Abstract
Background
Tolvaptan (TLV) is known to increase electrolyte-free water clearance. However, TLV actions on renal electrolytes including urine sodium (uNa) excretion and its consequences are less well understood. This subanalysis investigated the effect of add-on TLV compared to increased furosemide (FUR) on both electrolyte-free water and electrolyte clearance in patients with congestive heart failure (CHF) complicated by advanced chronic kidney disease (CKD).
Methods
The Kanagawa Aquaresis Investigators Trial of TLV on HF Patients with Renal Impairment (K-STAR) was a multicenter, open-labeled, randomized, and controlled prospective clinical study. Eighty-one Japanese patients with CHF and residual signs of congestion despite oral FUR treatment (≥ 40 mg/day) were recruited and randomly assigned to a 7-day add-on treatment with either ≤ 40 mg/day FUR or ≤ 15 mg/day TLV. Electrolyte-free water clearance, electrolyte osmolar clearance and electrolyte excretion were compared between the two groups before and after therapy.
Results
The change (Δ) in electrolyte-free water clearance was significantly higher in the add-on TLV group than in the add-on FUR group. However, Δelectrolyte osmolar clearance was also higher in the add-on TLV group than in the increased FUR group. This was primarily because ΔuNa excretion was significantly higher in the add-on TLV group than in the increased FUR group, since Δurine potassium excretion was significantly lower in the add-on TLV group than in the increased FUR group.
Conclusions
Add-on TLV may increase both renal water and Na excretion in CHF patients with advanced CKD to a greater degree than increased FUR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loop diuretics, such as furosemide (FUR), have long been used to treat fluid overload in patients with congestive heart failure (CHF). However, they have numerous adverse effects, including low blood pressure (BP), electrolyte disorders (e.g., hyponatremia), worsening renal function (WRF), and worsening nitrogen metabolism [1,2,3]. Vasopressin (AVP) V2 receptor (V2R) antagonists, such as tolvaptan (TLV), can be administered adjunctively with loop diuretics. TLV binds to AVP receptors in the collecting duct, thereby blocking water reabsorption and promoting electrolyte-free water excretion (aquaresis) [4]. It has been reported that add-on TLV did not affect urine solute excretion in a rat [4] and a CHF dog model [5]; however, its effects have not been fully investigated in patients with CHF and advanced chronic kidney disease (CKD). Therefore, the urine solute profiles of TLV as add-on therapy to FUR in patients with CHF and advanced CKD were determined in a subanalysis of the K-STAR study.
Methods
The Kanagawa Aquaresis Investigators Trial of TLV on Heart Failure (HF) Patients with Renal Impairment (K-STAR) was a multicenter, open-labeled, randomized, and controlled prospective clinical study that evaluated the efficacy and safety of add-on TLV therapy for HF. The main analysis [6] analyzed data from 81 Japanese patients with CHF complicated by advanced CKD [estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2]. Dialysis patients were not included. These patients had residual signs of congestion (e.g., pedal edema, pulmonary congestion, or distention of the jugular vein) despite oral FUR treatment (≥ 40 mg/day). Each was randomly assigned to 7 days of treatment with either ≤ 15 mg/day of add-on TLV or ≤ 40 mg/day of increased FUR.
Patients
Patients were excluded if they met any of the following criteria: use of assisted circulation devices; hypersensitivity to TLV or a similar drug; anuria; lack of thirst; inability to ingest water or oral medication; increase in serum sodium concentration (s[Na+]) by ≥ 12 mEq/L/24 h, or above the upper limits provided by the guidelines of medical institute; pregnancy or suspicion of pregnancy; use of TLV at inclusion; acute coronary syndrome and/or an elective percutaneous coronary intervention therapy during the study period; malignancy; or otherwise deemed unfit to participate in the study by a physician. Written informed consent was obtained from all patients before enrollment in the study. During the study, the administration of human atrial natriuretic peptides, phosphodiesterase III inhibitors, catecholamines, colforsin, and changing doses of all diuretics except FUR or TLV were prohibited. Other therapeutics were not restricted. Fifty patients completed the K-STAR study. However, there were data missing for 16 patients, resulting in 17 patients in each group in this subanalysis.
Procedures
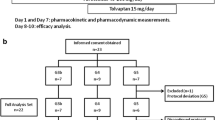
The study design of K-STAR is shown in Fig. 1. A screening test was performed first for each participant in the study. Before the administration of either increased FUR or add-on TLV, all participants received a constant dose of FUR during a three-day run-in period (days − 2–0). Subsequently, all participants received either ≤ 15 mg/day add-on TLV or ≤ 40 mg/day increased FUR orally for the next 7 days (days 1–7). The patients were hospitalized for at least 4 days before treatment and 2 days after treatment (days 8 and 9). Water intake was not limited for the patients who were assigned for the add-on TLV group during the study because fluid restriction during the therapy with TLV may increase the likelihood of overly rapid correction of s[Na+] and should generally be avoided.
The study was discontinued for any of the following reasons: (1) early termination, owing to complete or partial improvement of HF; (2) the stop criteria, (a) the patient no longer participated or withdrew consent, (b) adverse events that prevent continued treatment, (c) s[Na+] was either higher than 12 mEq/L within 24 h or above the upper limits permitted by medical institute after administration, or (d) recommendation by a physician; (3) the event criteria, (a) the need to use higher than regulated doses, (b) the need to add other drugs for HF, or (c) the need to use non-pharmacological therapies (e.g., positive pressure respiration therapeutic device).
Assessments
For this subanalysis, the patients were assessed for serum and urine chemistry. BP, heart rate, daily urine volume (UV), and water intake were also measured (days 1–9). Measurements taken before first dosing of treatment on day 1 were used as baseline values. Measurements of serum and urine chemistry, BP and heart rate taken before the first dosing on day 1, and that of daily UV and water intake taken between day 0 and the first dosing on day 1 were used as baseline values.
Serum levels of sodium (s[Na+]), potassium (s[K+]), chloride (s[Cl−]), urea nitrogen (sUN), and creatinine (sCr) were measured on day 1, 3, and 8. In addition, on day 1 and 8, urine sodium (uNa), urine potassium (uK), urine urea nitrogen (uUN), urine creatinine (uCr), urinary osmolality (uOsm), and serum osmolality (sOsm) were measured. The eGFR was calculated using a slightly modified equation for the Japanese population with renal disease: eGFR (mL/min/1.73 m2) = 194 × sCr− 1.094 × age− 0.287 (if female, multiply by 0.739) [7]. The electrolyte osmolar clearance (E-COsm) was calculated using the following equation: E-COsm (mL/min) = (uNa + uK) × UV/s[Na+]. The electrolyte-free water clearance (E-CH2O) was calculated using the following equation: E-CH2O (mL/min) = UV–E-COsm [8]. Pre-treatment values (day 1) were used as baseline values.
Statistical analyses
Changes in the serum chemistry values and the urine sample data obtained from the baseline values for patients in the same group were tested for statistical significance using the Wilcoxon signed rank test, whereas statistically significant changes between the two groups were determined using the Mann–Whitney test. For discrete variables, statistical significance between the two groups was determined using Fisher’s exact test. Statistical analyses were performed using Stata version 14 (StataCorp). Statistical significance was defined as P < 0.05.
Results
Between October 2012 and August 2014, 81 adult patients (49–93 years old) with CHF and advanced CKD with an eGFR less than 45 mL/min/1.73 m2) were recruited for the study and randomly assigned into two groups, namely, the increased FUR group (FUR group, n = 41) and the add-on TLV group (TLV group, n = 40). During the study period, 31 patients dropped out. In the FUR group, patients dropped out for the following reasons: symptom improvement (n = 4), WRF (n = 3), hypernatremia (n = 2), higher dose requirement (n = 2), and addition of other drug(s) for HF treatment (n = 1). Similarly, in the TLV group, patients dropped out for the following reasons: symptom improvement (n = 10), WRF (n = 1), hypernatremia (n = 3), impaired consciousness (n = 1), withdrawn consent (n = 2), and the need for higher doses (n = 2). The remaining 50 patients (29 in the FUR group and 21 in the TLV group) completed the study (Fig. 2). However, there were data missing for 16 patients (12 in the FUR group and 4 in the TLV group) for this subanalysis. The baseline characteristics of the remaining 34 patients (17 in the FUR group and 17 in the TLV group) in this subanalysis are summarized in Table 1.
FUR doses at baseline were not significantly different between the two groups. At the end of day 7, the mean increased FUR dose was 31.8 ± 2.5 mg/day in the FUR group and the mean add-on TLV dose was 9.3 ± 0.8 mg/day in the TLV group. Therefore, the mean total FUR dose was 87.1 ± 7.4 mg/day in the FUR group and 50.0 ± 5.6 mg/day in the TLV group (P < 0.001, Table 2).
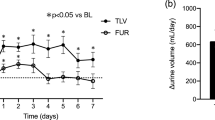
The change (Δ) in UV (between day 0 and 1 (before treatment), and between day 7 and day 8) was significantly higher in the TLV group than the FUR group [mean (SE): 450.6 (171.7) mL/day vs. − 121.3 (90.4) mL/min (P = 0.002)] (Fig. 3); however, Δwater intake (between day 0 and day 1 (before treatment), and between day 7 and 8) was not significantly different between the TLV group and the FUR group [mean (SE): 120.6 (103.7) mL/day vs. 110.6 (45.5) mL/day (P = 0.637)] (Fig. 3). ΔuOsm (from day 1 to 8) was greater in the TLV group than the FUR group [mean (SE): − 72.5 (15.8) mOsm/kg vs. -33.6 (14.6) mOsm/kg (P = 0.095)] (Fig. 4). ΔE-CH2O (from day 1 to 8) was significantly higher in the TLV group than the FUR group [mean (SE): 0.25 (0.07) mL/min vs. − 0.01 (0.04) mL/min (P = 0.001)] (Fig. 5). In addition, ΔE-COsm (from day 1 to 8) was higher in the TLV group than the FUR group [mean (SE): 0.06 (0.06) mL/min vs. − 0.08 (0.07) mL/min (P = 0.185)] (Fig. 5). Similarly, ΔuNa excretion (from day 1 to 8) was significantly higher in the TLV group than in the FUR group [mean (SE): 13.8 (10.9) mEq/day vs. − 16.2 (12.0) mEq/day, P = 0.040] and ΔuK excretion (from day 1 to 8) was significantly lower in the TLV group than in the FUR group [mean (SE): − 6.7 (2.4) mEq/day vs. 0.8 (1.2) mEq/day, P = 0.014] (Fig. 6). However, ΔuUN excretion was not significantly different between the TLV group and the FUR group [mean (SE): 58.8 (73.1) mg/day vs. − 86.0 (34.5) mg/day, P = 0.134].
The mean plasma renin activity (PRA) [mean (SE): 4.9 (1.3) ng/mL/h vs. 6.2 (1.4) ng/mL/h, P = 0.033] on day 8 was significantly higher than that on day 1 only in the FUR group.
Discussion
AVP-mediated activation of V2R in the collecting ducts stimulates the cAMP-dependent trafficking of aquaporin 2 (AQP2) water channels to the luminal membranes of the principal cells. It allows the diffusion of water along a solute concentration gradient. In contrast to loop diuretics, TLV promotes excretion of electrolyte-free water. It inhibits the trafficking of AQP2 to the luminal membranes of principal cells by blocking the action of AVP on V2R in the renal collecting duct, which leads to inhibition of water reabsorption [4]. Therefore, TLV seems to have no effect on urine solute excretion. As expected, in this study, not only ΔE-CH2O but also ΔUV were significantly greater in the add-on TLV group than in the increased FUR group, despite an insignificant difference in Δwater intake between the groups; however, ΔE-COsm was also greater in the add-on TLV group than in the increased FUR group (Figs. 3, 5). This was primarily because ΔuNa excretion was significantly higher in the add-on TLV group than in the increased FUR group (13.8 ± 10.9 vs. −16.2 ± 12.0 mEq/day, P = 0.040), since ΔuK excretion was significantly lower in the add-on TLV group than in the increased FUR group (− 6.7 ± 2.4 vs. 0.8 ± 1.2 mEq/day, P = 0.014) (Fig. 6). Ecelbarger et al. reported that AVP upregulated epithelial Na channels (ENaC) in the rat kidney [9]. In addition, a study by Bankir et al. [10] indicated the potent V2-dependent antinatriuretic effect of AVP in humans. Whether desmopressin (dDAVP), an AVP agonist, reduced uNa excretion was evaluated in healthy individuals and patients with central diabetes insipidus (CDI) or nephrogenic diabetes insipidus (NDI) owing to mutations of either the aquaporin 2 (AQP2) gene or V2R. dDAVP was found to reduce uNa excretion in healthy individuals and CDI and AQP2-mutated NDI patients, but not in V2R-mutated NDI patients [10]. Stockand also reported that AVP modulated discretionary Na reabsorption across principal cells mediated by ENaC via V2R [11]. There are some other possible reasons for the rise in uNa excretion induced by TLV. First is an influence of endogenous AVP mediated by V1a receptor (V1aR) [12]. By blocking V2R-mediated effects, TLV induces a secondary increase in endogenous AVP secretion, and thus likely more intense effects on V1aR. It has been well demonstrated that V1aR mediate a natriuretic response [13, 14]. This influence is even more marked in patients with chronic renal failure [15]. Second, Mori et al. speculated from the results of Cowley [16] that TLV stimulated natriuresis owing to increased renal blood flow (RBF) [17]. They also suggested that TLV improved FUR delivery due to increased RBF, which reduced FUR resistance. Moreover, congestion in the kidney has recently been focused on in the aspect of diuretic resistance. In 2016, Mori et al. reported that long-term TLV use decreased intracellular fluid and extracellular fluid in patients who underwent peritoneal dialysis with bioimpedance monitoring [18]. The authors also reported that TLV can improve left ventricular diastolic dysfunction. This was demonstrated by the ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity (E/e′) of cardiac ultrasound, especially in patients with E/e′ greater than 15. Mori et al. have shown a suggestive speculation regarding renal congestion [19]. Renal interstitial fluid flows from the medulla to the cortex [20], and water reabsorbed in the collecting ducts also flows from the medulla to the cortex. If renal venous pressure increases due to a congestive disorder, such as heart failure, renal interstitial pressure also increases [19]. This may result in Na retention via a reduction of renal medullary blood flow. TLV can improve renal congestion by blocking water reabsorption, and may increase renal Na excretion. Third, Shoaf et al. showed that TLV did not increase PRA; however, FUR increased PRA in healthy white men [21]. In this subanalysis, PRA was significantly increased only in the increased FUR group, although ΔBP over the study period (data not shown) and the percentage of patients who received renin–angiotensin–aldosterone system (RAS) inhibitors (Table 1) were not significantly different between the two groups. The significant increase in PRA could have caused the decrease in uNa excretion in the FUR group (Fig. 5). Generally, FUR increases urine solute excretion mainly by increasing uNa excretion; therefore, based on the results of this subanalysis, the FUR group might become more resistant to FUR.
Because TLV does not decrease RBF or GFR in patients with CHF [22], co-administration of TLV and FUR in CHF patients promoted diuresis at reduced FUR doses. In addition to this, compromised drug absorption in the intestine is observed in patients with CHF due to congestion [23]. Therefore, in this subanalysis, it was also possible for add-on TLV treatment to improve the bioavailability of FUR by reducing edema of the gastrointestinal tract. Thereby, resistance to FUR could also be reduced. However, in this subanalysis, Δbody weight, brain natriuretic peptide, left ventricular ejection fraction, early-to-late diastolic transmitral flow velocity of cardiac ultrasound, and cardiothoracic ratio of chest X-ray were not significantly different between the groups. It should be noted that ΔIn-Out balance between day 1 and 8 was significantly larger in the TLV group than in the FUR group (P = 0.015) (Supplementary Table 2). Based on the results of this subanalysis, some factors other than decongestion might contribute to the increase in UV in the TLV group. In fact, ΔuK excretion was significantly lower in the add-on TLV group than in the increased FUR group (Fig. 6). Field et al. reported that AVP stimulated uK secretion independent of urine flow rate, which suggested that AVP increased Na+/K+ exchange to prevent K retention during antidiuresis [24]. Blanchard et al. [25] investigated changes in urine in response to dDAVP administered alone (control period) or after 7-day pretreatment with amiloride (amiloride period) in healthy human subjects on a high Na/low K diet to suppress aldosterone secretion. In the control period, the uNa/K ratio decreased and the transtubular potassium gradient (TTKG) increased, suggesting that uK secretion was stimulated by dDAVP in the distal nephron, on which aldosterone acts. However, after 7-day pretreatment with amiloride, the uNa/K ratio significantly increased and TTKG significantly decreased, suggesting that amiloride prevented the effects of dDAVP on TTKG [25]. Udelson et al. investigated the efficacy of TLV monotherapy compared to FUR monotherapy and the combination of TLV and FUR in patients complicated by HF and systolic dysfunction with normal kidney function [26]. Compared with baseline uNa excretion values obtained after diuretic withdrawal, there were no significant differences in ΔuNa excretion from baseline during TLV therapy without a loop diuretic. The result of a significant reduction in aldosterone levels during TLV therapy [26] suggested that diuresis during TLV therapy may not occur simply by aquaresis, but rather may be partly due to no upregulatory effect of TLV on RAS. Therefore, according to the findings of this subanalysis, TLV probably increases uNa excretion and decreases uK excretion, and thus directly overcomes FUR resistance.
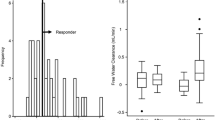
In addition to stimulating AQP2 expression in both the inner and outer medulla, AVP also stimulated the expression of urea transporters (UT-A1 and UT-A3) in the inner medulla [27, 28]. Urea transporters rapidly reabsorb urea, a process that is regulated by AVP. It is important to maintain a high urea concentration and to set up the osmotic gradient in the inner medullary interstitium [29]. Therefore, TLV should theoretically increase uUN excretion. Recently, in fact, a screening study was performed in rodents to identify UT-A1-selective inhibitors with suitable pharmacological properties [30]. Owing to their unique mechanism of action on the renal countercurrent mechanisms, UT-A1 inhibitors can increase urine volume by solute diuresis [31]. Mori et al. [17] conducted a study in patients undergoing peritoneal dialysis who were administered FUR. The study indicated that the responders to TLV (increased UV > 400 mL/day) had an increased uUN excretion, but the non-responders had a decreased uUN excretion. However, assessment of the actual uUN excretion was difficult due to a shift of UN between sUN and UN in the peritoneal dialysate. In this subanalysis, ΔuUN excretion was greater in the add-on TLV group than in the increased FUR group, but the difference was not significant.
UV is directly related to the total number of osmoles excreted in a day. Solutes responsible for driving water excretion could be electrolytes, such as Na and K, or urea [32]; therefore if uOsm decreases because of TLV, UV necessarily increases. Potential natriuresis may be less effective than aquaresis because the uOsm was decreased in the TLV group (Fig. 4). It may be difficult to identify whether TLV stimulated uNa excretion, based only on uOsm, not only because the aquaretic effect was stronger than that of osmotic diuresis, but also because the decrease in the osmotic gradient between the collecting duct and interstitium can make reabsorption of water in the inner medulla difficult. The augmented aquaretic effect and potential natriuretic effect of TLV may increase UV.
To the best of our knowledge, this is the first report of the urine solute excretion profiles of TLV as add-on therapy to FUR in patients with CHF complicated by advanced CKD (not including dialysis patients); however, it had several potential limitations. First, 31 patients did not complete the study; therefore, the remaining 50 patients might be the patients who had mild-to-moderate effects. In those patients, 16 patients had missing data, making the number of patients in each group of this subanalysis relatively small. Specifically, the FUR group had a large proportion of missing data. Mean sCr was significantly lower in the missing data subgroup (n = 12) than in the subgroup analysis (n = 17) (P = 0.040) (Supplementary Table 1). Therefore, the lower sCr in the missing data subgroup may have contributed to a more diuretic effect, especially natriuretic effect which was proved by higher E-COsm consisted of higher uNa excretion in the FUR group if the subgroup was included in the analysis because furosemide generally has a less natriuretic effect in patients with more advanced CKD. Second, the urine solutes were not measured every day, meaning detailed changes were not known. Third, data for dietary factors which could have affected the uNa and uK excretion were not evaluated due to the nature of the acute treatment phase of CHF. Fourth, the plasma aldosterone concentration was not determined, which may have limited the ability of this study to determine the influence of the increased FUR and add-on TLV.
Conclusion
Administration of TLV as an add-on therapy to FUR increased renal water and Na excretion more than increased FUR in patients with CHF complicated by advanced CKD.
References
Greenberg A. Diuretic complications. Am J Med Sci. 2000;319:10–24.
Wilcox CS. Metabolic and adverse effects of diuretics. Semin Nephrol. 1999;19:557–68.
Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf. 2010;9:259–73.
Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–7.
Onogawa T, Sakamoto Y, Nakamura S, Nakayama S, Fujiki H, Yamamura Y. Effects of tolvaptan on systemic and renal hemodynamic function in dogs with congestive heart failure. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S67–76.
Inomata T, Ikeda Y, Kida K, et al. Effects of additive tolvaptan versus increased furosemide on heart failure with diuretic resistance and renal impairment: results from the K-STAR study. Circ J 2017; 82:159–167.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Shimizu K, Kurosawa T, Sanjo T, Hoshino M, Nonaka T. Solute-free versus electrolyte-free water clearance in the analysis of osmoregulation. Nephron. 2002;91:51–7.
Ecelbarger CA, Kim GH, Masilamani S, et al. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46–53.
Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol. 2005;16:1920–8.
Stockand JD. Vasopressin regulation of renal sodium excretion. Kidney Int. 2010;78(9):849–56.
Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–91.
Musabayane CT, Forsling ML, Balment RJ. Arginine vasopressin increases renal sodium excretion in the anesthetized rat through V1 receptors. Ren Fail. 1997;19:23–32.
Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19:1721–31.
Nonoguchi H, Takayama M, Owada A, et al. Role of urinary arginine vasopressin in the sodium excretion in patients with chronic renal failure. Am J Med Sci. 1996;312:195–201.
Cowley AW Jr. Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273(1 Pt 2):R1-15.
Mori T, Oba I, Koizumi K, et al. Beneficial role of tolvaptan in the control of body fluids without reductions in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial. 2013;29:33–7.
Mori T, Kurasawa N, Ohsaki Y, et al. Role of chronic use of tolvaptan in patients with heart failure undergoing peritoneal dialysis. Adv Perit Dial. 2016;32:39–45.
Mori T, Ohsaki Y, Oba-Yabana I, Ito S. Diuretic usage for protection against end-organ damage in liver cirrhosis and heart failure. Hepatol Res. 2017;47:11–22.
Cowley AW, Roman RJ, Fenoy FJ, Mattson DL. Effect of renal medullary circulation on arterial pressure. J Hypertens Suppl. 1992;10:S187–S193.
Shoaf SE, Bramer SL, Bricmont P, et al. Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a non-peptide AVP antagonist, and furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol. 2007;50:213–22.
Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273-278.
Inomata T, Izumi T, Matsuzaki M, Hori M, Hirayama A. Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S57–65.
Field MJ, Stanton BA, Giebisch GH. Influence of ADH on renal potassium handling: a micropuncture and microperfusion study. Kidney Int. 1984;25:502–11.
Blanchard A, Frank M, Wuerzner G, et al. Antinatriuretic effect of vasopressin in humans is amiloride sensitive, thus ENaC dependent. Clin J Am Soc Nephrol. 2011;6:753–9.
Udelson JE, Bilsker M, Hauptman PJ, et al. A multicenter, randomized, double-blind, placebo-controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail. 2011;17:973–81.
Bankir LT, Trinh-Trang-Tan MM. Renal urea transporters. Direct and indirect regulation by vasopressin. Exp Physiol 2000; 85 Spec No:243S–252S.
Cai Q, Nelson SK, McReynolds MR, Diamond-Stanic MK, Elliott D, Brooks HL. Vasopressin increases expression of UT-A1, UT-A3, and ER chaperone GRP78 in the renal medulla of mice with a urinary concentrating defect. Am J Physiol Renal Physiol. 2010;299:F712–F719.
Fenton RA, Chou CL, Stewart GS, et al. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA. 2004;101:7469–74.
Esteva-Font C, Cil O, Phuan PW, et al. Diuresis and reduced urinary osmolality in rats produced by small-molecule UT-A-selective urea transport inhibitors. FASEB J. 2014;28:3878–90.
Esteva-Font C, Anderson MO, Verkman AS. Urea transporter proteins as targets for small-molecule diuretics. Nat Rev Nephrol. 2015;11:113–23.
Levin A, Klassen J, Halperin ML. Challenging consults: application of principles of physiology and biochemistry to the bedside. Osmotic diuresis: the importance of counting the number of osmoles excreted. Clin Invest Med. 1995;18:401–5.
Acknowledgements
Portions of this work were presented at the Annual Meeting of the American Society of Nephrology in Chicago, Illinois, in November 2016. We are thankful to Ms. Kazuko Nakamura for managing this study as a data and financial manager. We also greatly benefited from the expertise of Editage (http://www.editage.jp) with regard to English language editing.
Funding
The K-STAR study was supported by The Kidney Foundation, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Naoto Tominaga, Takayuki Inomata, Naoki Sato, and Yugo Shibagaki received lecture fees from Otsuka Pharmaceutical Co., Ltd. Yugo Shibagaki received research grants from Otsuka Pharmaceutical Co., Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 2301) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Registration of clinical trials
The original trial of this subanalysis [The Kanagawa Aquaresis Investigators Trial of TLV on Heart Failure (HF) Patients with Renal Impairment (K-STAR)] was registered with the University Hospital Medical Information Network (UMIN ID 000009201).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tominaga, N., Kida, K., Inomata, T. et al. Comparison of the effects of tolvaptan and furosemide on renal water and sodium excretion in patients with heart failure and advanced chronic kidney disease: a subanalysis of the K-STAR study. Clin Exp Nephrol 22, 1395–1403 (2018). https://doi.org/10.1007/s10157-018-1603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1603-1