Abstract
The present study was planned to investigate the molecular prevalence of canine monocytic ehrlichiosis (CME) in dogs in and around Hisar and to evaluate the haemato-biochemical profile for its better management. A total of 60 dogs presented to Medicine Section, TVCC, LUVAS, Hisar with the history of naturally acquired tick infestation and clinical signs consistent with CME were screened on the basis of blood smear examination, followed by molecular detection by nested PCR assay targeting a portion of 16S rRNA gene of Ehrlichia canis. Nested PCR detected 18 cases positive for E. canis with estimated 30% percent positivity as compared to 8.33% (5 out of 60) by blood smear examination. These 18 dogs confirmed for CME by nested PCR were assessed for clinical and haemato-biochemical profile. Breed-wise prevalence indicated maximum number of cases in Labrador retriever, followed by Pug, Rottweiler and German shepherd dog with more number of cases in male dogs. Age-wise prevalence revealed highest number of cases in more than 1 year age group, followed by 6 months to 1 year age group and least in less than 6 months aged dogs. Pyrexia, anorexia and pale to congested mucous membranes were the main clinical signs observed, followed by lethargy, vomiting. Less common clinical signs were epistaxis, lymphadenomegaly, hind limb weakness, malena, ocular discharge, followed by haematuria, corneal opacity, nasal discharge and coughing, icterus, dermal petechiae and ecchymoses. The haematological profile revealed macrocytic hypochromic anaemia, thrombocytopenia, normal leucocyte count with relative lymphocytosis, monocytosis and neutropenia. Serum biochemistry revealed significant rise in values of ALT, AST, GGT, bilirubin total, bilirubin indirect, alkaline phosphatase and A/G ratio in affected dogs as compared to healthy control, suggesting the hepatic dysfunction. The lipid metabolites and kidney function parameters were non-significantly altered from those of healthy control. A high positivity for E. canis detected by nested PCR in dogs in and around Hisar suggests the endemicity of the disease in dogs’ population in this region and warrants the screening for the disease in suspected dogs by this technique as compared to routine blood smear examination. The presented haemato-biochemical profile may be useful in presumptive diagnosis of the disease in dogs and their better clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tick-transmitted infections are emerging problems in dogs, causing serious disease not only in traditional tropical and semi-tropical regions, but also temperate climates and urban environments (Shaw et al. 2001). Canine monocytic ehrlichiosis (CME) is an important tick-borne disease with a worldwide distribution (Harrus and Waner 2010). The causative agent is the obligate pleomorphic rickettsia, Ehrlichia canis that is transmitted mainly by the bite of the brown dog tick, Rhipicephalus sanguineus (Groves et al. 1975). It is a multi-systemic disease manifesting in acute, subclinical or chronic form (Harrus and Waner 2010). The acute disease is characterized by high fever, depression, lethargy, anorexia, lymphadenomegaly, splenomegaly, epistaxis, dermal petechiae and ecchymoses. Ophthalmological lesions are frequent and include anterior uveitis, chorioretinitis, papilledema, retinal hemorrhage, presence of retinal perivascular infiltrates and bullous retinal detachment (Komnenou et al. 2007). Pale mucous membranes and weakness, bleeding and significant weight loss are common findings in the chronic phase (Harrus and Waner 2010).
Due to its different phases and multiple clinical manifestations, the diagnosis of the disease can be challenging. Traditional diagnostic techniques such as hematology, cytology, serology and isolation are valuable diagnostic tools for CME; however, a definitive diagnosis of E. canis infection requires molecular techniques (Harrus and Waner 2010). Molecular techniques like polymerase chain reaction (PCR) using parasite-specific primers provides a better diagnostic tool in terms of both sensitivity as well as specificity and have been widely used in the laboratory diagnosis of canine ehrlichiosis (Iqbal et al. 1994; Wen et al. 1997; Lakshmanan et al. 2007; Milanjeet et al. 2014). These assays are based on different target genes (e.g. 16S rRNA, p28, p30, dsb, VirB9); however, the 16S rRNA and the p30-based PCR assays are most commonly used (Harrus and Waner 2010). Limited investigation has been made in studying the molecular prevalence of CME in dogs and its haemato-biochemical profile in Hisar region of Haryana state, India. Therefore, the present study was planned to investigate the molecular prevalence of CME in naturally infected dogs in this region and to evaluate the haemato-biochemical profile for its better management.
Materials and methods
Selection of animals
The present study was conducted on dogs presented to Medicine Section, Teaching Veterinary Clinical Complex, Lala Lajpat Rai University of Veterinary and Animal Sciences (LUVAS), Hisar with the history of naturally acquired tick infestation and clinical signs consistent with haemoparasitic infection viz. fever, anorexia, lethargy, lymphadenomegaly, pale mucus membranes, dermal petechiae and ecchymoses, vomiting, malena, epistaxis, cachexia, corneal opacity etc. A total of 60 suspected dogs were screened for the haemoparasitic infection from June, 2015 to May, 2016. Six healthy adult dogs more than 1 year of age brought to TVCC for vaccination served as healthy control group.
Blood sampling, processing and preservation
About 6 ml blood was collected from cephalic/saphanous vein by using 22G S/V set and 5 ml disposable syringe aseptically. One ml of blood was collected into two tubes coated with K3 ethylenediamine-tetraacetic acid (K3EDTA) separately each for hematological examination and extraction of DNA for molecular detection of CME. 4 ml blood was collected in plain tube (without anticoagulant) with clot activator for harvesting serum. The blood samples collected in plain tube were kept undisturbed for 2 h and then, centrifuged at 3000 rpm for 5 min and the serum separated were decanted in 2 ml Eppendorf tubes and stored at −20 °C till analysis.
Thin blood smears from the micro-capillary circulation (ear tip) in duplicate from each suspected cases were prepared on clean, grease free microslides, air dried and fixed using methanol. The fixed blood smears were stained by Giemsa stain using 1:10 dilution for 30 min (Coles 1986). The slides were washed under running tap water, air dried and examined microscopically at 1000 times magnification under oil immersion for E. canis morulae in monocytes.
Haematology
The blood samples collected in tubes coated with K3EDTA were immediately analyzed for complete hematological examination using fully automated Heamatology cell counter (MS4s, Melet Schlosing Lab.). The erythrocytic indices measured were haemoglobin (Hb) in g/dl, total erythrocyte count (TEC) in M/mm3, hematocrit (Hct) in %, mean corpuscular volume (MCV) in fl, mean corpuscular hemoglobin (MCH) in pg and mean corpuscular hemoglobin concentration (MCHC) in g/dl. The leucocytic indices measured were total leucocyte count (TLC) in m/mm3, lymphocytes (L) in %, monocytes (M) in %, neutrophils (N) in %, eosinophils (E) in % and basophils (B) in %. The thrombocytic indices measured were thrombocyte count (THR) in m/mm3, mean platelet volume (MPV) in fl and plateletcrit (Pct) in %.
Serum biochemistry
The serum samples were analyzed for estimation of biochemical profile using fully automated random access clinical chemistry analyzer (EM Destiny 180, Erba Diagnostics Mannheim GmbH). The serum biochemical parameters of liver function measured were alanine aminotransferase (ALT) in U/L, aspartate aminotransferase (AST) in U/L, gamma glutamyl transferase (GGT) in U/L, bilirubin (total) in mg/dl, bilirubin (direct) in mg/dl, bilirubin (indirect) in mg/dl and alkaline phosphatase in U/L. The protein profile included the estimation of total protein in g/dl, albumin in g/dl, globulin in g/dl and albumin to globulin ratio (A/G). The serum biochemical parameters of kidney function measured were urea in mg/dl and creatinine in mg/dl. The lipid metabolites measured were triglycerides in mg/dl and total cholesterol in mg/dl.
Molecular detection of E. canis in blood of suspected dogs
The extraction of DNA from the blood was done using PurelinkTM Genomic DNA extraction kit (Invitrogen, USA) following the manufacturer’s protocol and processed for detection of E. canis by nested PCR assay targeting a portion of 16S rRNA gene as described by Murphy et al. (1998). The sequences of the primers were as follows:
-
Primary PCR assay
-
ECC: 5′AGAACGAACGCTGGCGGCAAGCC 3′
-
ECB: 5′ CGTATTACCGCGGCTGCTGGC 3′
-
Nested PCR assay
-
ECAN5: 5′-CAATTATTTATAGCCTCTGGCTATAGGA-3′
-
HE3: 5′-TATAGGTACCGTCATTATCTTCCCTAT-3′
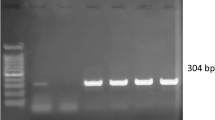
The extracted DNA from each sample was used as template in primary PCR amplification of 478 base-pair fragment of the 16S rRNA gene in the thermocycler (Quanta Biotech, UK). The reaction (10 μl) contained 0.5 μl of template DNA in 5.0 μl DreamTaq PCR master mix (Thermo Fisher Scientific) containing DreamTaq polymerase, optimized DreamTaq buffer, MgCl2 and dNTPs; 0.3 μl of each primer and DMSO and 3.6 μl nuclease-free water (NFW). The thermocycle profile consisted of initial denaturation at 94 °C for 2 min, followed by 34 cycles of denaturation at 94 °C for 1 min, annealing at 65 °C for 1 min and extension at 72 °C for 30 s. This was followed by a final extension at 72 °C for 6 min. The PCR reactions that resulted in positive amplification of the fragment of Ehrlichia species 16S rRNA was subjected to nested PCR for amplification of 389 base-pair fragment specific for E. canis using the species specific primers. The reaction (10 μl) contained 1.0 μl of template DNA in DreamTaq PCR master mix (Thermo Fisher Scientific) containing 5.0 μl DreamTaq polymerase, optimized DreamTaq buffer, MgCl2 and dNTPs; 0.3 μl of each primer and DMSO and 3.1 μl NFW. The thermocycling conditions consisted of initial denaturation at 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 56 °C for 45 s and extension at 72 °C for 30 s. This was followed by a final extension at 72 °C for 6 min. All the PCR products and a molecular weight marker (100 base-pair ladder) were electrophoresed through 1.5% agarose gels stained with ethidium bromide in Tris-acetate-EDTA (TAE) buffer for 35 min at 80 V and the DNA fragments were visualized under UV fluorescence using a gel documentation system (GeNei Uvitec, UK).
Statistical analysis
The data generated was analyzed statistically by suitable statistical methods using statistical software package (SPSS 16.0). For analysis of various parameters observed in diseased animals as compared to healthy control, the independent t test was applied. The results are presented as Mean ± S.E. at the significance level, P ≤ 0.05.
Results
Molecular prevalence of CME in dogs
A total of 60 dogs suspected for CME were evaluated for detection of E. canis in blood by nested PCR assay targeting a portion of 16S rRNA gene. Out of these, 20 were found to be positive for Ehrlichia infection by primary PCR using genus specific primers. These primers produced a 478 base-pair band in accordance with the positive control. All of the primary PCR-positive samples were subjected to nested PCR using species-specific primers and 18 out of 20, were found to be positive for E. canis infection producing an amplicon of 389 base-pair length (Fig. 1). Thus, the percent positivity for the detection of E. canis by nested PCR was estimated 30% (18/60) as compared to 8.33% (5/60) by blood smear examination as depicted in Table 1. Five dogs which were positive on peripheral blood smear examination were also found positive by nested PCR.
Clinical profile
The maximum number of cases were recorded in Labrador retriever (44.44%), followed by Pug (16.66%), Rottweiler (11.11%), German Shepherd (11.11%) and one case (5.56%) each in Lhasa Apso, Pomeranian, Gaddi. Age-wise prevalence recorded maximum number of cases in more than 1 year age group (50.00%), followed by 6 months to 1 year age group (38.89%) and least in less than 6 months age group. Sex-wise prevalence indicated more number of cases diagnosed in male dogs (66.67%) than female dogs (33.33%).
The clinical profile of dogs diagnosed with CME is depicted in Table 2. The most common clinical signs observed were pyrexia (66.66%), inappetence to anorexia (55.55%), pale to congested mucous membrane (44.44–50%), followed by lethargy (44.44%), vomiting (44.44%). Less common clinical signs were epistaxis, lymphadenomegaly, hind limb weakness, malena, ocular discharge, followed by haematuria, corneal opacity, nasal discharge and coughing, icterus, dermal petechiae and ecchymoses. Auricular discharge (5.55%) and prepucial swelling (5.55%) was observed in one dog respectively, however, these are not the characteristic sign of CME but the signs resolved after therapy. The external parasites found on these dogs were generally ticks (94.44%).
Haematological profile
Hematological profile of dogs diagnosed with CME is depicted in Table 3. The mean values of total erythrocyte count, Hematocrit, MCH, MCHC and thrombocyte count were significantly (P < 0.05) lower in affected dogs as compared to healthy control while the mean values of MCV, lymphocytes, monocytes and mean platelet volume was significantly (P < 0.05) higher in affected dogs as compared to healthy control. The mean values of remaining parameters in affected dogs were non-significantly different from those of healthy control.
Serum biochemical profile
Serum biochemical profile of dogs diagnosed with CME is depicted in Table 4. There was found significant (P < 0.05) rise in mean values of ALT, AST, GGT, bilirubin total, bilirubin indirect, alkaline phosphatase and A/G ration in affected dogs than those of healthy control group. The other parameters were non-significantly altered from those of healthy control.
Discussion
Nested PCR showed more sensitivity (30% positivity) in detection of E. canis infection as compared to peripheral blood smears (8.33% positivity). The present findings are in agreement with the observations of other workers (Lakshmanan et al. 2007; Parmar et al. 2013; Milanjeet et al. 2014) who reported nested PCR to be more diagnostic for CME than peripheral blood smear examination. The detection of morulae of E. canis in stained blood smears is a valuable diagnostic tool in the acute disease (Hildebrandt et al. 1973; Mylonakis et al. 2003); however, it lacks sensitivity for subclinical and chronic disease (Woody and Hoskins 1991). Poor sensitivity of blood smear examination might be due to low level parasitaemia that can be detected by highly sensitive nested PCR (Lakshmanan et al. 2007). High molecular prevalence for E. canis in suspected dogs suggests the endemicity of the infection in dogs’ population of this region. Dhankar et al. (2011) reported 23 out of 203 dogs examined in this and nearby region positive for canine ehrlichiosis based on blood and buffy coat smear examinations. A high molecular prevalence of E. canis infection in dogs varying from 20.6 to 50% has been reported from different regions of India (Lakshmanan et al. 2007; Abd Rani et al. 2011; Milanjeet et al. 2014). This variation in the prevalence might be attributed to sample size, geographical area, climatic conditions and time of sample collection.
CME was observed in wide variety of breeds with somewhat more predisposition in Labrador retriever followed by Pug, Rottweiler, German shepherd, Lhasa apso, Pomeranian and Gaddi. This finding is in disparity to other workers (Dhankar et al. 2011; Milanjeet et al. 2014; Bhadesiya and Raval 2015), who have reported highest incidence in German shepherd dogs. Such variations in breed-wise prevalence of CME in dogs might be due to variations in the population of different breeds or the preference of owner to different breeds in different areas. Age-wise prevalence of CME recorded in dogs of varying age i.e. from 3 months to 9 years with maximum number of cases in more than 1 year age group. This finding is contrary to other workers (Lakshmanan et al. 2006; Milanjeet et al. 2014; Bhadesiya and Raval 2015) who have reported highest prevalence in young dogs. The prevalence in the present study was found privileged in male than female dogs. This finding was in accordance with the workers (Thirunavukkarasu et al. 1993) who recorded higher incidence in males. In contrary, Milanjeet et al. (2014) and Bhadesiya and Raval (2015) have recorded higher prevalence in female than male dogs, however, no correlation has been estimated between the incidence of ehrlichiosis and sex of dogs. The present finding might be due to the over representation of male dogs in the study group or due to individual preference of pet owners for keeping male dogs. The most common clinical findings consistent with the CME were ticks infestation, pyrexia, anorexia, pale to congested mucous membrane etc. These findings was in agreement with the other researchers (Das and Konar 2013; Bhadesiya and Raval 2015). Pyrexia may be due to release of cytokine IL-1 in response to E. canis (Morais et al. 2010). Haemorrhages manifested as epistaxis, dermal petechiae and ecchymoses, haematuria and malena in cases of CME during the present study were also observed by Troy et al. (1980) which might be attributed to thrombocytopenia (Woody and Hoskins 1991; Ristic and Holland 1993; Harrus et al. 1996) and the deposition of immune complexes on vascular wall (Reardon and Pierce 1981). Corneal opacity observed in two cases has been reported by the other researchers (Hildebrandt et al. 1973; Das and Konar 2013) as a clinical sign in CME. This corneal opacity might be due to due to edema and/or deposition of cellular precipitates (Waner et al. 1999).
Lower levels of total erythrocyte count, Hematocrit, MCH, MCHC and higher levels of MCV observed in the present study indicates macrocytic, hypochrmic anemia in affected dogs which is contrary to the usual finding of normocytic, normochromic anemia as reported by many workers (Thirunavukkarasu et al. 1994; Nakaghi et al. 2008; Gaunt et al. 2010; Silva et al. 2012; Milanjeet et al. 2014). Such type of anaemia might be due to haemorrhagic manifestation of disease (Bhardwaj 2013) owing to thrombocytopenia observed in the present study. An immunological mechanism may be involved through production of antibodies and their subsequent binding on the membranes of erythrocytes which may result in destruction of these cells resulting anemia (Taylor et al. 2007). Thrombocyte count revealed a clear picture of thrombocytopenia in affected dogs which is considered as one of the characteristic feature of the disease (Woody and Hoskins 1991; Ristic and Holland 1993; Waner et al. 1995; Harrus et al. 1996; Agnihotri et al. 2012; Srikala et al. 2012; Bhardwaj 2013; Prashar et al. 2015). Platelet consumption, increased splenic sequestration and decreased platelet lifespan are the possible attributes for thrombocytopenia (Harrus et al. 1999). Leucogram in affected dogs revealed leucocytosis due to significant lymphocytosis and monocytosis in addition to neutropenia. This was in agreement with the other workers (Dixit et al. 2012; Parmar et al. 2013) who reported a significant increase in lymphocytes in dogs with ehrlichiosis. Neutropenia observed in dogs affected with CME was in agreement with the workers (Dixit et al. 2012; Osathanon et al. 2013), indicative of continued pathological changes (Harrus et al. 1999). On contrary, Das and Konar (2013) reported neutrophilic leucocytosis in dogs affected with CME. Lymphocytosis in the present study is feature of chronic E. canis infection (Avery and Avery 2007). Numerous studies have shown that naturally occurring E. canis infection can result in lymphocytosis (Heeb et al. 2003; Weiser et al. 1991; Frank and Breitschwerdt 1999), although not all case series describe lymphocytosis (Breitschwerdt et al. 1998). Therefore, an important discrepancy for lymphocytosis in dogs is the E. canis infection.
There was increase in serum ALT, AST and alkaline phosphatase values in affected dogs, suggesting hepato-biliary dysfunction. These findings were in agreement with other workers (Srikala et al. 2012; Bhardwaj 2013; Agnihotri et al. 2012). Increased levels of AST and ALT might be due to histopathological changes in liver as a result of the infiltration of perivascular mononuclear cells (Nair et al. 2016). Serum protein profile indicated hypoproteinemia owing to hypoaluminemia and hypoglobunemia in affected dogs which is in agreement with the workers (Castro et al. 2004; Srikala et al. 2012; Srivastava and Srivastava 2011; Agnihotri et al. 2012; Bhadesiya and Raval 2015). The hypoalbuminemia seen in CME might be the consequence of peripheral loss of albumin to edematous inflammatory fluids as a result of increased vascular permeability (Woody and Hoskins 1991), blood loss, or decreased protein production due to concurrent liver disease as suggested by elevated hepatic enzymes. Total bilirubin levels in affected dogs were higher as compared to healthy control which is due to rise in indirect bilirubin levels owing to immune mediated RBC lysis. Mean urea and creatinine value in affected dogs were higher than healthy control dogs. These findings were in agreement with the workers (Srivastava and Srivastava 2011; Agnihotri et al. 2012). The increase in urea and creatinine values may be due to membrano-proliferative glomerulopathy and interstitial nephritis. It has been suggested that the presence of inflammatory infiltrates rich in lymphocytes might be responsible for immuno-pathogenesis of renal lesion in dogs with CME. Furthermore, hypoalbuminemia also seems to be a marker of renal damage in dogs infected with E. canis (Silva et al. 2016).
Conclusion
It may be concluded that nested PCR detected high positivity for E. canis in suspected dogs, suggesting the endemicity of the disease in dog population in and around Hisar region. The presented haemato-biochemical profile may be useful in presumptive diagnosis of the disease in dogs and their better clinical management.
References
Abd Rani PAM, Irwin PJ, Coleman GT, Gatne M, Traub RJ (2011) A survey of canine tick-borne diseases in India. Parasites Vectors 4:141–148
Agnihotri D, Khurana R, Jain VK, Singh G (2012) Concurrent infection of Ehrlichia canis and ancylostomosis in a dog. Indian Vet J 89(11):89–90
Avery AC, Avery PR (2007) Veterinary clinics small animal practice determining the significance of persistent lymphocytosis. Vet Clin Small Anim 37:267–282
Bhadesiya CM, Raval SK (2015) Hematobiochemical changes in ehrlichiosis in dogs of Anand region, Gujrat. Vet World 8(6):713–717
Bhardwaj RK (2013) Therapeutic management of acute canine monocytic ehrlichiosis. Indian Vet J 90(2):138–139
Breitschwerdt EB, Hegarty BC, Hancock SI (1998) Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol 36:2645–2651
Castro DB, Machado RZ, Tomaz de Aquino LP, Alessi AC, Costa MT (2004) Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol 119(1):73–86
Coles EH (1986) Veterinary clinical pathology, 4th edn. WB Saunders Company, London, pp 46–47
Das M, Konar S (2013) Clinical and hematological study of canine ehrlichiosis with other hemoprotozoan parasites in Kolkata, West Bengal, India. Asian Pac J Trop Biomed 3(11):913–915
Dhankar S, Sharma RD, Jindal N (2011) Some epidemiological observations on canine ehrlichiosis in Haryana and Delhi states. Haryana Vet 50:9–14
Dixit AK, Dixit P, Sukla PC (2012) Canine monocytic ehrlichiosis and its therapeutic management in a dog. Intas Polivet 13(1):140–141
Frank JR, Breitschwerdt EB (1999) A retrospective study of ehrlichiosis in 62 dogs from North Carolina and Virginia. J Vet Intern Med 13:194–201
Gaunt SD, Beal MJ, Stillman BA, Lorentzen L, Diniz PPVP, Chandrashekar R, Breitschwerdt EB (2010) Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasites Vectors 3:33
Groves MG, Dennis GL, Amyx HL, Huxsoll DL (1975) Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res 36:937–940
Harrus S, Waner T (2010) Diagnosis of canine monocytotropic ehrlichiosis: an overview. Vet J 187:292–296
Harrus S, Waner T, Eldor A, Zwang E, Bark H (1996) Platelet dysfunction associated with experimental acute canine ehrlichiosis. Vet Rec 139:290–293
Harrus S, Waner T, Bark H, Jongejan F, Cornelissen AW (1999) Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J Clin Microbiol 37:2745–2749
Heeb HL, Wilkerson MJ, Chun R (2003) Large granular lymphocytosis, lymphocyte subset inversion, thrombocytopenia, dysproteinemia, and positive Ehrlichia serology in a dog. J Am Anim Hosp Assoc 39:379–384
Hildebrandt PK, Huxsoll DL, Walker JS, Nims RM, Taylor R, Andrews M (1973) Pathology of canine ehrlichiosis (Tropical canine pancytopenia). Am J Vet Res 34:1309–1320
Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y (1994) Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clinical Microbiol 32:1658–1662
Komnenou AA, Mylonakis ME, Kouti V, Tendoma L, Leontides L, Skountzou E, Dessiris A, Koutinas AF, Ofri R (2007) Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): a retrospective study of 90 cases. Vet Ophthalmol 10:137–142
Lakshmanan B, John L, Gomathinayagam S, Dhinakarraj G (2006) Prevalence of Ehrlichia canis in Chennai. Indian Vet J 7:307–312
Lakshmanan B, John L, Gornathinayagam S, Dhinakarraj G (2007) Molecular detection of Ehrlichia canis from blood of naturally infected dogs in India. Vet Archive 83:353–354
Milanjeet HS, Singh NK, Singh ND, Singh C, Rath SS (2014) Molecular prevalence and risk factors for the occurrence of canine monocytic ehrlichiosis. Vet Med 59:129–136
Morais CDN, Castro DRJ, Mundim VA, Bastos DEJ, Ferreira AF, Souza AM, Salaberry SRS, Lima-Ribeiro CMA (2010) Clinical and hematological aspects of dogs naturally infected with Ehrlichia spp. and Leptospira interrogans. Biosci J 27(3):452–459
Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA (1998) A molecular and serological survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol 79:325–339
Mylonakis ME, Koutinas AF, Billinis C, Leontides LS, Kontos V, Papadopoulos O, Rallis T, Fytianou A (2003) Evaluation of cytology in the diagnosis oxf acute canine monocytic ehrlichiosis (Ehrlichia canis): a comparison between five methods. Vet Microbiol 91(2–3):197–204
Nair AD, Cheng C, Ganta CK, Sanderson MW, Alleman AR, Munderloh UG, Ganta RR (2016) Comparative experimental infection seen in dogs with E. canis, E. chaffensis, A. platys and A. phagocytophilum. PLoS One 11(2):e0148239
Nakaghi AC, Machado RZ, Costa MT, Andre MR, Baldani CD (2008) Canine ehrlichiosis: clinical, haematological, serological and molecular aspects. Cien Rural Santa Maria 38(3):766–770
Osathanon R, Moonarmart W, Suksantilap N, Krajangpit N, Lekcharoensook P, Julapanthong P, Wongrerkngam N (2013) Evaluation of hematology profiles and measurement of serum cardiac troponin level in canine monocytic ehrlichiosis. Thai J Vet Med 43(3):411–419
Parmar C, Pednekar R, Jayraw A, Gatne M (2013) Comparative diagnostic methods for canine ehrlichiosis. Turk J Vet Anim Sci 37:282–290
Prashar R, Sudan V, Jaiswal KA, Srivastava A, Shanker D (2015) Evaluation of clinical, biochemical and haematological markers in natural infection of canine monocytic ehrlichiosis. J Parasitic Dis. doi:10.1007/s12639-015-0688-7
Reardon MJ, Pierce KR (1981) Acute experimental canine ehrlichiosis. I. Sequential reaction of the hemic and lymphoreticular systems. Vet Pathol 18:48–61
Ristic M, Holland CJ (1993) Canine ehrlichiosis. In: Woldehiwet Z, Ristic M (eds) Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, New York, pp 169–186
Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB (2001) Tick-borne infectious diseases of dogs. Trends Parasitol 17:74–80
Silva GCF, Benitez AN, Girotto A, Taroda A, Vidotto MC, Garcia JL, Freitas JC, Headley SA, Vidotto O (2012) Occurrence of Ehrlichia canis and Anaplasma platys in household dogs from Northern Parana. Rev Bras Parasitol Vet 21:379–385
Silva LS, Pinho FA, Maria G, Prianti MG, Braga JFV, Pires JV, França SA, Silva SMMS (2016) Renal histopathological changes in dogs naturally infected with Ehrlichia canis. Braz J Vet Pathol 9(1):2–15
Srikala D, Satish Kumar K, Amruth Kumar VV, Tirumala Rao DS (2012) Clinical and therapeutic aspects of canine monocytic ehrlichiosis. Indian J Vet Med 32(2):109–110
Srivastava MK, Srivastava A (2011) Canine ehrlichiosis in dog. Indian J Vet Med 31(2):128–129
Taylor MA, Coop RL, Wall RL (2007) Parasites of dogs and cats. In: Taylor MA, Coop RL, Wall RL (eds) Veterinary parasitology, 3rd edn. Blackwell Publishing, Oxford, p 356–458
Thirunavukkarasu PS, Dhanapalan P, Gnanaprakasam V (1993) Incidence of canine ehrlichiosis in Madras city. Cheiron 22:222–224
Thirunavukkarasu PS, Nambi AP, Rajan TSS, Gnanaprakasam V (1994) Clinical and haematological findings in canine ehrlichiosis in Madras city. Indian Vet J 71:825–828
Troy GC, Vulgamott JC, Turnwald GH (1980) Canine ehrlichiosis: a retrospective study of 30 naturally occurring cases. J Am Anim Hosp Assoc 16:181–187
Waner T, Harrus S, Weiss DJ, Bark H, Keysary A (1995) Demonstration of serum anti-platelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol 48:177–182
Waner T, Harrus S, Bark H (1999) Canine monocytic ehrlichiosis—an overview. Israel Vet Med A 54(4):1–8
Weiser MG, Thrail MA, Dulton R, Beck ER, Wise LA, Vansteenhouse JL (1991) Granular lymphocytosis and hyperproteinemia in dogs with chronic ehrlichiosis. J Am Anim Hosp Assoc 27(1):84–88
Wen B, Rikihista Y, Mott JM, Grene R, Kim HY, Zhi N, Couto C, Unver A, Bartsch R (1997) Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol 35:1852–1855
Woody BJ, Hoskins JD (1991) Ehrlichial diseases of dogs. Vet Clin North Am Small Anim Pract 21:75–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, L., Goel, P., Jhambh, R. et al. Molecular prevalence and haemato-biochemical profile of canine monocytic ehrlichiosis in dogs in and around Hisar, Haryana, India. J Parasit Dis 41, 647–654 (2017). https://doi.org/10.1007/s12639-016-0860-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-016-0860-8