Abstract
Mipomersen sodium (Kynamro™) (henceforth mipomersen) is a second-generation antisense oligonucleotide inhibitor of apolipoprotein B-100, which is the main structural component of atherogenic lipid particles. Mipomersen is administered via subcutaneous injection and is indicated as adjunctive treatment for homozygous familial hypercholesterolaemia (HoFH). The drug was developed by Isis Pharmaceuticals, which now collaborates with Genzyme Corporation for on-going development and product marketing. Multinational phase III trials of mipomersen as adjunctive therapy were completed in patients with HoFH, severe FH, heterozygous FH (HeFH) with coronary artery disease (CAD), and in those with hypercholesterolaemia at high risk of CAD. Mipomersen 200 mg once weekly has been approved in the USA as an adjunct to lipid-lowering medications and diet in HoFH patients and is undergoing regulatory review in the EU for the same indication. Genzyme is also conducting a multinational phase III, open-label extension study to evaluate long-term treatment in HoFH and HeFH patients, as well as a multinational trial to evaluate a three-times-per-week mipomersen regimen in patients with severe FH. This article summarises the milestones in the development of once-weekly, subcutaneous mipomersen leading to this first approval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Apolipoprotein B-100 (apoB-100) is an amphipathic protein and, in humans, it is produced mainly in the liver, providing the structural core for atherogenic lipoprotein particles, including low-density lipoprotein (LDL), very-low density lipoprotein (VLDL) and lipoprotein(a) [Lp(a)] [1, 2]. In the bloodstream, apoB-100 remains bound to its lipoprotein particle and, therefore, can be used as a marker to estimate the total number of atherogenic particles [3].
Familial hypercholesterolaemia (FH) is an autosomal dominant disease characterized by lifelong elevated serum apoB-100 and LDL-cholesterol (LDL-C), resulting in a markedly increased risk for atherosclerosis and cardiovascular disease (CVD) [4]. In Western Europe, the prevalence of heterozygous FH (HeFH) is approximately 1 in 500, and for homozygous FH (HoFH), it is approximately 1 in 1,000,000 [4]. Current guidelines for the treatment of adults recommend that LDL-C levels be reduced by at least 50 % of baseline [5]. Lifestyle modifications, including a low-fat diet, tend to achieve only modest reductions in LDL-C in patients with FH, and drug therapy is usually required [4]. Statin therapy effectively reduces LDL-C and dramatically improves the prognosis of patients with HeFH [6]. However, many FH patients do not achieve goal LDL-C levels with statin therapy, and in some patients statin therapy may be poorly tolerated or contraindicated [4]. Second-line therapies include ezetimibe, bile acid sequestrants, nicotinic acid and fibrates, either with a statin or as monotherapy [4]. Regular apheresis or plasmapheresis provide another option to decrease LDL-C levels and, in selected cases, liver transplantation may be considered [4].
Mipomersen sodium (Kynamro™) (henceforth mipomersen) is an oligonucleotide antisense inhibitor directed against apoB-100 messenger RNA (mRNA) and complementary to the coding region of the mRNA for apoB-100 [7]. Following the binding of mipomersen to its complementary mRNA, degradation by RNase-H is induced, resulting in inhibition of apoB-100 protein synthesis [7]. Mipomersen was developed by Isis Pharmaceuticals, which now works in collaboration with Genzyme Corporation for on-going development and product marketing.
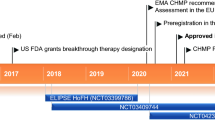
In the USA, the New Drug Application for mipomersen was submitted in March 2012 and, in October 2012, the USA FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted to recommend the approval of mipomersen for the treatment of HoFH [8]. Mipomersen was approved by the FDA in January 2013 for use as an adjunct to lipid-lowering medications and diet to reduce LDL-C, apoB-100, total cholesterol (TC) and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with HoFH (but not HeFH) [9]. The FDA also accepted the reduction of LDL-C as an endpoint for clinical trials. Approval included the requirement for a Risk Evaluation and Mitigation Strategy (REMS) to ensure the benefits of the drug outweigh the risk of hepatotoxicity. The approved mipomersen dosage is 200 mg once weekly via subcutaneous injection [7].
In July 2011, a marketing authorization application (MAA) was submitted to the European Medicines Agency (EMA) for subcutaneous mipomersen 200 mg weekly for the treatment of HoFH in patients already maintaining a stable regimen of maximally tolerated lipid-lowering therapies, and for the treatment of severe HeFH [10]. The latter indication was subsequently voluntarily removed by the company. In December 2012, the Committee for Medicinal Products for Human Use (CHMP) adopted a negative opinion regarding the MAA for the HoFH indication [11]; at Genzyme’s request, the CHMP opinion is being re-examined [12]. Genzyme is also preparing for filings in markets beyond the USA and the EU.
Mipomersen has also been evaluated for the treatment of atherosclerosis. Isis completed preclinical studies, in which the drug reduced apoB-100 and atherosclerotic plaques in animal models of atherosclerosis [13, 14]. However, no recent development has been reported for this indication.
An oral formulation of mipomersen completed preclinical testing, and Isis announced the initiation of a phase I study in March 2005. Preliminary results were announced in February 2006 [15]. However, the development of this formulation has been discontinued.
Features and Properties of Mipomersen Sodium
Alternative names | ISIS 301012; ISIS301012; Kynamro™ |
Class | Antisense-oligonucleotides |
Mechanism of Action | Antisense RNA inhibitor |
Indication | Homozygous familial hypercholesterolaemia |
Route of Administration | Subcutaneous |
Pharmacodynamics | Reduces levels of apolipoprotein B-100, LDL-C, VLDL-C and TC |
Pharmacokinetics (following once-weekly subcutaneous injection) | Cmax reached in 3–4 h |
Plasma t½α 2–5 h | |
Plasma Cmin increases over time and steady state typically reached within 6 months | |
Adverse events | |
Most frequent | Flu-like symptoms, injection-site reactions |
Occasional | Elevated aminotransferase levels |
ATC codes | |
WHO | C10A-X (other lipid modifying agents) |
EphMRA | C10A9 (all other cholesterol/triglyceride regulators) |
Molecular formula | C230 H305 N67 Na19 O122 P19 S19 |
CAS registry number | 629167-92-6 |
1.1 Company Agreements
Isis and Genzyme agreed that Isis would contribute up to $US50 million in additional development funding for mipomersen, bringing Isis’ development funding commitment up to $US125 million. Thereafter, Isis and Genzyme will share development costs equally. The initial Isis development funding commitment and the shared funding will end when the programme is profitable. In exchange for this additional contribution, Isis will be eligible to receive $US75 million in milestone payments [16].
The alliance between Isis and Genzyme began in January 2008 and centred on the development and commercialization of mipomersen. Under the terms of the initial agreement, Genzyme was responsible for development of the compound over a 2-year period. Following clearance of the deal in February 2008 under the Hart–Scott–Rodino Antitrust Improvement Act, Genzyme purchased $US150 million of Isis common stock and made an additional upfront payment of $US175 million. Isis was eligible to earn up to $US825 million in development and regulatory milestone payments and up to $US750 million in commercial milestone payments. Genzyme was to share profits with Isis and would also have preferred access to future drugs developed by the company [17, 18].
Isis entered into an agreement with Symphony Capital Partners in April 2006. Symphony provided $US75 million to fund the development of mipomersen and two other drugs that Isis was developing for its metabolic disease programme. As a result of this transaction, Symphony GenIsis was formed and mipomersen was licensed to the new entity. However, in September 2007, Isis reacquired full ownership of mipomersen [19, 20].
1.2 Patent Information
1.2.1 USA Patents
A USA patent (USA Patent No. 7,407,943) entitled “Antisense Modulation of Apolipoprotein B Expression” was issued by the United States Patent and Trademark Office (USPTO) in August 2008. The patent claims the use of antisense compounds 12–30 nucleobases in length and 100 % complementary to the nucleic acid sequence of human apoB-100 mRNA. This patent will expire in August 2021. The USPTO issued USA Patent No. 7,511,131 in March 2009 that claims, among other embodiments, the composition of matter of mipomersen. This patent expires in the USA in December 2025. Additional patent applications in the mipomersen portfolio are pending.
1.2.2 Worldwide Patents
Patents that claim the composition of matter of mipomersen have been issued in Australia (Australian Patent No. 2003294281), Japan (Japanese Patent No. 4986109) and India (Indian Patent No. 219847); patent applications are also pending in Europe, Australia, Canada and South Africa. These patents will all expire in November 2023. Additional patent applications in the mipomersen portfolio are pending worldwide.
2 Scientific Summary
2.1 Pharmacodynamics
In vitro, mipomersen selectively reduced apoB-100 mRNA, protein and secreted protein in a concentration- and time-dependent manner [7].
Subcutaneous mipomersen (50–400 mg/week) reduced levels of apoB-100, LDL-C and TC in a phase I study in 36 volunteers with elevated cholesterol levels [21]. The onset of action of mipomersen was rapid, with reductions in apoB-100 and lipids evident immediately after the loading regimen [21].
Mipomersen significantly reduced LDL-C, which appeared to occur preferentially via a decrease in small, dense, atherogenic LDL particles in a randomized, double-blind, placebo-controlled, dose escalation, phase I trial in healthy volunteers [22]. After a 200 mg dose, plasma apoB-100 and LDL-C levels were reduced by 50 % and 35 %, respectively, with a similar decrease in LDL particle number, predominantly the small, dense LDL particle fraction (p < 0.05 vs placebo). The duration of response for mipomersen was >30 days [22].
Mipomersen did not prolong the corrected QT interval to a clinically relevant extent at a concentration of 3.8 times the maximum plasma concentration of the maximum recommended subcutaneous dose (200 mg) [7].
2.1.1 Preclinical
Mipomersen significantly decreased plasma Lp(a) and oxidised phospholipids in transgenic mice overexpressing human apoB-100 or both human apoB-100 and human Lp(a), to near undetectable levels by week 5 [23]. Mipomersen also significantly reduced levels of oxidised phospholipids per apoB-100 particle [23].
Mipomersen 50 mg/kg/week for 14 weeks significantly reduced levels of apoB-100 (69 %; p < 0.001) and aortic plaque volume (75 %; p = 0.033) in transgenic mice compared with control treated mice [14]. Mipomersen was administered to mice bred with no LDL receptor and expressing human apoB-100 who developed extensive atherosclerotic plaques. Mipomersen also substantially reduced circulating inflammatory cytokines, including interferon-γ, interleukin (IL)-6, and tumour necrosis factor (TNF)-α [14]. Mipomersen produced linearly dose-dependent reductions in apoB-100, atherogenic lipid levels, triglycerides and atherosclerotic plaques in atherosclerotic mice [13].
2.2 Pharmacokinetics
Mipomersen plasma exposure increases with increasing dose in the range of 30 to 400 mg in healthy volunteers and in patients with FH and non-FH [7]. Peak plasma concentrations of mipomersen are reached within 3–4 h of subcutaneous administration [7]. The estimated plasma bioavailability of subcutaneous mipomersen 50–400 mg relative to intravenous mipomersen ranged from 54 to 78 %.
At clinically relevant concentrations (1–8 μg/mL), mipomersen is highly bound to plasma proteins (≥90 %) [7]. The distribution plasma half-life (t½) of mipomersen is approximately 2–5 h [7]. Steady state is typically reached within 6 months of weekly dosing.
Mipomersen is not a substrate for cytochrome P450 (CYP) metabolism [7], and, in healthy volunteers, mipomersen did not inhibit any of the major CYP enzymes [24]. The drug is primarily excreted in the urine, but, in humans, urinary recovery was <4 % within 24 h of a dose [7]. After subcutaneous administration, the elimination t½ for mipomersen is approximately 1–2 months [7].
2.2.1 Drug Interactions
There were no clinically relevant pharmacokinetic interactions between mipomersen (administered intravenously) and oral doses of simvastatin or ezetimibe in healthy volunteers [24].
2.3 Therapeutic Trials
2.3.1 Phase III Trials
Weekly subcutaneous mipomersen 200 mg add-on therapy to lipid-lowering medication significantly reduced LDL-C levels by 25–37 % from baseline (primary endpoint), as well as reducing other atherogenic lipids and lipoproteins in patients with hypercholesterolaemia in 26-week, randomized, double-blind, placebo-controlled studies in adults [25–27] or adults and adolescents [28].
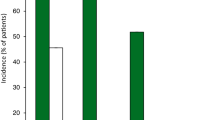
In patients (n = 58) with severe hypercholesterolaemia taking maximally tolerated lipid-lowering medication, mean LDL-C levels were reduced by 35.9 % in the mipomersen group (7.2 mmol/l at baseline) compared with an increase of 12.5 % in the placebo group (6.5 mmol/l at baseline) (p < 0.001) [25]. Similarly, in patients (n = 158) with hypercholesterolaemia and at high cardiovascular risk (56 % had type 2 diabetes mellitus and 40 % were taking maximum statin dosages), mean LDL-C levels were reduced by 37 % in the mipomersen group compared with 5 % in the placebo group (p < 0.001) (reported in an abstract; baseline levels not reported) [26]. Half of all patients receiving mipomersen achieved a mean LDL-C level of <1.8 mmol/l compared with 4 % of placebo recipients [26].
In adults (n = 124) with HeFH and coronary heart disease (CHD), mean LDL-C levels were reduced from baseline by 28 % in the mipomersen group (3.96 mmol/l at baseline) compared with an increase of 5.2 % in the placebo group (3.70 mmol/l at baseline) (p < 0.001) [27]. Similarly, in adults and adolescents (n = 51) with HoFH, mean LDL-C levels were reduced by 24.7 % in the mipomersen group (11.4 mmol/l at baseline) compared with 3.3 % in the placebo group (10.4 mmol/l at baseline) (p = 0.003) [28].
In all of the phase III trials, mipomersen treatment also led to significant reductions compared with placebo in apoB-100, Lp(a), TC and non-HDL-C (p < 0.05–0.0001) [25–28]. Furthermore, when added to maximally tolerated doses of statin therapy, mipomersen was associated with a reduction in the proportion of patients requiring LDL apheresis [25, 29]. For example, at the end of the study by McGowan et al. [25], the proportion of patients who still met LDL-apheresis criteria was 28 % in the mipomersen group versus 67 % in the placebo group.
Mipomersen was associated with sustained reductions in LDL-C in patients (n = 141) with FH uncontrolled on maximally tolerated lipid-lowering medication in a long-term, open-label, single-arm extension trial (reported in an abstract) [30]. Mean LDL-C levels decreased by 26 % at week 26 and remained 26–28 % lower than at baseline at weeks 52, 76 and 104. From week 26 to 104, mean levels of apoB-100 decreased by 28–31 % and Lp(a) by 14–21 % [30].
2.3.2 Phase II Trials
Weekly mipomersen added to lipid-lowering therapy demonstrated a dose-dependent efficacy that appeared to improve as the length of treatment increased in randomized, double-blind, placebo-controlled, dose-escalation studies of adults with hypercholesterolaemia [31, 32]. Eight doses of mipomersen 200 or 300 mg, but not 50 or 100 mg, given over 6 weeks led to significant reductions from baseline in levels of atherogenic lipids in patients (n = 44) with HeFH receiving stable lipid-lowering therapy [32]. Mean LDL-C levels (primary endpoint) were reduced from baseline (4.5 mmol/l) by 21 % (p < 0.05) in the 200-mg dosage group and 34 % (p < 0.01) in the 300-mg dosage group. In the corresponding dosage groups, apoB-100 levels were reduced by 23 % (p < 0.05) and 33 % (p < 0.01), non-HDL-C levels were reduced by 21 and 31 % (p < 0.05 for both), and TC levels were reduced by 16 and 25 % (p < 0.05 for both) [32]. Extended treatment with weekly mipomersen 300 mg for an additional 7 weeks produced significant mean reductions from baseline in LDL-C (37 %; p < 0.001), apoB-100 (37 %; p < 0.01), non-HDL-C (35 %; p < 0.001), TC (29 %; p < 0.01) and Lp(a) (29 %; p < 0.05) [32].
In a similarly designed study in a similar population, patients (n = 74) received seven doses of mipomersen 30–400 mg over 5 weeks, or 15 doses of mipomersen 200 mg over 13 weeks in addition to stable dosages of statin therapy [31]. Mipomersen dosages ≥100 mg per week produced significant reductions from baseline in levels of atherogenic lipids. For example, after 5 weeks’ treatment in the mipomersen 100–400 mg dosage groups, mean LDL-C levels were reduced from baseline (≈3.48 mmol/l) by 21–52 % and apoB-100 levels (1.09 mmol/l at baseline) were reduced by 19–54 %. In the cohort receiving mipomersen 200 mg for 13 weeks, LDL-C levels were reduced by 35.8 % and apoB-100 levels were reduced by 35.7 %, and these reductions were both greater than reductions reported at 5 weeks in the equivalent dosage group (levels of significance not reported) [31].
Additional randomized, double-blind, placebo-controlled studies evaluated mipomersen monotherapy [33–35]. Two dose-escalation studies, one in patients (n = 50) with mild to moderate hyperlipidaemia [33] and one in volunteers (n = 36) with mild dyslipidaemia [34] demonstrated dose-dependent reductions from baseline in atherogenic lipids. Significant reductions in mean LDL-C levels were reported in mipomersen dosage groups ≥200 mg (p ≤ 0.001 vs. placebo), but not lower dosage groups, after 13 [33] or 5 [34] weeks of treatment.
Monotherapy with mipomersen was also evaluated in statin-intolerant hypercholesterolaemia patients (n = 33) at high risk for CVD [35]. Weekly mipomersen 200 mg for 26 weeks significantly reduced mean LDL-C levels from baseline (6.3 mmol/l) by 47.3 % in the mipomersen group compared with 2.0 % in the placebo group (p < 0.001) [35]. Significant reductions in mean apoB-100 (46.4 %), non-HDL-C (45.6 %), TC (36.9 %), Lp(a) (27.1 %) and VLDL-cholesterol (27 %) were also reported (p < 0.01 for all vs. placebo) [35].
Key clinical trials of mipomersen sodium
Indication | Phase | Status | Country | Trial identifier | Company |
|---|---|---|---|---|---|
Severe heterozygous familial hypercholesterolemia | III | Recruiting | Multinational | NCT01475825 | Genzyme |
Severe hypercholesterolaemia | III | Completed | Multinational | NCT00794664 | Genzyme, Isis Pharmaceuticals |
Hypercholesterolaemia and high cardiovascular risk | III | Completed | USA | NCT00770146 | Genzyme, Isis Pharmaceuticals |
Homozygous familial hypercholesterolaemia | III | Completed | Multinational | NCT00607373 | Genzyme, Isis Pharmaceuticals |
Heterozygous familial hypercholesterolaemia and coronary artery disease | III | Completed | USA, Canada | NCY00706849 | Genzyme, Isis Pharmaceuticals |
Familial hypercholesterolaemia or severe hypercholesterolaemia | III | On-going extension | Multinational | NCT00694109 | Genzyme, Isis Pharmaceuticals |
Hypercholesterolaemia; statin intolerant patients | II | Completed | Netherlands | NCT00707746 | Genzyme, Isis Pharmaceuticals |
Hyperlipidaemia | II | Completed | Netherlands | NCT00362180 | Genzyme, Isis Pharmaceuticals |
Heterozygous familial hypercholesterolaemia | II | Completed | USA, Netherlands | NCT00281008 | Genzyme, Isis Pharmaceuticals |
Hypercholesterolaemia | II | Completed | Netherlands, Germany | NCT00216463 | Genzyme, Isis Pharmaceuticals |
Hypercholesterolaemia | II | Completed | Netherlands, USA | NCT00231569 | Genzyme, Isis Pharmaceuticals |
2.4 Adverse Events
Across all phase III studies, the most common adverse events associated with 26 weeks of weekly mipomersen 200 mg were mild to moderate injection-site reactions and flu-like symptoms [25–28]. In the study by McGowan et al. [25] in 58 patients with severe hypercholesterolaemia taking maximally tolerated lipid-lowering medication, 90 % of patients experienced injection-site reactions (including pain 59 %, erythema 56 % and pruritus 33 %), which generally resolved in 2–3 days. Flu-like symptoms, including flu-like illness, pyrexia, chills, myalgia arthralgia and malaise, were reported in 46 % of mipomersen recipients compared with 4 % of placebo recipients [25]. The onset of flu-like symptoms generally occurred early in treatment and did not worsen with repeated administration.
Serious adverse events were reported in a small number of patients in each study [25–28], but were usually not considered to be related to study medication. Two patients in the study by McGowan et al. [25] developed a serious adverse event that was considered to be related or possibly related to mipomersen treatment. One patient developed increased levels of AST and ALT and hepatic steatosis and another patient had a cerebrovascular accident and angina pectoris. In this study, eight patients in the mipomersen group discontinued therapy due to an adverse event [25].
Elevations in liver transaminases were reported in some mipomersen-treated patients in all phase III trials [25–28]. ALT elevations of ≥3 times the upper limit of normal occurred in 12–28 % of patients across the four studies, but there were no reports of concomitant significant bilirubin elevations [25–28]. In the study by Stein et al. [27] in patients with FH, one mipomersen-treated patient developed ALT levels that were ≥10 times the upper limit of normal, and results suggested that increased ALT levels were associated with reductions in apoB-100 levels [27].
In an on-going phase III extension study in 141 patients, median liver fat increased from 1 % at baseline to 5 % and 12 % at weeks 26 and 52, respectively, but decreased to 5 % after 104 weeks of therapy [30]. During extended treatment, ALT elevations of ≥3 times the upper limit of normal occurred in 13 % of patients, and none had significant increases in bilirubin levels [30].
Weekly mipomersen 200 mg for 13 weeks was associated with a trend towards increased intrahepatic triglyceride (IHTG) content in a randomized, double-blind, placebo-controlled phase II trial in 21 patients with FH [36]. At week 15, IHTG content increased 0.8 percentage points in the mipomersen group compared with a decrease of 0.1 percentage points in the placebo group (p = 0.0513). The IHTG content of one patient was above the upper limit of normal [36].
In phase II dose-escalation studies, mipomersen was generally well tolerated across multiple doses, and adverse events were consistent with those reported in phase III trials [31–33].
2.5 On-Going Trials
The long-term safety and efficacy of mipomersen is being evaluated in an open-label extension study in HoFH and HeFH patients on maximally tolerated doses of lipid lowering therapy who completed a mipomersen phase III trial [30].
In December 2011, Genzyme initiated a multinational, placebo-controlled phase III clinical trial to investigate an alternative treatment regimen of mipomersen (70 mg subcutaneously three times weekly) as adjunctive therapy in patients with severe HeFH (FOCUS FH; NCT01475825). Patients will receive mipomersen three times per week every other week for the first 8 weeks, then three times every week for 52 weeks, or mipomersen once every week (control). Approximately 480 patients will be enrolled in the USA, Canada, Europe, Israel, South Africa, South America and the Asia-Pacific region. The trial protocol was approved under a Special Protocol Assessment (SPA) with the FDA and is designed to support broadening the patient population beyond the first indication and support an alternative dosing regimen of three times weekly.
In addition, a phase III trial in Germany (sponsored by the University of Munich) was commenced in May 2012 to assess the efficacy of mipomersen in patients with LDL-C ≥3.37 mmol/l and atherosclerosis, treated with weekly LDL apheresis (NCT01598948).
3 Current Status
Mipomersen received its first global approval on the 29th of January 2013 for the treatment of HoFH in the USA.
References
Gelsinger C, Steinhagen-Thiessen E, Kassner U. Therapeutic potential of mipomersen in the management of familial hypercholesterolaemia. Drugs. 2012;72(11):1445–55.
Ricotta DN, Frishman W. Mipomersen: a safe and effective antisense therapy adjunct to statins in patients with hypercholesterolemia. Cardiol Rev. 2012;20(2):90–5.
Visser ME, Witztum JL, Stroes ESG, et al. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J. 2012;33(12):1451–8.
Parhofer KG. Mipomersen: evidence-based review of its potential in the treatment of homozygous and severe heterozygous familial hypercholesterolemia. Core Evid. 2012;7:29–38.
Robinson JG, Goldberg AC. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl.):S18–29.
Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia. BMJ. 2008;. doi:10.1136/bmj.a2423.
Corporation Genzyme. Kynamro™ (mipomersen sodium): US prescribing information. Cambridge: Genzyme Corporation; 2013.
Genzyme, Isis Pharmaceuticals Inc. FDA advisory committee recommends Kynamro™ for homozygous familial hypercholesterolemia [media release]. Accessed 18 Oct 2012. http://www.isispharm.com.
US FDA. FDA approves new orphan drug Kynamro to treat inherited cholesterol disorder [media release]. Accessed 29 Jan 2013. http://www.fda.govt.
Genzyme, Isis Pharmaceutcials Inc. Genzyme and Isis announce submission of European marketing authorization application for mipomersen (Kynamro™) [media release]. Accessed 28 Jul 2011. http://www.genzyme.com.
European Medicines Agency. Refusal of the marketing authorisation for Kynamro (mipomersen) [media release]. Accessed 13 Dec 2012.
Genzyme, Isis Pharmaceutcials Inc. Genzyme and Isis provide update on CHMP opinion on Kynamro™ (mipomersen) [media release]. Accessed 14 Dec 2012. http://www.genzyme.com.
Isis Pharmaceuticals Inc. Isis reports reduction of apoB-100 levels in a murine model resulting in up to 92% reduction of atherosclerosis [media release]. Accessed 18 Apr 2008. http://www.isispharm.com.
Isis Pharmaceuticals Inc. ISIS 301012 reduces atherosclerotic plaques in animal models [media release]. Accessed 27 Apr 2006. http://www.isispharm.com.
Isis Pharmaceuticals I. Isis Pharmaceuticals’ oral formulation of ISIS 301012 reduces cholesterol in humans [media release]. Accessed 7 Feb 2006. http://www.isispharm.com.
Genzyme Corporation, Isis Pharmaceuticals Inc. Genzyme and Isis complete licensing of mipomersen [media release]. Accessed 24 Jun 2008. http://www.genzyme.com.
Genzyme Corporation, Isis Pharmaceuticals Inc. Genzyme and Isis announce Hart-Scott-Rodino approval of collaboration [media release]. Accessed 1 Feb 2008. http://www.genzyme.com.
Genzyme Corporation, Isis Pharmaceuticals Inc. Genzyme and Isis announce strategic alliance including exclusive worldwide license of mipomersen [media release]. Accessed 7 Jan 2008. http://www.genzyme.com.
Isis Pharmaceuticals Inc. Isis acquires Symphony GenIsis [media release]. Accessed 27 Sep 2007. http://www.isispharm.com.
Isis Pharmaceuticals Inc. Isis Pharmaceuticals and Symphony GenIsis enter into $75 million product development collaboration [media release]. Accessed 7 Apr 2006. http://www.isispharm.com.
Isis Pharmaceuticals Inc. ISIS 301012 produces significant and durable reductions in cholesterol in humans [media release]. Accessed 9 Jun 2005. http://www.isispharm.com.
Bradley J, Crooke R, Graham M. Small, dense, LDL particle concentration is significantly reduced after treatment with an antisense inhibitor of ApoB in healthy volunteers [abstract no. 727]. Circulation. 2005;112(Suppl.):133–4.
Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118(7):743–53.
Yu RZ, Geary RS, Flaim JD, et al. Lack of pharmacokinetic interaction of mipomersen sodium (ISIS 301012), a 2’-O-methoxyethyl modified antisense oligonucleotide targeting apolipoprotein B-100 messenger RNA, with simvastatin and ezetimibe. Clin Pharmacokinet. 2009;48(1):39–50.
McGowan MP, Tardif J-C, Ceska R, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS ONE. 2012;. doi:10.1371/journal.pone.0049006.
Cromwell WC, Thomas GS, Boltje I, et al. Safety and efficacy of mipomersen administered as addon therapy in patients with hypercholesterolemia and high cardiovascular risk+. Annual Scientific Sessions of the National Lipid Association, 31 May–3 Jun 2012; Scottsdale.
Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulations. 2012;126(19):2283–92.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006.
Parhofer KG, Vogt A. Mipomersen, an APOB synthesis inhibitor, evaluation of potential to reduce necessity for lipid-apheresis in patients with heterozygous FH and CAD [abstract]. 79th European Athersclerosis Society Congress, 26–29 Jun 2011. Gothenburg; 2011.
Duell PB, Santos RD, East C, et al. Long-term safety and efficacy of mipomersen in patients with familial hypercholesterolemia uncontrolled by maximally tolerated lipid lowering therapy. Annual Scientific Sessions of the National Lipid Association, 31 May–3 Jun 2012. Scottsdale.
Akdim F, Stroes ESG, Sijbrands EJG, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55(15):1611–8.
Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105(10):1413–9.
Akdim F, Tribble DL, Flaim JD, et al. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011;32(21):2650–9.
Kastelein JJP, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114(16):1729–35.
Visser ME, Wagener G, Baker BF, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2012;33(9):1142–9.
Visser ME, Akdim F, Tribble DL, et al. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51(5):1057–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
This report has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch. © Springer International Publishing Switzerland 2013
Rights and permissions
About this article
Cite this article
Hair, P., Cameron, F. & McKeage, K. Mipomersen Sodium: First Global Approval. Drugs 73, 487–493 (2013). https://doi.org/10.1007/s40265-013-0042-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0042-2