Abstract
The present study was aimed at detecting natural cases of canine monocytic ehrlichiosis (CME) by molecular and cytological methods and assessment of clinico-patho-biochemical parameters among the affected population. Nested polymerase chain reaction was found to be highly sensitive (47.7 %) in detecting the acute cases, followed by buffy coat (29.5 %) and blood smear examination (22.7 %). CME incidence rate was found to be 8.4 %. Hemogram revealed significant (P < 0.01) depletion in the levels of hemoglobin, hematocrit, total erythrocyte count, monocyte and platelet count in diseased dogs as compared to healthy controls. Plasma biochemistry revealed hypoproteinemia, hypoalbuminemia and hyperglobulinemia along with significantly (P < 0.05) higher blood urea nitrogen and alanine aminotransferase values. CME revealed non-significant increase in the levels of erythrocytic lipid peroxides, reduced glutathione and activity of superoxide dismutase, whereas activities of glutathione-S-transferase and glutathione reductase showed significant (P < 0.05) increase and decrease, respectively as compared to healthy controls. Plasma levels of cortisol, insulin and blood level of copper showed non-significant (P > 0.05) reduction, whereas blood level of zinc showed significant (P < 0.05) reduction as compared to healthy controls. These findings revealed that changes in erythrocytic oxidant–antioxidant profile and blood mineral concentration in canine monocytic ehrlichiosis are largely non-significant and inconsistent. Hence, further study is required to elucidate their role in pathogenesis of canine monocytic ehrlichiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tick-borne diseases are highly prevalent in tropical countries like India causing loss of animal performance and mortality. Canine ehrlichioses is an example of such a tick-borne disease. Among all the ehrlichial species, Ehrlichia canis is the most well-studied, pathogenic and worldwide prevalent in dogs which causes canine monocytic ehrlichiosis (CME). These small pleomorphic coccoid bacteria reside and replicate within monocytes, macrophages and granulocytes resulting in severe leukopenia and thrombocytopenia [1]. It is a potentially fatal disease and induces a life-long carrier state in dogs. Thus, it warrants a rapid and accurate diagnosis for initiating appropriate therapy and a favorable prognosis [2].
Currently, most of the epidemiological data available on E. canis are from the southern part of India and there is complete dearth of information with respect to epidemiology of CME in northern part of the country. Moreover, knowledge on vector-borne diseases of companion animals in India remains incomplete [3]. Recently, molecular techniques like the polymerase chain reaction (PCR) and more specifically nested PCR and real time PCR have become the most promising diagnostic tools of choice because of their higher sensitivity and specificity in detecting target pathogens [4].
Reactive oxygen species (ROS) are free radicals generated from regular metabolism in cells as a consequence of electron transfer reactions that can be scavenged by cellular defense systems and antioxidants [5]. The dynamic balance between the generation and elimination of ROS may be broken under certain pathological conditions leading to oxidative stress. These excess free radicals interact with endogenous bio-molecules affecting various cellular components and destruction of the defense system leading to cell death [6]. As ROS are involved in the etiology of many diseases, determination of oxidant/antioxidant balance, trace elements in peripheral blood of dogs and their correlation in cases of CME, therefore, would facilitate better understanding of the disease pathogenesis.
Taking all the above facts into consideration, the present study was aimed at investigating clinico-incidental aspects of the disease, its comparative diagnosis by nested PCR/buffy coat (BC)/blood smear (BS) examination, effect of the disease on hemato-biochemistry, and assessment of erythrocytic oxidant/antioxidant status and blood mineral among the affected population.
Material and Methods
Animals/Study Area
The study was carried out from January 2010 to May 2011 at Referral Veterinary Polyclinic, Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India. Dogs with the history of tick infestation, erratic fever, epistaxis and refractoriness to conventional treatment were screened in the present study. Those dogs which were found positive for E. canis infection in blood/BC smear examination and/or nested PCR were included in this study. Further, biometric data for the animals including age, sex, breed as well as seasonal occurrence of canine ehrlichiosis were recorded and incidence rate was estimated in the referred canine population. For comparison, 10 healthy control dogs brought for vaccination to the clinic were selected.
Twenty dogs, irrespective of age, sex and breed, naturally infected with acute form of CME were selected as diseased group (T1) for estimation of erythrocytic oxidant/antioxidant, blood mineral and plasma hormone. Ten apparently healthy, PCR- negative dogs presented for routine health check -up and vaccination served as healthy control (T2).
Blood Sampling, Plasma Separation
Four milliliters of blood per infected animal was collected aseptically by venipuncture in sterile vial with K3 EDTA as an anticoagulant, out of which 1 mL was used for separation of BC for DNA isolation and kept at –20 °C for further use. Rest 3 mL of blood was used for separation of plasma (plasma biochemistry), for hematological study, erythrocyte preparation for assessment of oxidant/antioxidant status and estimation of blood mineral.
Diagnosis
Diagnosis of CME was based on anamnesis, clinical signs, blood smear cytology, laboratory tests and nested PCR.
Cytological Examination
Both PB (smear made from ear tip prick) and BC smears were stained with Giemsa stain (Stock Giemsa stain solution is diluted with distilled water @1:9 to make working solution) and examined for ehrlichial inclusions under ×100 oil immersion lens.
DNA Extraction, Quantification and Purification
Genomic DNA was isolated from BC (200–300 µL) of the suspected dogs by phenol:chloroform:isoamyl alcohol extraction method [7] and stored at −20 °C. Concentrations of total nucleic acid isolated were then quantified by measuring optical density (OD) value at 260 nm and the purity was checked by taking the ratio of OD values at 260 and 280 nm using a Nanodrop (Nanoview, General Electronics, USA). The samples having OD ratio 1.7–1.9 were used for the experiment.
PCR Amplification
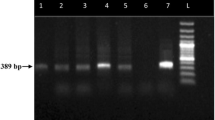
In order to detect and identify the etiologic agent of CME in suspected dogs, nested PCR technique was followed with initial amplification using modified universal eubacterial primers (EC 9 and EC 12) and reamplification with species specific primer specific for E. canis. Universal primer pair EC9 (5′ AAGGATCCTACCTTGTTACGACTT 3′) and EC12 (5′ AATCTAGAGTTTGATCMTGG 3′) was used to amplify nearly the entire 16S rRNA gene of eubacteria [8] which yielded a 1500 bp product. In order to sequence the 1500 bp of the corresponding fragment, an internal primer pair ECA [(Forward primer): 5′ CAA TTA TTT ATA GCC TCT GGC TAT AGG AA 3′)] and HE3 [(Reverse primer): 5′ TAT AGG TAC CGT CAT TAT CTT CCC TAT 3′] [9] was used to amplify a 389 bp portion of the E. canis 16 s rRNA gene.
Amplification by PCR was preformed with a 25 µL reaction mixture containing 0.5–1 µg of template DNA, 1 µL each of forward (10 pmol/µL) and reverse primer (10 pmol/µL), 12.5 µL of 2× PCR master mix (0.05U/µL Taq DNA polymerase, 4 mM MgCl2, 0.4 mM dNTP) (Fermentas Life Sciences) and nuclease free water to make final volume of 25 µL. First step of the PCR was performed under following conditions: initial denaturation 94 °C for 5 min, denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min, extension at 72 °C for 1 min, thirty-five cycles followed by final extension at 72 °C for 10 min.
In the nested reaction, 1 µL of PCR product was used along with1 µL each of primer ECA (10 pmol/µL) and HE3 (10 pmol/µL), 12.5 µL of 2× PCR master mix (Fermentas) and nuclease free water to make final volume of 25 µL. For the nested PCR, the conditions followed were; initial denaturation: 95 °C for 2 min., denaturation: 94 °C for 40 s., annealing: 58 °C for 1 min., extension: 72 °C for 40 s., repeated for 40 cycles and final extension at 72 °C for 10 min. Each time the reaction was run with positive control (50 pg of E. canis DNA) in addition to negative control without DNA template.
All PCR products were electrophoresed through ethidium bromide stained 2 % agarose gel and visualized under UV fluorescence.
Hemato-Biochemical Study
Hematological parameters were estimated as per the standard methods [10]. Plasma biochemistry was estimated using Cogent Diagnostic Kits (Span Diagnostic Limited, Surat, India).
Preparation of Hemolysate and RBC Suspension for Assessment of Oxidant/Antioxidant Status
The anticoagulated whole blood from affected animals was centrifuged at 3000 rpm for 10 min with the removal of plasma and buffy coat followed by washing of red blood cells thrice with ice-cold isotonic sodium chloride solution (NSS). The RBC pellet was diluted with ice-cold distilled water in 1:10 ratio for the preparation of 10 % stock hemolysate for the estimation of superoxide dismutase (SOD), lipid peroxidation (LPO), Glutathione-S-transferase (GST) and glutathione reductase (GR) and rest of the RBC pellet was diluted with ice-cold NSS in 1:1 ratio to get RBC suspension for estimation of reduced glutathione (GSH). Hemoglobin concentration of hemolysate was estimated by cyanomethemoglobin method [11].
Assay of Oxidant–Antioxidant Parameters
The concentration of malonaldehyde (MDA; nmol of MDA/mg of Hb) [12], SOD activity [13], GST [14] and GR [15] were measured in the hemolysate, whereas, GSH (µmol/mL packed RBC) was measured in the RBC suspension [16].
Estimation of Blood Minerals
One milliliter of the blood was digested with triple acid extract comprised of nitric acid, sulphuric acid and perchloric acid in micro-kjeldahl flask and micro-minerals (Cu and Zn) were estimated by atomic absorption spectrophotometer (Electronic Corporation of India Limited, Model 4141) in air-acetylene flame.
Hormonal Estimation
Plasma levels of cortisol (nmol/L) and insulin (μIU/mL) were determined by radio-immuno assay kit (Beckman Coulter, USA) using competitive binding immunoenzymatic assay procedure using a gamma counter (STRATEC Biomedical AG, Germany; Model, SR 300).
Statistical Analysis
The values obtained were expressed as mean ± SE and analyzed using appropriate statistical procedures [17]. The level of statistical significance for all the comparisons was established at P ≤ 0.05 or P ≤ 0.01.
Results and Discussion
Clinico-Incidental Study
A total of 833 dogs suspected for CME were screened, out of which 70 were found positive in PB/BC examination with an incidence rate of 8.40 %. The incidence rate of CME varies from region to region. In contrast to the present findings (8.40 %), incidence rates of 18.90 and 6 % were observed in Nagpur [18] and Chennai [19], respectively. Month-wise incidence (Fig. 1) revealed highest incidence rate in the month of April (24.28 %) and no incidence in the months of November and December. Season-wise incidence revealed highest incidence in summer (April–June) followed by that of winter and monsoon season. Out of 12 different breeds (Table 1), German Shepherd (25.71 %) suffered the most followed by Labrador (24.28 %). There was no gender predilection. The overall sex ratio (male:female) was 1.12:1.0. Age of affection ranged from 3 months to 11 years and higher incidence was observed in the age group of 12–36 months. In an effort to study the ecology of the disease in dogs, data with respect to age, sex, breed and season was collected. The lack of incidence in November and December might be due to reduced vector activity whereas high incidence in summer [20] might be due to increased activity of the vector tick [2]. The higher susceptibility of German Shepherd to this disease was well established [21, 22], which might be explained on the basis of the breed’s poor cellular immune response against E. canis [21]. The variation in the occurrence of ehrlichiosis in different breeds of dog might be due to variation in the population size of different breeds which was in agreement with previous reports [18, 19]. Similar high incidence in the younger age group (up to 36 months) was also reported in earlier studies [23].
Out of 70 confirmed positive cases, 52 (74.28 %) were pure cases of CME, rest 18 were of mixed infections with different organisms, e.g. Babesia gibsoni (7.14 %), Hepatozoon canis (5.71 %), Anaplasma phagocytophilum (5.71 %), Trypanosoma evansi (2.85 %), B. canis (2.85 %) and Leptospira icterohaemorrhagiae (1.42 %). In majority of cases, the E. canis organisms were observed as intracytoplasmic varying shaped morulae (Fig. 2). Concurrent infections of E. canis with other tick-borne diseases were reported from time to time. Concomitant infections of E. canis with H. canis [24], Babesia spp. [25], and Trypanosomosa spp. [26] were reported from different parts of the country.
Clinical signs (Table 2) varied from case to case with most consistent findings of anorexia/inappetance, followed by dehydration, presence of ticks on body, pyrexia and hemorrhage. Hemorrhages were manifested in the form of epistaxis, melena and cutaneous petechiae. The wide variation in clinical picture may be due to many factors like age, breed, immune competence of dogs, clinical phase of the disease, variation in virulence between different strains and coinfection with other ehrlichial species or diseases [2]. Pyrexia, the most common indicator of acute phase of the disease, was observed in 81.42 % which was in agreement with Chaudhury [20]. Pale mucous membranes (37.14 %) were associated with chronic phase of the disease during the development of pancytopenia [27]. Conditions like hepatomegaly and splenomegaly [2] as well as lymphadenomegaly [22] were detected mostly in acute conditions. Bleeding tendencies may be attributed to a combination of mild thrombocytopenia and vasculitis [28]. Out of the 70 positive cases, six dogs succumbed to death (four pure CME, one with additional infection of B. gibsoni and other with T. evansi), 50 % of which were German Shepherd echoing the more severe form with poor prognosis in this breed [2].
Detection of Organism and Comparison Between Molecular and Cytological Examinations
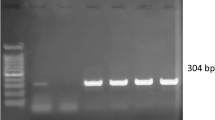
A total of 44 dogs were evaluated for CME by nested PCR. The amplification of the 16S rRNA fragment with specific primers for E. canis produced a specific, visible, single band pattern of approximately 389 bp (Fig. 3). Out of 44 samples which were subjected to comparative study, the nested PCR revealed (21/44; 47.72 %) better sensitivity as compared to BC (13/44; 29.54 %) and PB examinations (10/44; 22.72 %) in detecting E. canis organism which signified and underscored the former’s usefulness. The sensitivity of nested PCR was found to be better than the cytological methods in detecting acute form of CME which was in agreement with an earlier report [29]. Wen et al. [9] found nested PCR as one of the most preferred methods as it can detect as little as 0.2 pg of purified E. canis DNA which was 100 times more sensitive than one-step PCR.
Agarose gel electrophoresis result showing positive amplification of Nested-PCR products from dogs. Lane M 100 bp ladder; Lane P Known positive control of E. canis; Lane N Known negative control of E. canis; Lane 1 and 5 DNA of Tick and blood from the same dog; Lane 2–4 and 6 Positive samples of E. canis
Hemato-Biochemistry
Hematological indices showed mild to marked anemia, leukopenia to leukocytosis, lymphopenia to marked lymphocytosis and mild to moderate thrombocytopenia in acute stage of the disease (Table 3). Levels of neutrophil, lymphocyte, eosinophil and total leucocyte count (TLC) were marginally altered in comparison to healthy controls. Plasma biochemical profile (Table 4) revealed significantly (P < 0.01) decreased values of total protein and albumin, whereas significantly (P < 0.05) increased values were observed in the level of BUN and activity of ALT as compared to healthy control. However, alterations in the levels of ALP and creatinine were non-significant as compared to healthy control (Table 4). Significant variations in the leukocytic indices might be due to different stage of the diseased condition at the time of presentation [2, 30]. Anemic changes and thrombocytopenia were significant when compared with healthy control echoing the earlier finding [31]. Thrombocytopenia (94 %) was found to be most consistent and acted as prognostic marker of the disease [22], which has been attributed to an immunopathological mechanism with overproduction of antiplatelet IgG antibodies induced by E. canis [32].
Similar hypoproteinemia and hypoalbunemia conditions were reported earlier [2, 30] and similar increase in BUN and ALT levels was also found [2, 23]. The increase in BUN was prerenal in nature and occurred mostly in the acute condition [27]. The hypoalbuminemia may be attributed to anorexia and associated decrease in protein intake [22], hepatic insufficiency, nephropathy with protein loss, gastrointestinal loss and vasculitis [33].
Oxidant/Antioxidant Status in Affected Animals
The mean values of the oxidant/antioxidant parameters for dogs are illustrated in Table 5. Except GST and GR, rest of oxidative stress parameters like LPO, GSH and SOD showed non-significant (P > 0.05) difference between affected and healthy dogs. In diseased dogs, activity of GST was significantly (P < 0.05) increased, whereas activity of GR was significantly (P < 0.01) reduced in comparison to that of healthy dogs. Free radicals generated from regular biochemical and physiological reactions in the cell are capable of oxidative damage [34], but are protected by a broad variety of endogenous antioxidant enzyme systems like glutathione peroxidase, GR, GST, GSH and SOD [5]. Free radicals also initiate and promote lipid peroxidation causing cellular injury by inactivation of membrane enzymes and receptors, depolymerizaton of polysaccharide, as well as protein cross-linking and fragmentation [35]. MDA, a breakdown product, which is frequently quantified as a measure of lipid hydroperoxides is a good predictor of oxidative damage. The finding of non-significant increase in the level of MDA in diseased dogs, as observed in the present study, was in corroboration with the earlier in vitro [36] and in vivo [37] studies. This indicates that a minimal role is played by these free radicals in CME disease process. Killing of intracellular bacteria like E. canis by monocytes involves both oxygen-dependent and oxygen-independent mechanisms. However, it was hypothesized that oxidative stress plays only a minor role in killing of intracellular ehrlichiae and hence ROS production is not enhanced significantly [38]. GSH, a first line endogenous antioxidant defense can effectively neutralize free radicals either directly or indirectly through enzymatic reactions and protects cells from oxidative damage [39]. SOD accelerates the dismutation of superoxide radicals (O2 −) to H2O2 [40]. Non-significant increase in the levels of SOD and GSH in the diseased dogs might be due to their decreased utilization in the absence of oxidative stress. However, the significant increase in the activity of GST and decrease in GR activity indicated involvement of oxidative stress to a certain extent. But, as the majority of oxidant–antioxidant status (LPO, GSH and SOD) was not affected, the role of oxidative stress in the pathogenesis of acute form of CME might be minimal.
Plasma Hormonal and Blood Mineral Profile of Affected Animals
Plasma minerals and hormones of diseased as well as healthy dogs are presented in Table 6. Blood levels of copper and zinc in diseased dogs exhibited non-significant (P > 0.05) and significant (P < 0.05) decrease, respectively as compared to healthy controls. Both copper and zinc help in various vital functions of the body as a co-factor of many enzymes including Cu–Zn superoxide dismutase and metabolic activities. Non-significant reduction in blood copper level substantiated the present finding of minimal role played by free radicals in this disease pathogenesis as Cu is an integral part of various enzymes like SOD. Zinc is involved in combating infection and acts as a cofactor of many enzymes in the body [41]. The significant decrease in zinc level may be attributed to the increased use of zinc in different metabolic enzymes and low Zn intake due to inappetence/anorexia or stress. To the author’s knowledge, report on the levels of these minerals in dogs with CME or any other rickettsial disease is lacking to compare the present findings. However, low levels of Zn and Cu have been reported in case of other tick-borne diseases like Babesia gibsoni [42] and Hepatozoon canis [43] in dogs.
The mean plasma cortisol and insulin concentration of dogs with CME were non-significantly (P > 0.05) lower than that observed in healthy dogs. Plasma cortisol level is elevated in stressful conditions like infection, thermal stress, illness, fever, trauma, septicemia, inappetence and anorexia. The insulin and cortisol level showed wide variations in the diseased animals, and did not differ significantly from those observed in healthy dogs. The non-significant decrease in the levels of cortisol in CME positive animals also supported absence of oxidative stress in the disease process. The decrease in plasma insulin concentration might be attributed to body’s attempt to raise the blood glucose concentration or a result of the malfunctioning of the adrenals, pancreas and thyroid [44].
Conclusion
In conclusion, the study highlights the importance of nested PCR in diagnosing CME, incidence of CME in northern part of India and clinico-hemato-biochemical variations in affected dogs. However, changes exhibited by Ehrlichia canis infection on erythrocytic oxidant–antioxidant profile, plasma hormonal profile and blood mineral concentration in CME are largely non-significant and inconsistent, which need to be further explored to provide an insight into the disease pathology.
References
Morgan RV (2008) Handbook of small animal practice, 5th edn. Saunders Elsevier, USA, pp 1122–1124
Neer MT, Harrus S (2006) Ehrlichiosis, neorickettsiosis, anaplasmosis and Wolbachia infection. In: Greene CE (ed) Infectious diseases of the dog and cat, 3rd edn. Elsevier, Philadelphia, pp 203–216
Rani P, Irwin PJ, Gatne M, Coleman GT, Traub RJ (2010) Canine vector-borne diseases in India: a review of the literature and identification of existing knowledge gaps. Parasites Vectors 3:28
Inokuma H, Beppua T, Okudaa M, Shimada Y, Sakata Y (2003) Epidemiological survey of Anaplasma platys and Ehrlichia canis using ticks collected from dogs in Japan. Vet Parasitol 115:343–348
van Haaften RI, Haenen GR, Evelo CT, Bast A (2003) Effect of vitamin E on glutathione dependent enzymes. Drug Metab Rev 35:215–253
Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38:96–109
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Anderson BE, Dawson JE, Jones DC, Wilson KH (1991) Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol 29:2838–2842
Wen B, Rikihisa Y, Mott JM, Greene R, Kim HY, Zhi N, Couto GC, Unver A, Bartsch R (1997) Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol 35:1852–1855
Benjamin MM (2000) Outline of veterinary clinical pathology, 3rd edn. Kalyani Publishers, New Delhi, pp 48–56
Tentori L, Salvati AM (1981) Hemoglobinometry in human blood. In: Antonini E, Rossi-Bernardi L, Chiancone E (eds) Methods in enzymology. Academic Press, New York, pp 707–715
Placer ZA, Cushman LL, Johnson B (1966) Estimation of product of lipid peroxidation (malonaldehyde) in biochemical system. Anal Biochem 16:359–364
Minami M, Yoshikawa H (1979) Simplified assay method of superoxide dismutase activity of clinical use. Clin Chim Acta 92:337–342
Habig WH, Miheal JP, Jacoby WB (1974) Glutathione-S-transferase, the first enzymatic step in the mercapturic acid formation. J Biol Chem 249:7130–7139
Goldberg DM, Spooner RJ (1983) Assay of glutathione reductase. In: Bergmeyer HV (ed) Methods of enzymatic analysis, 3rd edn. Verlag Chemie, New York, pp 258–265
Prins HK, Loos JA (1969) Glutathione. In: Yunis JG (ed) Biochemical methods in red cell genetics. Academic Press, New York, pp 127–129
Snedecor GW, Cochran WG (1994) Statistical methods, 8th edn. Iowa State University Press, Ames
Samradhini D, Maske DK, Shobha R, Shinde PN (2005) Bionomics and hemodynamicsin blood protozoal infections in dogs from Nagpur. Indian J Anim Health 44:57–66
Kumar KS, Vairamuthu S, Kathiresan D (2009) Prevalence of hemoprotozoans in canines in Chennai city. Tamilnadu J Vet Anim Sci 5:104–108
Chaudhury S (2006) Studies on clinico-therapeutic aspects of babesiosis in dogs. Dissertation, Division of Veterinary Medicine, Deemed University, Indian Veterinary Research Institute, Izatnagar
Nyindo M, Huxsoll DL, Ristic M, Kakoma I, Brown JL, Carson CA, Stephenson EH (1980) Cell-mediated and humor immune responses of German shepherd dogs and beagles to experimental infection with Ehrlichia canis. Am J Vet Res 41:250–254
Harrus S, Kass PH, Klement E, Waner T (1997) Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators of the disease. Vet Rec 141:360–363
Chandrasekar M, Nambi AP, Ramprabhu R, Dhanapalan P (2002) Epizootiological studies on canine ehrlichiosis. Indian Vet J 79:1311–1312
Behera SK, Monsang SW, Kumar M, Sahu BD (2011) Changes in oxidative stress indices and hemato-biochemical profile in a dog with concurrent infection of Ehrlichia canis and Hepatozoon canis. Indian J Field Vet 6:59–62
Harikrishnan TJ, Pazhanivel N, Chellappa J (2005) Concomitant Babesia gibsoni and Ehrlichia canis infection in a dog. Vet Arhiv 75:513–520
Balachandran C, Pazhanivel N, Babulal DR (2007) Concomitant ehrlichiosis and trypanosomosis in a dog. Indian Vet J 84:877
Nelson RW, Couto CG (2009) Small animal internal medicine, 4th edn. Mosby Elsevier, St. Louis, pp 1325–1327
Brandao LP, Hasegawa MY, Hagiwara MK, Kohayagawa A (2006) Platelet aggregation studies in acute experimental canine ehrlichiosis. Vet Clin Pathol 35:78–81
Lakshmanan B, John L, Gomathinayagam S, Dhinakarraj G (2007) Molecular detection of Ehrlichia canis from blood of naturally infected dogs in India. Vet Arhiv 77:307–312
Sacchini F, Cessford RJ, Robinson BM (2007) Outbreak of canine monocytic ehrlichiosis in Saudi Arabia. Vet Clin Pathol 36:331–335
Pasa S, Azizoglu A (2003) Clinical and some hematologic findings in dogs with ehrlichiosis: 4 cases. Indian Vet J 80:33–35
Harrus S, Waner T, Weiss DJ, Keysary A, Bark H (1996) Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol 51:13–20
Frank JR, Breitschwerdt EB (1999) A retrospective study of ehrlichiosis in 62 dogs from North Carolina and Virginia. J Vet Intern Med 13:194–201
Kovacic P, Cooksy A (2005) Iminium metabolite mechanism for nicotinic toxicity and addiction: oxidative stress and electron transfer. Med Hypotheses 64:104–111
Luqman S, Rizvi SI (2006) Protection of lipid peroxidation and carbonyl formation in protein by capsaicin in human erythrocytes subjects to oxidative stress. Phytother Res 20:303–306
Hasegawa MY, Kohayagawa A, Brandao LP, Morgulis MSFA, Hagiwara MK (2005) Evaluation of neutrophil oxidative metabolism in canine monocytic ehrlichiosis. Vet Clin Pathol 34:213–217
Kumar A, Varshney JP, Patra RC (2006) A comparative study on oxidative stress in dogs infected with Ehrlichia canis with or without concurrent infection with Babesia gibsoni. Vet Res Commun 30:917–920
Brouqui P, Dumler JS (1997) The immune response to E. chaffeensis. In: Rickettsial Infection and Immunity, Plenum Press, New York, pp 163–172
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants and nutrition. Nutrition 18:872–879
Linares MV, Sanchez DJ, Belles M, Albina ML, Gomez M, Domingo JL (2007) Pro-oxidant effects in the brain of rats concurrently exposed to uranium and stress. Toxicology 236:82–91
Scott ME, Koski KG (2000) Zinc deficiency impairs immune responses against parasitic nematode infections at intestinal and systemic sites. J Nutr 130:1412S–1420S
Chaudhuri S, Varshney JP, Patra RC (2008) Erythrocytic antioxidant defense, lipid peroxides level and blood iron, zinc and copper concentrations in dogs naturally infected with Babesia gibsoni. Res Vet Sci 85:120–124
Seyrek K, Karagenç T, Paşa S, Kiral F, Atasoy A (2009) Serum zinc, iron and copper concentrations in dogs infected with Hepatozoon canis. Acta Vet Brno 78:471–475
Kumar A, Varshney JP, Varshney VP (2006) Endocrine dysfunction in chronic severe ehrlichiosis with or without babesiosis in dogs. Vet Res Commun 30:911–916
Acknowledgments
The authors would like to thank authorities of Indian Veterinary Research Institute (ICAR), Izatnagar, Bareilly, Uttar Pradesh for providing basic infrastructure and fund to undertake the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Behera, S.K., Dimri, U., Banerjee, P. et al. Molecular Detection and Assessment of Hemato-Biochemistry, Oxidant/Antioxidant Status in Natural Canine Monocytic Ehrlichiosis Cases from Northern India. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 361–368 (2017). https://doi.org/10.1007/s40011-015-0605-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0605-y