01864746; PENELOPE-B; EudraCT2013-001040-62) [41]. The trial will enrol approximately 800 patients in 11 countries [41] and recruitment has begun in Germany.
In addition, the Institute for Cancer Research and the Royal Marsden National Health Service (NHS) Foundation Trust, US National Surgical Adjuvant Breast and Bowel Breast Project (NSABP) foundation in collaboration with Pfizer are planning a randomized phase II study to evaluate palbociclib plus letrozole versus letrozole as neoadjuvant therapy in patients with ER-positive, HER2-negative early-stage breast cancer (NCT02296801; PALLET; UKCRN17716; EudraCT2014-000887-16).
A Phase II study is also ongoing to assess the efficacy of palbociclib plus an aromatase inhibitor in patients with HR-positive, HER2-negative, stage II/III invasive breast cancer (NCT02040857) [42]. Other studies include two phase II studies assessing the efficacy of neoadjuvant endocrine therapy with or without palbociclib in patients with stage II/III primary breast cancer (PREDIXLumA; EudraCT2014-000809-12 and PREDIXLumB; EudraCT2014-000810-72).
2.5.1.4 Other studies
A phase III trial is investigating the efficacy and safety of palbociclib in men with HER2-positive metastatic breast cancer (EudraCT2014-004226-18). In addition, a phase I study has been initiated to investigate the efficacy of the combination of weekly paclitaxel and alternating palbociclib in patients with Rb-positive metastatic breast cancer (NCT01320592) [43], as well as a phase Ib trial assessing the safety and tolerability of palbociclib plus trastuzumab-emtansine in patients with recurrent or metastatic HER2-positive breast cancer who have received prior treatment with trastuzumab or other HER2-directed therapies (NCT01976169).
2.5.2 Non-Small Cell Lung Cancer
In June 2014, the Lung Cancer Master Protocol (Lung-MAP) phase II/III trial was initiated by the Southwest Oncology Group in the US, in collaboration with the US National Cancer Institute, Pfizer, Amgen, Genentech, AstraZeneca and MedImmune (NCT02154490). The trial is investigating biomarker-driven targeted therapy to treat patients with stage IIIB or IV squamous non-small cell lung cancer who have failed first-line therapy with a platinum-based regimen for metastatic disease. Five experimental drugs, including palbociclib, will be investigated initially. Up to 5–7 additional drugs may be added to the protocol over the first 5 years of the study. It is estimated that 500–1000 patients will be screened each year for more than 200 cancer-related genetic mutations. Patients will then be assigned to a treatment arm based on the genomic profile. In one arm of the trial (S1400C arm), patients with CDK4 or 6, CCND1, CCND2 or CCND3-positive tumours will be randomised to treatment with either oral palbociclib or docetaxel. The estimated completion date of the trial is June 2022 [44].
2.5.3 Other Cancers
Other ongoing palbociclib studies include phase II monotherapy studies in patients with metastatic urothelial carcinoma after failure of first-line chemotherapy (NCT02334527), in patients with inoperable, recurrent/refractory advanced hepatocellular carcinoma (NCT01356628) [45] and in patients with recurrent ovarian cancer (NCT01536743). Ongoing combination therapy studies include a phase II study of androgen therapy with or without palbociclib in patients with Rb-positive metastatic prostate cancer (NCT02059213), a phase I/II study of palbociclib plus cetuximab in patients with incurable Squamous Cell of Head and Neck (NCT02101034), a phase I/II study of palbociclib plus standard vemurafenib therapy in patients with BRAF V600E/K-mutated, RB-positive metastatic melanoma (NCT02202200; OPTIMUM) and a phase I/II study of palbociclib in combination with trametinib in patients with solid tumours (phase I) or advanced or metastatic melanoma (phase II) (NCT02065063; Study 200344). Also underway are several phase I studies including those assessing the combination of palbociclib, oxaliplatin and fluorouracil in patients with advanced colorectal cancer (NCT01522989), the combination of palbociclib and bortezomib in patients with relapsed mantle cell lymphoma (NCT01111188), and the combination of palbociclib, lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma (NCT02030483).
3 Current Status
Palbociclib, in combination with letrozole, received its first global approval on 3 February 2015 in the USA for the treatment of postmenopausal women with ER-positive, HER2-negative advanced breast cancer as initial endocrine-based therapy for their metastatic disease. Development continues or is planned in breast cancer, non-small cell lung cancer, other solid tumours and haematological malignancies.
References
Cadoo KA, Gucalp A, Traina TA. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer (Dove Med Press). 2014;6:123–33.
Sutherland RL, Musgrove EA. CDK inhibitors as potential breast cancer therapeutics: new evidence for enhanced efficacy in ER+ disease. Breast Cancer Res. 2009;11(6):112.
Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20(13):3379–83.
Pfizer Inc. IBRANCE® (palbociclib): US prescribing information. 2015. http://www.accessdata.fda.gov. Accessed 12 Feb 2015.
Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33(15):1890–903.
Pfizer. Pfizer receives US FDA accelerated approval of IBRANCE® (palbociclib) [media release]. http://www.pfizer.com. Accessed3 Feb 2015.
Pfizer. Pfizer reports third-quarter 2014 results [media release]. http://press.pfizer.com. Accessed 28 Oct 2014.
Pfizer. Pfizer announces submission of palbociclib new drug application to the FDA [media release]. http://www.pfizer.com. Accessed 18 Aug 2014.
Pfizer. Pfizer reports second-quarter 2014 results [media release]. http://press.pfizer.com. Accessed 29 Jul 2014.
Amgen. Amgen’s third quarter 2013 revenues increased 10 percent to $4.7 billion and adjusted earnings per share (EPS) increased 16 percent to $1.94 [media release]. http://www.amgen.com. Accessed 22 Oct 2013.
Pfizer. Pfizer and GSK to initiate study of novel combination therapy in patients with melanoma [media release]. http://www.pfizer.com. Accessed 21 Nov 2013.
Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77.
Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–38.
Lee NV, Yuan J, Eisele K, et al. Mechanistic exploration of combined CDK4/6 and ER inhibition in ER-positive breast cancer [abstract no. LB-136]. 105th Annual Meeting of the American Association for Cancer Research, AACR. 2014;74(19 Suppl 1).
Zoellner AK, Dietzfelbinger R, Hutter G, et al. The CDK 4/CDK 6 Inhibitor PD0332991 significantly reduces cell growth via a G1 phase arrest in Mantle Cell Lymphoma cell lines and additively increases effects of Ibrutinib [abstract no. V667]. Onkologie. 2013;36(Suppl 7):198.
Zoellner AK, Nestorova V, Hutter G, et al. The CDK4/CDK6 Inhibitor PD0332991 significantly reduces cell growth in GCB and ABC DLBCL cell lines and additively increases effects of GS-1101 [abstract no. V666]. Onkologie. 2013;36(Suppl 7):198.
Logan JE, Mostofizadeh N, Desai AJ, et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res. 2013;33(8):2997–3004.
Comstock CE, Augello MA, Goodwin JF, et al. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene. 2013;32(48):5481–91.
Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One. 2013;8(10):e77639.
Baughn LB, Di Liberto M, Wu K, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66(15):7661–7.
Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17(6):1591–602.
Von Euw EM, Conklin D, Rong HM, et al. Identification of markers of sensitivity and resistance to palbociclib (PD0332991) in melanoma [abstract no. 1321]. Cancer Res. 2014;74(19 Suppl 1).
Flaherty KT, Lorusso PM, DeMichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18(2):568–76.
Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer. 2011;104(12):1862–8.
O’Dwyer PJ, LoRusso P, DeMichele A, et al. A phase I dose escalation trial of a daily oral CDK 4/6 inhibitor PD-0332991 [abstract no. 3550]. 2007;25(18S).
Ruiz-Garcia A, Plotka A, Pawlak S, et al. Effect of food on the bioavailability of palbociclib 125 mg capsules in healthy volunteers [abstract no. 463P]. Ann Oncol. 2014;25(suppl 4):iv154.
Sun W, Wang DD. A population pharmacokinetic (PK) analysis of palbociclib (PD-0332991) in patients (pts) with advanced solid tumors [abstract no. 462P]. Ann Oncol. 2014;25(Suppl 4):iv154.
Hoffman JT, Plotka A, O’Gorman M, et al. A phase 1 randomized, open-label, 2-sequence, 2-period crossover study of the effect of multiple doses of palbociclib (PD-0332991) on midazolam pharmacokinetics in healthy women of non-childbearing potential [abstract no. Abstract CT419]. Cancer Res. 2014;74(19 Suppl).
Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2014. doi:10.1158/1078-0432.ccr-14-2258.
Gopalan PK, Pinder MC, Chiappori A, et al. A phase II clinical trial of the CDK 4/6 inhibitor palbociclib (PD 0332991) in previously treated, advanced non-small cell lung cancer (NSCLC) patients with inactivated CDKN2A [abstract no. 8077]. J Clin Oncol. 2014;32(15 Suppl 1).
Vaughn DJ, Hwang W, Lal P, et al. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer. 2014. doi:10.1002/cncr.29213.
Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31(16):2024–8.
Niesvizky R, Ely S, Jayabalan DS, et al. A Phase I Trial of PD 0332991, a Novel, Orally-Bioavailable CDK4/6-Specific Inhibitor Administered in Combination with Bortezomib and Dexamethasone to Patients with Relapsed and Refractory Multiple Myeloma [abstract no. 1877]. In: 51st annual meeting and exposition of the American Society of Hematology. 2009.
Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119(20):4597–607.
Martin P, DiLiberto M, Mason CE, et al. The combination of palbociclib plus bortezomib is safe and active in patients with previously treated mantle cell lymphoma: final results of a phase I trial [abstract no. 4393]. In: 55th annual meeting and exposition of the American Society of Hematology. 2013.
Finn R, Crown J, Ettl J, et al. Clinical patterns of palbociclib associated neutropenia in the PALOMA-1/TRIO-18 trial [abstract no. 368P]. Ann Oncol. 2014;25(suppl 4):iv122.
Turner N, André F, Loibl S, et al. Phase 3, multicenter, randomized, double-blind, placebo-controlled trial of fulvestrant with or without PD-0332991 (palbociclib) ± goserelin in women with hormone receptor-positive, HER2-negative metastatic breast cancer (MBC) whose disease progressed after prior endocrine therapy [abstract no. OT3-2-10]. Cancer Res. 2013;73(24 Suppl).
Martin M, Beslija S, Carrasco E, et al. Phase III study of palbociclib in combination with exemestane vs. capecitabine, in hormonal receptor (HR) positive/HER2 negative metastatic breast cancer (MBC) patients with resistance to non-steroidal aromatase inhibitors (NSAI): PEARL study (geicam/2013-02_cecog/bc.1.3.006) [abstract no. 409TiP]. Ann Oncol. 2014;25(Suppl 4):iv134–iv5.
Malorni L, Sanna G, Pestrin M, et al. Phase 2 study of palbociclib (CDK 4/6 inhibitor) for ER positive, HER2- negative post-menopausal advanced breast cancer patients recurring after hormonal therapy (to reverse endocrine resistance - TREnd trial) [abstract no. Abstract OT2-6-01]. Cancer Res. 2013;73(24 Suppl).
Von Minckwitz G, Bear H, Bonnefoi H, et al. PENELOPE: Phase III study evaluating palbociclib (PD-0332991), a cyclin-dependent kinase (CDK) 4/6 inhibitor in patients with hormone-receptor-positive, HER2-normal primary breast cancer with high relapse risk after neoadjuvant chemotherapy (GBG-78/BIG1-13) [abstract no. OT2-6-11]. Cancer Res. 2013;73(24 Suppl 1).
Mayer EL, Gropper AB, Tung NM, et al. Adjuvant palbociclib (P) plus endocrine therapy (ET) for hormone receptor positive (HR+) breast cancer: a phase II feasibility study [abstract no. TPS654]. J Clin Oncol. 2014;32(15 Suppl 1).
Clark AS, O’Dwyer PJ, Heitjan D, et al. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer [abstract no. 527]. J Clin Oncol. 2014;32(5 Supp).
Foundation for the National Institutes of Health. Groundbreaking collaborative clinical trial launched [media release]. www.fnih.org. Accessed16 Jun 2014.
Littman S, Burkart A, Hyslop T, et al. The CD4/6 inhibitor, PD0332991, has activity in metastatic hepatocellular carcinoma: preliminary results of a phase II trial [abstract no. P-0119]. Ann Oncol. 2013;24(Suppl 4):iv69.
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. Dhillon is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Dhillon, S. Palbociclib: First Global Approval. Drugs 75, 543–551 (2015). https://doi.org/10.1007/s40265-015-0379-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0379-9