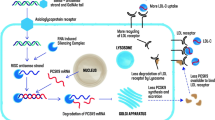
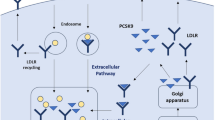
Abstract
Inclisiran (Leqvio®) is a first-in-class, subcutaneously administered, small interfering RNA (siRNA) that prevents hepatic synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9), thereby decreasing circulating low-density lipoprotein cholesterol (LDL-C). In the EU, inclisiran is indicated in adults with primary hypercholesterolemia or mixed dyslipidemia, as an adjunct to diet. It is intended for use in patients unable to reach LDL-C goals on maximally tolerated statin therapy, with or without other lipid-lowering therapies (LLTs). In patients who are statin intolerant or for whom a statin is contraindicated, it can be used with or without other LLTs. In clinical trials, twice-yearly injections of inclisiran (after initial doses at days 1 and 90) approximately halved LDL-C levels in patients with, or at high risk of developing, atherosclerotic cardiovascular disease (ASCVD) who had hypercholesterolemia, irrespective of whether or not their existing treatment included a statin. The safety and tolerability profile of the drug was similar to placebo, although mild to moderate, transient injection-site adverse reactions were more frequent with inclisiran. Pending confirmation of the expected reduction in cardiovascular (CV) events with inclisiran, it is a valuable additional/alternative antihyperlipidemic agent to a statin, as its infrequent maintenance dosing regimen confers a convenience advantage over other non-statin LLTs.
Plain Language Summary
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of death and disability. ‘Statins’ are the drugs of choice for reducing elevated levels of low-density lipoprotein cholesterol (LDL-C) in patients with, or at risk of developing, ASCVD. However, due to multiple factors, including adverse events and/or poor adherence, many patients don’t achieve their guideline target LDL-C level on conventional (statin-based) therapy and novel, non-statin lipid-lowering therapies (LLTs) are needed. Inclisiran (Leqvio®) is a small interfering RNA (siRNA) drug that works as an LLT by stopping the liver from making an enzyme [proprotein convertase subtilisin/kexin type 9 (PCSK9)] that otherwise reduces its ability to remove LDL-C from the blood. Subcutaneously injecting inclisiran every 6 months (after initial doses at days 1 and 90) was generally well tolerated and approximately halved LDL-C levels in patients with, or at high risk of developing, ASCVD who had hypercholesterolemia, regardless of whether or not their conventional therapy included a statin. Inclisiran is a potentially valuable additional/alternative antihyperlipidemic agent to a statin because of its infrequent, and therefore more convenient, dosing schedule versus other non-statin LLTs, including anti-PCSK9 monoclonal antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.21862563. |
First-in-class siRNA directed against PCSK9 mRNA |
Convenient maintenance dosing every 6 months (after initial doses at days 1 and 90) |
Markedly lowers LDL-C levels in patients with, or at high risk of developing, ASCVD who have hypercholesterolemia, irrespective of whether or not their conventional therapy includes a statin |
Generally well tolerated; only adverse reactions at the injection site occur more often than with placebo |
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD) continues to be the leading cause of mortality and a major cause of morbidity worldwide [1, 2]. Elevated low-density lipoprotein cholesterol (LDL-C) levels are one of the most important modifiable risk factors related to the development of ASCVD [3]. Accordingly, lowering LDL-C levels is a key therapeutic strategy in the primary and secondary prevention of major adverse cardiovascular events (MACEs) in patients with ASCVD or at risk of ASCVD, with benefits proportionally related to greater absolute reductions in LDL-C levels, as well as greater baseline risk [1, 2, 4]. As per current guidelines, specific targets for LDL-C are, respectively, < 70 mg/dL and < 55 mg/dL in patients at high and very high risk of future ASCVD events in the EU [2] and, in effect, < 70 mg/dL in patients at very high risk of future ASCVD events in the USA [1].
The highest tolerable dose of a statin, in addition to lifestyle modifications, is the current standard of care for achieving LDL-C goals [1]. However, a substantial proportion (≈ 75–85%) of statin-treated patients at high or very high cardiovascular (CV) risk, such as those with established ASCVD or familial hypercholesterolemia (FH), fail to reach guideline-recommended LDL-C goals [5,6,7,8], and therefore remain at increased risk of MACEs. One of the factors contributing to the suboptimal response to statins appears to be poor adherence to treatment, with ≈ 50% of patients discontinuing their prescribed regimen within the first year, in some cases due to adverse events (AEs), most commonly statin-associated muscle symptoms [9, 10]. As such, there is a need for additional, non-statin lipid-lowering therapies (LLTs) to reduce the residual risk of MACEs in patients, particularly those in high-risk populations, who do not achieve their target LDL-C level on maximally tolerated statin therapy or are unable to tolerate statin therapy.
Non-statin LLTs used as alternatives or adjuncts to statins include those that, like statins, lower plasma LDL-C levels by reducing the synthesis of cholesterol (e.g. bempedoic acid), while others work by decreasing the intestinal absorption of cholesterol (ezetimibe) or by increasing the hepatic clearance of cholesterol (bile acid sequestrants) [1, 2, 11]. Another strategy for lowering plasma LDL-C levels based on enhanced cholesterol clearance involves the targeting of proprotein convertase subtilisin/kexin type 9 (PCSK9) [9, 12,13,14]. Secreted predominantly from the liver, PCSK9 is a serine protease that has a central regulatory role in LDL receptor (LDLR) recycling within hepatocytes, which is the major route for LDL-C clearance from the circulation [15]. Normally, LDL-C binds to LDLRs on the surface of hepatocytes and the resulting ligand-receptor complexes are internalized by endocytosis before the LDL-C is degraded in the lysosome, while the LDLR is recycled back to the cell surface [15]. PCSK9 inhibits this process due to its ability to bind to LDLRs, both intracellularly and extracellularly, and to chaperone them into lysosomes for degradation, thereby preventing their recycling to the cell membrane and, as a consequence, decreasing LDL-C clearance [15]. As such, reducing PCSK9-mediated degradation of LDLRs leads to higher hepatic LDLR expression and, hence, to lower plasma LDL-C levels [12, 14, 15].
Initially, PCSK9 was targeted using monoclonal antibodies (mAbs) that inhibit its interaction with the LDLR and clear it from the circulation via the reticuloendothelial system [12, 13]. Anti-PCSK9 mAbs have been shown to reduce LDL-C levels in patients with ASCVD and FH, as well as decreasing the incidence of CV events in patients with ASCVD [12, 13]. However, they have a short duration of effect and require relatively frequent subcutaneous administration (every 2–4 weeks); accordingly, a risk for low compliance remains [9, 12, 13].
More recently, PCSK9 has been targeted using small interfering or ‘silencing’ RNA (siRNA) to prevent hepatic production of this enzyme [9, 14, 16]. Inclisiran (Leqvio®), a first-in-class, subcutaneously administered siRNA-based therapeutic oligonucleotide, specifically inhibits synthesis of PCSK9 in the liver, leading to increased hepatic uptake of circulating LDL-C and, hence, lowered plasma LDL-C levels [14, 16, 17]. In the EU, inclisiran is approved as an adjunct to diet for the treatment of adults with primary hypercholesterolemia [heterozygous FH (HeFH) or non-familial hypercholesterolemia] or mixed dyslipidemia as follows: (i) in combination with a statin or statin with other LLTs in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin; and (ii) alone or in combination with other LLTs in patients who are statin intolerant, or for whom a statin is contraindicated [18]. Similarly, in the USA, inclisiran is approved as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with HeFH or clinical ASCVD who require additional lowering of LDL-C [19]. In line with these indications, this review focuses on the efficacy and safety of inclisiran in patients with, or at high risk of developing, ASCVD who have elevated LDL-C levels despite receiving the maximum tolerated dose of statin therapy, with or without other LLTs. Relevant pharmacologic properties of the drug are also discussed.
2 Pharmacodynamic Properties of Inclisiran
Inclisiran is a double-stranded, chemically synthesised, long-acting anti-PCSK9 siRNA molecule composed of 21–23 nucleotides that have been modified for durability and low immunogenicity [9, 14, 16,17,18,19,20]. The antisense strand of inclisiran specifically corresponds to human PCSK9 mRNA, while the sense strand is conjugated with triantennary N-acetylgalactosamine carbohydrates (tri-GalNAc); binding of the tri-GalNAc ligand to hepatocyte-specific asialoglycoprotein receptors enables selective uptake of the drug by liver cells. In hepatocytes, inclisiran engages the endogenous RNA interference pathway by binding to the RNA-induced silencing complex, which then directs the catalytic breakdown of PCSK9 mRNA [9, 14, 16,17,18,19,20].
A single 200 mg, 300 mg or 500 mg dose of subcutaneous inclisiran sodium administered on day 1 or two 100 mg, 200 mg or 300 mg doses administered on days 1 and 90 resulted in significant (p < 0.001 vs placebo) and sustained reductions in LDL-C and PCSK9 levels in ORION-1, a randomized, double-blind, placebo-controlled, phase II dose-finding trial in patients with, or at high risk of developing, ASCVD who had elevated LDL-C levels despite receiving the maximum tolerated dose of statins, with or without other LLTs (n = 501) [21]. The ORION-1 study population therefore consisted both of secondary prevention patients (i.e. those with ASCVD) and of high-risk primary prevention patients [i.e. those with type 2 diabetes mellitus, FH or a 10-year risk of a CV event of ≥ 20%, as assessed by the Framingham Risk Score for CV disease; referred to as ‘risk equivalent’ (RE)]. The protocol-stipulated serum LDL-C level was lower in the former than the latter (> 70 mg/dL vs ≥ 100 mg/dL) [21].
The least-square mean (LSM) reduction from baseline in LDL-C levels at day 180 with two-dose inclisiran 300 mg was more marked than that with single-dose inclisiran 300 mg (52.6% vs 38.4%) [21]. The LDL-C reduction with two-dose inclisiran 300 mg was also more pronounced than that with the other remaining regimens evaluated, namely two-dose inclisiran 200 mg (44.9%), two-dose inclisiran 100 mg (35.5%), single-dose inclisiran 500 mg (41.9%) and single-dose inclisiran 200 mg (27.9%). A similar pattern was seen with respect to LSM reductions from baseline in PCSK9 levels at day 180, which were 69.1%, 66.2% and 53.2% with, respectively, two-dose inclisiran 300 mg, 200 mg and 100 mg, and 59.3%, 56.0% and 47.9% with, respectively, single-dose inclisiran 500 mg, 300 mg and 200 mg [21].
In terms of the timecourse of inclisiran action in ORION-1, the reductions in LDL-C and PCSK9 levels on day 14 following single-dose inclisiran 300 mg or the first injection of the two-dose inclisiran 300 mg regimen, for example, were nearly as great in magnitude as those subsequently seen on days 30 and 60 [21]. Thereafter, LDL-C and PCSK9 levels began a slow but steady return towards baseline, although this trend was reversed following the second injection of the two-dose inclisiran 300 mg regimen on day 90, which yielded a treatment effect (as assessed on days 120, 150 and 180) at least comparable to that observed with the first injection [21]. Consistent with the results at day 180, the largest reductions in LDL-C and PCSK9 levels at 1 year in ORION-1 were observed with two-dose inclisiran 300 mg (31.4% and 37.7%, respectively; both p < 0.001 vs baseline) [22].
Longer-term, inclisiran produced sustained reductions in LDL-C and PCSK9 levels over a period of 4 years in ORION-3, a phase II, open-label, extension study in which a total of 290 patients who had previously received single- or two-dose inclisiran regimens in, and had completed, ORION-1 were enrolled and received twice-yearly doses of inclisiran 300 mg [23]. On day 210 of ORION-3, the mean reduction from baseline (day 1 of ORION-1) in LDL-C level was 47.5% among 277 patients who had LDL-C assessments at both time points. The time-averaged reduction in LDL-C over the 4-year period was 44.2%, and the mean reduction from baseline to year 4 (day 1440 of ORION-3) in PCSK9 level was 69.5% [23].
3 Pharmacokinetic Properties of Inclisiran
The pharmacokinetics of a single subcutaneous dose of inclisiran were approximately dose-proportional over the dose range of 24–756 mg, and pharmacokinetic findings for multiple-dose administration were similar to those for single-dose administration [18, 19]. The peak plasma concentration of inclisiran was reached ≈ 4 h after administration of the approved dose (i.e. 300 mg of inclisiran sodium; Sect. 6); concentrations reached undetectable levels 24–48 h post-dosing [18, 19]. No accumulation of inclisiran occurred with multiple dosing [18, 19, 24].
Inclisiran is 87% protein bound in vitro at clinically relevant plasma concentrations and has an apparent volume of distribution of ≈ 500 L [18, 19]. It has been shown to have high uptake into, and selectivity for, the liver, and is mainly metabolized by non-specific nucleases into inactive shorter nucleotides. Inclisiran has a terminal elimination half-life of ≈ 9 h; 16% of the dose is cleared through the kidneys [18, 19].
The pharmacokinetics of inclisiran are not significantly affected by age, gender, bodyweight or race [18]. Similarly, inclisiran dose adjustments are not required in patients with mild [creatinine clearance (CLCR) 60–89 mL/min], moderate (CLCR 30–59 mL/min) or severe (CLCR 15–29 mL/min) kidney impairment [18, 25], or in those with mild (Child–Pugh class A) or moderate (Child–Pugh class B) hepatic impairment [18, 26]. The use of inclisiran in patients with severe (Child–Pugh class C) hepatic impairment has not been studied [18] (Sect. 6).
Inclisiran is not anticipated to be a substrate for, or an inhibitor or inducer of, cytochrome P450 enzymes. Additionally, it is not a substrate for, or an inhibitor or inducer of, common drug transporters. As such, inclisiran is not expected to have clinically significant drug-drug interactions with other medicinal products, including statins [18].
4 Therapeutic Efficacy of Inclisiran
The efficacy of subcutaneously administered inclisiran in patients requiring additional LDL-C reduction in order to reach their treatment goals has been evaluated in three randomized, double-blind, placebo-controlled, multicenter, phase III trials: one in patients with ASCVD (ORION-10 [27]) (Sect. 4.1); one in patients with either ASCVD or ASCVD risk equivalent (RE) [ORION-11 [27]]; and one in patients with HeFH (ORION-9 [28]) (Sect. 4.2). Differences in the target populations notwithstanding, these studies had similar designs to facilitate data pooling [24] (Sect. 4.3). All participants randomly allocated to inclisiran received the approved dose of the drug (i.e. 300 mg of inclisiran sodium, which is equivalent to 284 mg of inclisiran free salt; Sect. 6).
4.1 ASCVD/Risk Equivalent
ORION-10 (n = 1561) was conducted in the USA, while ORION-11 (n = 1617) was conducted in Europe and South Africa [27]. Both studies enrolled adults with a history of ASCVD who had elevated serum LDL-C levels (≥ 70 mg/dL) despite receiving the maximum tolerated dose of statin therapy, with or without other LLTs (e.g. ezetimibe). Participants not receiving a statin had to have documented evidence of intolerance to all doses of at least two different statins. ORION-11 also enrolled adults with a history of ASCVD RE (Sect. 2); these individuals were required to have a higher serum LDL-C level than those with ASCVD (≥ 100 mg/dL). All patients had to have a fasting triglyceride level of < 400 mg/dL and to not be receiving an anti-PCSK9 mAb [27].
Based on the 1-year follow-up results of a phase II dose-finding trial (ORION-1) (Sect. 2) [21, 22], eligible participants were randomized to receive a total of four doses of inclisiran 300 mg or placebo, which were administered on days 1, 90, 270 and 450 [27]. Randomization was stratified according to background statin use (both studies) and geographic region (ORION-11 only). The final follow-up visit was on day 510 (month 17); the end-of-trial visit was on day 540 (month 18). The co-primary efficacy endpoints were the percent change from baseline in LDL-C at day 510 and the time-adjusted percent change in LDL-C from baseline between days 90 and 540 [27].
Patient characteristics at baseline were similar in the two treatment groups in each trial, although there were some differences between the two trials [27]. The mean ages of the study populations in ORION-10 and -11 were 66 years and 65 years; 31% and 28% of patients were women; and 86%/13% and 98%/1% were white/black [18, 27]. In line with the inclusion criteria, all participants in ORION-10 and 87% of those in ORION-11 had ASCVD; the remaining 13% of participants in ORION-11 had ASCVD RE. More than two-thirds (69%) of patients in ORION-10 and three-quarters (78%) of those in ORION-11 were receiving high-intensity statin therapy; 19% and 16% were receiving medium-intensity statin therapy; and 11% and 5% were not receiving a statin (i.e. were statin intolerant) [18, 27]. Ten percent and 7% of patients in ORION-10 and -11 were taking ezetimibe [27].
Twice-yearly administration of inclisiran (after initial doses at days 1 and 90) had a sustained effect and approximately halved LDL-C levels in patients with ASCVD or ASCVD RE who were already receiving a maximally tolerated dose of statin therapy and/or alternative LLT [27]. Concerning the co-primary efficacy endpoints in ORION-10 and -11, this dosing regimen significantly reduced LDL-C levels at day 510 by ≈ 50% and, likewise, significantly lowered time-adjusted LDL-C levels between days 90 and 540 by ≈ 50% compared with placebo (Table 1).
Findings for both co-primary endpoints appeared consistent across a range of subgroups, including those based on age, sex, background statin therapy (yes vs no; high vs not high intensity), metabolic disease (diabetes, metabolic syndrome or neither) and kidney function in both trials, and those based on risk category (ASCVD or ASCVD RE) and geographic region (Europe or South Africa) in ORION-11 only [27]. In ORION-10, for example, the LSM placebo-corrected percent change from baseline in LDL-C at day 510 was − 57.0% in the overall study population (n = 1561) as compared with −57.3% and −54.8% in the subgroups of patients receiving and not receiving statin therapy (n = 1393 and 168) and −58.2% and −54.9% in the subgroups of patients receiving and not receiving high intensity statin therapy (n = 1084 and 477) [observed case data]. In ORION-11, the corresponding results were −52.7% in the overall population (n = 1617) versus −53.3% and −41.6% in patients receiving and not receiving statin therapy (n = 1532 and 85) and −53.4% and −45.4% in patients receiving and not receiving high intensity statin therapy (n = 1463 and 154) [27]. Also in ORION-11, the LSM placebo-corrected percent change from baseline in LDL-C at day 510 was −53.3% in the subgroup of patients with ASCVD (n = 1414) as compared with −47.2% in the subgroups of patients with ASCVD RE (n = 203) [27].
Regarding other lipid parameters, inclisiran therapy significantly lowered levels of total cholesterol, non-HDL-cholesterol (non-HDL-C) and apolipoprotein B (ApoB) at day 510 (Table 1). Changes from baseline in triglyceride (TG) and lipoprotein (a) [Lp(a)] levels are also shown in Table 1. Inclisiran significantly (p < 0.001) reduced PCSK9 levels by a mean of 83.3% and 79.3% versus placebo at day 510 in ORION-10 and -11, respectively [27].
4.2 HeFH
ORION-9, which was conducted in Europe and South Africa, enrolled 482 adults aged ≥ 18 years diagnosed with HeFH who had elevated serum LDL-C levels (≥ 100 mg/dL) despite receiving statin therapy at the maximum tolerated dose, with or without other LLTs (e.g. ezetimibe) [28]. Participants not receiving a statin had to have documented evidence of intolerance to all doses of at least two different statins. All patients were required to have a fasting triglyceride level of < 400 mg/dL and to not be receiving an anti-PCSK9 mAb [28].
As in ORION-10 and -11 (Sect. 4.1), eligible patients in ORION-9 were randomized to receive inclisiran 300 mg or placebo on days 1, 90, 270 and 450 [28]. The final follow-up visit was on day 510; the end-of-trial visit was on day 540. The co-primary efficacy endpoints were the percent change from baseline in LDL-C at day 510 and the time-adjusted percent change in LDL-C from baseline between days 90 and 540 [28].
Baseline characteristics of the patients in the two treatment groups were similar [28]. The mean age of the overall study population was 55 years; 53% of patients were women; and 94%/3% were white/black [18, 28]. Just over one-quarter of the patients (27%) had ASCVD. Almost three-quarters of patients (74%) were receiving high-intensity statin therapy; 15% were receiving medium-low intensity statin therapy; and 10% were statin intolerant (i.e. not receiving a statin) [18, 28]. Just over half of the patients (53%) were taking ezetimibe [28].
Administration of inclisiran every 6 months (after initial doses at days 1 and 90) had a long-lasting effect and nearly halved LDL-C levels in patients with HeFH who were already receiving a maximally accepted dose of statin therapy and/or other LLTs [28]. Regarding the co-primary efficacy endpoints, this dosing regimen significantly reduced LDL-C levels at day 510 by 48% and, similarly, significantly lowered time-adjusted LDL-C levels between days 90 and 540 by 44% compared with placebo (Table 1).
Results for both co-primary endpoints appeared consistent across a range of subgroups, including those based on age, sex, background statin therapy, metabolic disease, kidney function, and risk category (ASCVD or ASCVD RE) [24]. For example, the LSM placebo-corrected percent change from baseline in LDL-C at day 510 was −48.9% in the overall study population (n = 482) as compared with −49.7% and −42.7% in patients receiving and not receiving statin therapy (n = 436 and 52) and −50.7% and −43.4% in patients receiving and not receiving high intensity statin therapy (n = 356 and 126) [observed case data] [24]. Notably, the beneficial effect of inclisiran was also consistent across a range of subgroups based on HeFH genotype [29]. For example, the LSM placebo-corrected percent changes from baseline in LDL-C at day 510 were −41.2% in patients with two variants (i.e. those with double or compound heterozygosity; n = 37), −46.0% in patients with LDLR pathogenic variants (n = 231), −48.3% in patients with LDLR probably pathogenic variants (n = 17) and −42.3% in patients with LDLR variants of uncertain significance (n = 8) [29].
With respect to other lipid parameters, inclisiran significantly lowered levels of total cholesterol, non-HDL-C and ApoB at day 510 (Table 1). Changes from baseline in TG and Lp(a) levels are also shown in Table 1. Inclisiran significantly (p < 0.001) reduced PCSK9 levels by a mean of 78.4% relative to placebo at day 510 in ORION-9 [28].
4.3 Pooled Analyses
4.3.1 Lipid-Lowering Outcomes
The results of the individual studies have been affirmed in a pooled analysis of ORION-9, -10 and -11 (Table 1). In terms of responder rates, 61.5% of inclisiran recipients (n = 1833) as compared with only 2.2% of placebo recipients (n = 1827) achieved a ≥ 50% reduction in LDL-C levels at day 510 [30]. The proportions of inclisiran- and placebo-treated patients reaching specified LDL-C thresholds at day 510 are shown in Fig. 1. Alongside LDL-C, there were also significant (p < 0.001) reductions in non-HDL-C, ApoB, Lp(a) and TG levels (Table 1), as well as a significant (p < 0.0001) increase in HDL-C levels (by a median of 6.2% vs placebo at day 510) [30]. Inclisiran significantly (p < 0.001) reduced PCSK9 levels by a mean of 80.9% relative to placebo at day 510 [30].
Proportions of inclisiran- and placebo-treated patients who reached specific LDL-C thresholds at day 510 in the ORION-9, -10 and -11 studies (pooled analysis [30]). LDL-C low-density lipoprotein cholesterol
4.3.2 Cardiovascular Events
ORION-9, -10 and -11 were not formally designed to assess the impact on CV outcomes of LDL-C-lowering with inclisiran; hence, the effect of the drug on the incidence of MACE was a prespecifed, non-adjudicated, exploratory endpoint in each of the three studies [31]. Individual and pooled analyses of these trials used CV events that were reported as AEs by a study physician, and MACE was defined as a composite of cardiac death, cardiac arrest, non-fatal myocardial infarction (MI), and fatal and non-fatal stroke, using a basket of MedDRA-defined CV terms. Total fatal and non-fatal MI, and stroke, also collected as AEs, were evaluated, albeit these were not prespecified endpoints [31].
The majority (≈ 85%) of the 3660 patients in the intent-to-treat (ITT) population of the pooled analysis had ASCVD (89.5% with a prior or current history of coronary heart disease, 16.4% with cerebrovascular disease, 10.5% with peripheral artery disease and 37.4% with diabetes); ≈ 15% had ASCVD RE (28.2% with diabetes, 68.4% with FH and 20.6% with an estimated 10-year risk of a CV event > 20%). The analysis of MACE was based on the safety population, which comprised five fewer patients than the ITT population [31].
The results of the pooled analysis suggested that lowering LDL-C with inclisiran had potential benefits in terms of reducing MACE collected as AEs [31]. Specifically, inclisiran significantly reduced the incidence of composite MACE by 26% compared with placebo [7.1% vs 9.4%; hazard ratio (HR) 0.75; 95% CI 0.60–0.94]. In contrast to composite MACE, however, inclisiran did not significantly reduce either the incidence of fatal and non-fatal MIs [1.8% vs 2.3%; HR 0.81; 95% CI 0.51–1.29] or that of fatal and non-fatal stroke [0.7% vs 0.8%; HR 0.80; 95% CI 0.39–1.67] [31].
5 Tolerability of Inclisiran
Inclisiran was generally well tolerated in patients with hypercholesterolemia enrolled in the phase III ORION-9, -10 and -11 trials, demonstrating an AE profile that, with the exception of adverse reactions at the injection site, was similar to that of placebo [27, 28, 30]. The safety of inclisiran did not differ between patients aged ≥ 65 years or ≥ 75 years and younger patients who received the drug [18].
The pooled safety population from these three studies comprised 3655 patients (1833 and 1822 receiving inclisiran and placebo). The inclisiran group, which included 1682 patients who were exposed to the drug for 540 days [19], yielded ≈ 2653 patient-years of exposure to this agent [30]. Just over three-quarters of patients treated with inclisiran (78%) and placebo (77%) reported ≥ 1 treatment-emergent AE (TEAE); TEAEs were mostly mild or moderate in severity in both treatment groups [30].
Diabetes (i.e. worsening of glycaemic control as defined in the clinical protocol), the most common TEAE in both inclisiran and placebo recipients, occurred with a similar frequency in the two treatment groups (11.6% vs 11.4%) [risk ratio 1.02; 95% CI 0.85–1.22] [30]. Among the most common TEAEs that occurred more frequently with inclisiran than with placebo (Fig. 2), only those at the injection site were considered to be adverse reactions associated with the drug [30]. In the inclisiran group, the most frequently reported adverse reactions at the injection site were injection-site reactions (3.1%; Fig. 2), injection-site pain (2.2%), injection-site erythema (1.6%) and injection-site rash (0.7%); the total incidence of adverse reactions at the injection site was 8.2% (vs 1.8% in the placebo group) [18, 19]. All adverse reactions at the injection site were of mild or moderate severity and self-limiting [18].
Treatment-emergent adverse events reported in ≥ 3% of inclisiran-treated patients and more frequently than with placebo in the ORION-9, -10 and -11 studies (pooled analysis [30]). Diabetes mellitus describes protocol-defined worsening of glycaemic control. ISR injection site reaction, UTI urinary tract infection
Similar proportions of patients in the inclisiran and placebo groups reported ≥ 1 TEAE related to liver (3.8% vs 4.2%) or kidney (3.3% vs 3.3%) events [24]. Moreover, there was no indication that inclisiran therapy was associated with liver or kidney toxicity, based on the finding that abnormal results of laboratory tests assessing liver and kidney function were comparably infrequent in the two groups [30]. Specifically, 0.5% of inclisiran recipients versus 0.4% of placebo recipients had alanine aminotransferase elevations > 3 × the upper limit of normal (ULN), 0.4% versus 0.5% had aspartate aminotransferase elevations > 3 × ULN, 0.4% versus 0.3% had alkaline phosphatase elevations > 2 × ULN, 0.8% versus 0.8% had bilirubin elevations > 2 × ULN and 2.0% versus 2.3% had creatinine elevations > 2 mg/dL. Likewise, there was no indication that inclisiran therapy was associated with muscle damage, with similar proportions of inclisiran and placebo recipients experiencing creatine kinase elevations > 5 × ULN (1.3% vs 1.2%) [30].
One-fifth of inclisiran recipients (20.4%) compared with just under one-quarter of placebo recipients (23.0%) reported ≥ 1 serious TEAE [30]. TEAEs leading to discontinuation of study medication occurred in 2.5% of inclisiran recipients versus 1.9% of placebo recipients, the most common being adverse reactions at the injection site (0.2% vs 0%) [18, 19, 30]. Deaths occurred in 1.5% of patients in both treatment groups [30].
In line with data from the phase II ORION-1 study [21, 32] (Sect. 2), inclisiran therapy did not adversely affect hematologic parameters (i.e. differential white blood cell and platelet counts) or high-sensitivity C-reactive protein [33] (a validated measure of CV inflammation), as assessed in the pooled analysis of the three phase III trials [34].
With respect to immunogenicity, 33 (1.8%) of the 1830 inclisiran-treated patients in ORION-9, -10 and -11 who were assessed for anti-drug antibodies (ADAs) tested positive prior to dosing; 90 (4.9%) tested positive during 540 days of treatment [18, 19]. Of note, 31 inclisiran-treated patients with a negative sample at baseline had a persistent ADA response (defined as two confirmed positive samples ≥ 16 weeks apart or a single confirmed positive final sample) [19]. There was no evidence that the presence of ADAs impacted the efficacy or safety of inclisiran [18, 19].
Longer-term, the safety profile of twice-yearly inclisiran over a period of 4 years in the phase II ORION-3 extension study (Sect. 2) was consistent with that seen over a period of 1.5 years in the three phase III studies [23]. The most common drug-related TEAEs were general disorders and administration site conditions (in 15.1% of patients; mostly injection-site reactions), which were mostly mild-to-moderate in severity and self-limiting [23].
6 Dosage and Administration of Inclisiran
Both in the EU [18] and USA [19], the recommended dose is 300 mg of inclisiran sodium (corresponding to 284 mg of inclisiran free salt) administered as a single subcutaneous injection on day 1, day 90 and every 6 months thereafter. It is intended for administration by a healthcare professional. Inclisiran should be used with caution in patients with severe kidney impairment due to the limited experience with the drug in this population [18]. Moreover, hemodialysis should not be performed for at least 72 h after administration [18]. Inclisiran should also be used with caution in patients with severe (Child–Pugh class C) hepatic impairment due to the absence of data in this population [18].
Consult local prescribing information for more details regarding the administration and use of inclisiran. In the EU, this not only includes instructions for missed doses, but also for transitioning treatment from anti-PCSK9 mAb therapy [18].
7 Place of Inclisiran in the Management of Hypercholesterolemia
Inclisiran, a first-in-class, subcutaneously administered siRNA that specifically prevents the synthesis of PCSK9 enzyme in the liver (thereby lowering circulating levels of LDL-C), has a long duration of action that permits twice-yearly maintenance dosing after initial doses at days 1 and 90 (Sects. 2, 4 and 6). In recognition of the novel mechanism of action of inclisiran, it may be referred to as a ‘PCSK9 synthesis inhibitor’ to differentiate it from other anti-PCSK9 therapies in the form of anti-PCSK9 mAbs, which are referred to as ‘PCSK9 inhibitors’ [35].
In three phase III studies (ORION-9, -10 and -11), administration of inclisiran every 6 months (after initial doses at days 1 and 90) had a long-lasting effect and approximately halved LDL-C levels relative to placebo over a period of 540 days (18 months) in patients with, or at high risk of developing, ASCVD who had elevated LDL-C levels despite receiving maximally tolerated statin therapy, with or without other LLTs (Sect. 4). Notably, inclisiran demonstrated robust efficacy across a range of subgroups, including patients who were receiving high-intensity statin therapy and those who were not receiving statin therapy (i.e. were statin intolerant) (Sects. 4.1 and 4.3). High-risk primary prevention patients enrolled in ORION-9 and -11 had HeFH and ASCVD RE (including FH), respectively; findings for the ASCVD RE group in ORION-11 have been published in more detail separately [36].
In a pooled analysis of ORION-9, -10 and -11, more than two-thirds of inclisiran recipients achieved an LDL-C level of < 70 mg/dL; just over half achieved a more stringent target of < 50 mg/dL (Sect. 4.3). Inclisiran also significantly reduced levels of other atherolipoproteins, including Lp(a), apoB and non-HDL-C (Sect. 4.3). Currently, it is unclear whether reductions in Lp(a) will contribute further benefits in terms of reducing ASCVD events beyond those resulting from reducing LDL-C levels [37].
Inclisiran was generally well tolerated in ORION-9, -10 and -11 (Sect. 5). It had an AE profile similar to that of placebo, with the only clear exception being a higher frequency of mild to moderate, self-limiting adverse reactions at the injection site (Sect. 5). Moreover, inclisiran was not associated with adverse changes in laboratory markers, including liver/kidney function tests, platelet count and high-sensitivity C-reactive protein levels, and reported ADAs were non-neutralizing in nature (Sect. 5).
Based on the sustained decreases in LDL-C levels, and acceptable safety profile observed in ORION-9, -10 and -11, inclisiran has been approved for use in the EU and USA (Sect. 1). Notably, the 18-month evaluation period for key efficacy and safety outcomes in these pivotal phase III studies has been extended to 4 years in ORION-3. In this open-label extension of ORION-1, inclisiran provided effective and sustained reductions in LDL-C and PCSK9 levels in patients with ASCVD (or ASCVD RE) and elevated LDL-C levels despite maximally tolerated statin therapy (Sect. 2), with no new safety signals observed (Sect. 5). The long-term efficacy and safety of inclisiran in patients is also being evaluated over a period of 3 years in ORION-8 (NCT038141870), an ongoing, phase III, open-label, extension study in 3275 patients with, or at high risk of developing, ASCVD and elevated LDL-C levels who have completed one of the three pivotal trials.
The aim of administering adjunctive and/or alternative LLTs in patients, particularly those at high risk of future ASCVD events, who fail to reach their target LDL-C on maximally tolerated statin therapy or who are statin intolerant, is to reduce the residual risk of MACEs (Sect. 1). In this regard, the pivotal studies were not designed to demonstrate the CV benefit expected to accompany the decrease in LDL-C levels, although there was encouraging evidence from a pooled analysis of the same, with a prespecified, non-adjudicated, exploratory composite endpoint of MACE collected as AEs occurring significantly less frequently on inclisiran than placebo (Sect. 4.3). Currently, the LDL-C lowering effect of inclisiran in reducing CV events is being formally investigated in two randomized, double-blind, multinational, phase III secondary prevention trials: ORION-4 and VICTORION-2 PREVENT (NCT05030428) [37, 38]. In ORION-4, ≈ 15,000 participants with ASCVD will be randomized to inclisiran or placebo and followed for a median time of 5 years. The primary endpoint is time to first occurrence of a 4-point MACE (coronary heart disease, death, MI, fatal- or non-fatal stroke or urgent coronary revascularisation procedure) [37, 38]. Similarly, in VICTORION-2 PREVENT, an estimated 15,000 participants with established CV disease (myocardial infarction, stroke or peripheral arterial disease) and an elevated LDL-C level (≥ 70 mg/dL) despite receiving maximally tolerated statin therapy will be randomized to inclisiran or placebo and followed for up to 6 years. The primary endpoint is time to first occurrence of a 3-point MACE (CV death, non-fatal MI or non-fatal ischemic stroke) or that of an urgent coronary revascularisation procedure [37, 38].
Other ongoing phase III multicenter studies of interest include two randomized, open-label trials [VICTORION INITIATE (NCT04929249) and VICTORION-INCEPTION (NCT04873934)] and one non-randomized, non-interventional cohort study [VICTORION IMPLEMENT (NCT05362903)]. Briefly, VICTORION INITIATE (estimated 444 participants) is assessing the effectiveness of an ‘inclisiran first’ implementation strategy (i.e. adding the drug immediately upon failure to achieve LDL-C < 70 mg/dLwith maximally tolerated statin therapy alone) versus usual care in patients with ASCVD, while VICTORION INCEPTION (estimated 384 participants) is assessing the effectiveness of a hospital post-discharge care pathway involving aggressive LDL-C management that includes inclisiran with usual care versus usual care alone in patients with a recent acute coronary syndrome. Primary endpoints in both studies include the percent change in LDL-C levels. VICTORION IMPLEMENT (estimated 2030 participants) is evaluating treatment with inclisiran and other LLTs in a real-world setting; primary endpoints include the date of first ASCVD diagnosis and the number of patients with CV events.
The approvals of inclisiran in the EU and USA post-date the latest versions of the European Society of Cardiology (ESC)/European Atherosclerosis Society dyslipidemia guidelines [2] and the American College of Cardiology/American Heart Association cholesterol guidelines [1]. Although not specifically mentioning the drug, these guidelines do nonetheless contain advice on the use of anti-PCSK9 therapies in the form of anti-PCSK9 mAbs, which is in line with the licensed indications for inclisiran. For example, according to the EU dyslipidemia guidelines, the addition of a PCSK9 inhibitor is recommended for patients with clinical ASCVD or HeFH at very high risk of future ASCVD events who are not achieving their LDL-C goal on a maximum tolerated dose of a statin and ezetimibe [2]. Similarly, according to the US cholesterol guidelines, the addition of a PCSK9 inhibitor is reasonable in patients with clinical ASCVD who are at very high risk of future ASCVD events but whose LDL-C level remains ≥ 70 mg/dL on maximally tolerated statin and ezetimibe therapy [1]. By contrast, the latest version of the ESC guidelines on CV disease prevention in clinical practice [35] was issued after the approval(s) of inclisiran and does include mention of the drug. As with the EU dyslipidemia [2] and US cholesterol [1] guidelines, although relevant recommendations in the EU prevention guidelines [35] only refer to uses of anti-PCSK9 therapies in the form of PCSK9 inhibitors, these uses are consistent with the licensed indications for inclisiran [35].
In the absence of ‘head-to-head’ comparisons with other LLTs, indirect comparisons (network meta-analyses) suggest that the LDL-C lowering efficacy of inclisiran in patients with, or at risk of, ASCVD (including those with HeFH) who have elevated LDL-C levels despite receiving maximally tolerated statin therapy is comparable to that of anti-PCSK9 mAbs (alirocumab and evolocumab), but superior to that of ezetimibe and bempedoic acid [39, 40]. Similarly, in a meta-analysis of HeFH trials, LDL-C reductions with inclisiran did not differ significantly from those with alirocumab and evolocumab across genetic variants [41]. In contrast to anti-PCSK9 mAbs, definitive data demonstrating a reduction in CV morbidity and mortality are awaited for inclisiran. This notwithstanding, the infrequent dosing regimen is considered to be a convenience advantage for the drug over anti-PCSK9 mAbs, which require self-injection every 2–4 weeks (Sect. 1), as well as statins, which require daily oral administration [9, 37, 38, 42]. As such, the reduced injection burden of inclisiran administered as an adjunct or alternative to statin therapy is anticipated to have a favourable impact on long-term adherence and compliance. Complete adherence/compliance may be feasible if it is administered by a healthcare professional, as intended (Sect. 6) [43], and this in turn may result in improved CV outcomes. Although this remains an unproven assumption, interestingly, the effectiveness of, and adherence for, inclisiran plus standard-of-care LLTs (including bempedoic acid, ezetimibe and anti-PCSK9 mAbs) versus standard-of-care LLTs alone in routine clinical practice is being evaluated in an observational, matched prospective study [VICTORION REAL (NCT05399992); estimated 2100 participants].
Thus far, concerns over the high acquisition costs of anti-PCSK9 therapies relative to less effective, but also less expensive LLTs, such as ezetimibe and/or bempedoic acid, has been a major limitation to their widespread adoption [44, 45]. In this regard, the maximum price at which inclisiran added to standard of care is cost-effective versus standard of care alone in patients with ASCVD in the USA is US$5,910, US$9,346, and US$12,781 at willingness-to-pay thresholds of $50,000, $100,000 and $150,000 per quality-adjusted life year, respectively, according to the most recent determination [46]. In the UK, the National Institute for Health and Clinical Excellence has recommended the use of inclisiran in line with its approved indication, despite considerable uncertainties in current cost-effectiveness estimates due to the lack of head-to-head studies with other LLTs and definitive data showing a reduction in CV events [47]. However, the price at which inclisiran is provided to NHS England is not publically known, as it is subject to a confidential commercial arrangement with the manufacturer [47].
In conclusion, inclisiran is a valuable additional/alternative antihyperlipidemic agent, pending demonstration of the expected reduction in CV events in ongoing studies in patients with ASCVD (including those already on maximally tolerated statin therapy), It combines a sustained reduction in LDL-C levels with an infrequent dosing regimen that confers a convenience advantage over existing LLTs and may, therefore, result in improved adherence and compliance with treatment.
Data Selection Inclisiran: 255 records identified
Duplicates removed | 69 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 11 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 128 |
Cited efficacy/tolerability articles | 11 |
Cited articles not efficacy/tolerability | 36 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Inclisiran, Leqvio, ALN-60212, ALN-PCSsc, hypercholesterolemia, dyslipidemia. Records were limited to those in English language. Searches last updated 24 Jan 2023 | |
References
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–143.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardio-vascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111–88.
Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56.
Catapano AL, Tokgözoğlu L, Mello A, et al. Pharmaceutical strategies for reducing LDL-C and risk of cardiovascular disease. Atherosclerosis. 2019;39(Suppl):100002.
Fox KM, Tai MH, Kostev K, et al. Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate intensity statins. Clin Res Cardiol. 2018;107(5):380–8.
Marz W, Dippel FW, Theobald K, et al. Utilization of lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients at high and very-high cardiovascular risk: real-world evidence from Germany. Atherosclerosis. 2018;268:99–107.
Cannon CP, Khan I, Klimchak AC, et al. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:959–66.
Bruckert E, Parhofer KG, Gonzalez-Juanatey JR, et al. Proportion of high-risk/very high-risk patients in Europe with low-density lipoprotein cholesterol at target according to European guidelines: a systematic review. Adv Ther. 2020;37:1724–36.
Hardy J, Niman S, Pereira E, et al. A critical review of the efficacy and safety of inclisiran. Am J Cardiovasc Drugs. 2021;21(16):629–42.
Halava H, Huupponen R, Pentti J, et al. Predictors of first-year statin medication discontinuation: a cohort study. J Clin Lipidol. 2016;10(4):987–95.
Bardolia C, Amin NS, Turgeon J. Emerging non-statin treatment options for lowering low-density lipoprotein cholesterol. Front Cardiovasc Med. 2021;8:789931.
Coppinger C, Movahed MR, Azemawah V, et al. A comprehensive review of PCSK9 inhibitors. J Cardiovasc Pharmacol Ther. 2022;27:1–14.
Catapano AL, Pirillo A, Norata GD. New pharmacological approaches to target PCSK9. Curr Atheroscler Rep. 2020;22:24.
German CA, Shapiro MD. Small interfering RNA therapeutic inclisiran: a new approach to targeting PCSK9. BioDrugs. 2020;34(1):1–9.
Macchi C, Ferri N, Sirtori CR, et al. Proprotein convertase subtilisin/kexin type 9: a view beyond the canonical cholesterol-lowering impact. Am J Pathol. 2021;191(8):1385–97.
Dyrbus KM, Gasior MM, Penson PM, et al. Inclisiran: new hope in the management of lipid disorders? J Clin Lipidol. 2020;14:16–27.
Lamb YN. Inclisiran: first approval. Drugs. 2021;81(3):389–95.
Novartis Europharm Limited. Leqvio 284 mg solution for injection in pre-filled syringe: EU prescribing information. 2021. https://www.ema.europa.eu. Accessed 24 Jan 2023.
Novartis Pharmaceuticals Corporation. LEQVIO (inclisiran) injection, for subcutaneous use: US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214012lbl.pdf. Accessed 24 Jan 2023.
Macchi C, Sirtori CR, Corsini A, et al. A new dawn for managing dyslipidemias: the era of RNA-based therapies. Pharmacol Res. 2019;150:104413.
Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40.
Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 2019;4(11):1067–75.
Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023. https://doi.org/10.1016/S2213-8587(22)00353-9.
European Medicines Agency. Leqvio (inclisiran) assessment report. 2020. https://www.ema.europa.eu. Accessed 24 Jan 2023.
Wright RS, Collins MG, Stoekenbroek RM, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin Proc. 2020;95(1):77–89.
Kallend D, Stoekenbroek R, He Y, et al. Pharmacokinetics and pharmacodynamics of inclisiran, a small interfering RNA therapy, in patients with hepatic impairment. J Clin Lipidol. 2022;16(2):208–19.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran reduces LDL-cholesterol independent of genotype in subjects with heterozygous familial hypercholesterolaemia [abstract no. 018 / #1707]. Atherosclerosis. 2020;315:e7–8.
Wright RS, Ray KK, Raal FJ, et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. 2021;77(9):1182–93.
Ray KK, Raal FJ, Kallend DG, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2022;44(2):129–38.
Landmesser U, Haghikia A, Leiter LA, et al. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: a pre-specified analysis from ORION-1. Cardiovasc Res. 2021;117(1):284–91.
Ridker PM, Koenig W, Kastelein JJ, et al. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur Heart J. 2018;39:4109–11.
Landmesser U, Conde LG, Wright RS, et al. Effect of inclisiran on haematological and immunological biomarkers: a pooled analysis of ORION-9, -10 and -11 trial data [abstract no. S025 / #1062]. Atherosclerosis. 2021;331:e37.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–37.
Ray KK, Kallend D, Leiter LA, et al. Effect of inclisiran on lipids in primary prevention: the ORION-11 trial. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac615.
Brandts J, Ray KK. Clinical implications and outcomes of the ORION phase III trials. Future Cardiol. 2021;17(5):769–77.
Scicchitano P, Milo M, Mallamaci R, et al. Inclisiran in lipid management: a literature overview and future perspectives. Biomed Pharmacother. 2021;143:1–15.
Campbell C, Desai NR, Electricwala B, et al. The comparative efficacy of inclisiran, PCSK9 inhibiting monoclonal antibodies, and ezetimibe for the treatment of high cholesterol in adults with or at risk of ASCVD: a systematic literature review and network meta-analysis. J Am Coll Cardiol. 2022;79(9 Suppl. A):1551.
Burnett H, Fahrbach K, Cichewicz A, et al. Comparative efficacy of non-statin lipid-lowering therapies in patients with hypercholesterolemia at increased cardiovascular risk: a network meta-analysis. Curr Med Res Opin. 2022;38(5):777–84.
Brandts J, Dharmayat KI, Vallejo-Vaz AJ, et al. A meta-analysis of medications directed against PCSK9 in familial hypercholesterolemia. Atherosclerosis. 2021;325:46–56.
Merćep I, Friščić N, Strikić D, et al. Advantages and disadvantages of inclisiran: a small interfering ribonucleic acid molecule targeting PCSK9—a narrative review. Cardiovasc Ther. 2022;2022:8129513.
Cupido AJ, Kastelein JJP. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions from the ORION trials. Cardiovasc Res. 2020;116(11):e136–9.
Azari S, Rezapour A, Omidi N, et al. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: a systematic review. Heart Fail Rev. 2020;25:1077–88.
Lin G, Kazi D, Jih J, et al. Inclisiran and bempedoic acid for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value; evidence report: Institute for Clinical and Economic Review. Mar 2, 2021. http://icer.org/wp-content/uploads/2020/10/ICER_High-Cholesterol_Final-Evidence-Report_030221.pdf. Accessed 24 Jan 2023.
Campbell C, Desai NR, Electricwala B, et al. Cost-effectiveness of inclisiran in atherosclerotic cardiovascular patients with elevated low-density lipoprotein cholesterol despite statin use: a threshold analysis [abstract]. J Am Coll Cardiol. 2022;79(9 Suppl. A):1559.
National Institute for Health and Clinical Care Excellence. Inclisiran for treating primary hypercholesterolaemia or mixed dyslipidaemia. Technology appraisal guidance [TA733]. 2021. https://www.nice.org.uk/. Accessed 24 Jan 2023.
Acknowledgements
During the peer review process, the manufacturer of inclisiran was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: M. Rusica, Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Milan, Italy; P. Sabouret, Heart Institute, Cardiology Department, Pitié-Salpétrière, Sorbonne University and Collège National des Cardiologues Français Paris, France; P. Scicchitano, Cardiology Department, Hospital “F. Perinei”, Altamura, BA, Italy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Inclisiran: A Review in Hypercholesterolemia. Am J Cardiovasc Drugs 23, 219–230 (2023). https://doi.org/10.1007/s40256-023-00568-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00568-7