Abstract
The present paper deals with the salt effect on root, stem and leaf anatomy of Spergularia marina. Salt tolerant populations of Spergularia marina from low (2.0–4.2 dS/m), medium (9.3–10.7 dS/m) and highly (18.4–26.2 dS/m) saline soils were evaluated for anatomical modifications. Root anatomical characteristics as cortex thickness and xylem vessel diameter were decreased in high saline environments. Increased aerenchyma and periderm thickness in the root were critical for checking water loss and enhancing water storage capability. In stem, higher salinity decreased the thickness of the epidermis and cortex. Increased aerenchyma and increased thickness of vascular tissue seemed to be crucial for its better survival under saline environments. The thickness of sclerenchyma was unchanged under low and moderate salinity but considerably increased under high salinity. Leaf anatomy shows that salt stress resulted in an increase of cuticle and parenchyma thickness as well as an increase of vascular bundle sheath thickness. The presence of the cells with calcium oxalate crystals in the stem and leaf increased at higher salinity. Additionally, under high salinity it was observed that both stomatal index and stomatal dimensions were considerably reduced. These results show that salinity stress shows significant anatomical modifications in Spergularia marina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the most important abiotic stresses, limiting plant growth and productivity in arid and semi-arid regions [1]. High salt content, especially chloride and sodium sulphates, affect plant growth by modifying their morphological [2], anatomical [2, 3] and physiological traits [4, 5]. Therefore, plants display specific adaptations at structural and physiological level showing altered physiological and biochemical mechanisms, thus enabling to grow well in high salinity and maintaining their reproductive capacity [6, 7].
Morphological and anatomical modifications in the plant body are capable of minimizing detrimental effects of salt stress [8]. Salt tolerant species show a range of anatomical adaptive features like increased succulence (both in root and stem), thick cuticle and deposition of wax, salt-secretory trichomes and glands, thick and many layered epidermis and well developed water storing tissues in the cortex, widening of casparian band and enhanced development of root endodermis [9, 10]. Many studies have shown that anatomical alterations such as inhibited differentiation, change in diameter and number of xylem vessels are the result of high salinity. Based on these results it has been assumed that water transport capacity can be affected by this change in the xylem structure [11, 12]. Additionally, several studies reported that anatomical structures of plant organs, especially of leaves, change, thus enabling the plant to adapt to its environment [13], because the leaf is the main photosyntetic organ of the plant [14]. Morpho-anatomical alterations of halophytes include an increase of cell volume, especially of spongy and water parenchyma, an increase in leaf thickness and a decrease in the number of stomata [15–17]. Also, under saline conditions, mesophyll resistance to the gaseous exchange increases as a consequence of structural changes in the mesophyll cells [18]. However, promoting effects of salt stress on leaf thickness [19, 20] and stomata number [19] have also been recorded.
Spergularia marina belonging to the family Caryophyllaceae, is a coastal annual halophytic herb, and is widely distributed among the sea shores of Turkey [21, 22]. It is commonly found in cultivated fields and waste lands of saline and sandy habitats [23]. In this species the salt resistance mechanism is often related with the relationship between Na+ and K+ accumulation and growth [24]. Keiffer and Ungar [25] showed that seeds of S. marina failed to germinate in 2 % NaCl. It has been reported that S. marina can grow in a wide range of salinity [22]. Therefore the aim of the present study was to investigate the effects of salinity on root, stem and leaf anatomy under increasing salinity levels. Further the main aim of the present study is to investigate the reaction of S. marina under different salinity levels and also to contribute to the understanding of the salt tolerance adaptation mechanism.
Material and Methods
The plant species S. marina was collected from different localities of the Kızılırmak Delta, Samsun, Turkey in flowering period during the year 2010–2011(Fig. 1). Soil salinity was determined from 28 soil samples randomly collected in the study area. Soil samples were taken from the root zone of each population from different habitats. The electrical conductivity meter (EC meter) was used to determine the EC in the obtained soil extract. Therefore, three salinity levels were determined as 2.0–4.2 dS/m (low salinity), 9.3–10.7 dS/m (moderate salinity) and 18.4–26.2 dS/m (high salinity) in the study area. Subsequently the first S. marina population was collected from the low saline soils (coordinates 41° 40′ 09″ N, 36° 01′ 16″ E, ECe 2.0–4.2 dSm−1); second population was collected from the moderate saline soils (coordinates 41° 40′ 05″ N, 36° 02′ 19″ E, ECe 9.3–10.7 dSm−1) and the third population was collected from the high saline soils (coordinates 41° 40′ 11″ N, 36° 01′ 57″ E, ECe 18.4–26.2 dSm−1).
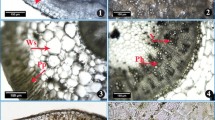
Stem cross-sections showing changes in S. marina plants grown at low salinity (a), moderate salinity (b) and high salinity (c), respectively. Ac aeriferous cavities, Co cortex, Ph phloem, Pt pith, Sc sclerenchyma, X xylem. Sclerenchyma thickness is seen to be highly thick as compared to the stem at low salinity in a. The aeriferous cavities at the cortex parenchyma were correlated with an increase in salt levels (a–c)
For the anatomical studies, slides were made from transverse sections of middle parts of fully developed roots, stems and leaves and surface sections of leaf material preserved in 70 % ethanol. A light microscope (Nikon SE, Japan) was used for all microscopic observations and obtained images were photographed using a Nikon Coolpix P5100 digital camera. Image J program was used to measure of various cells and tissues on the figures. All anatomical measurements were determined on at least 30 specimens. The stomatal index (SI) was calculated using the following formula [26]:
where SI is the stomatal index, S is the number of stomata per unit area and E is the number of ordinary epidermal cells per unit area.
The statistical package programme SPSS (P < 0.05) was used to compare mean values with one-way ANOVA. The Duncan post hoc tests was used to compare mean values in case of significant differences.
Results
Root Anatomy
Root anatomical characters significantly affected by increased salinity levels are shown in Table 1. Increasing salinity generally increased periderm thickness particularly at the highest salt level (Table 1; Fig. 2a–c). On the contrary, the cortex thickness was decreased reasonably with the increase in salinity levels (Table 1). The aeriferous cavities in S. marina root increased with increasing salt levels (Table 1; Fig. 2a–c). At the highest salinity level a remarkable decrease was determined in the xylem vessel diameter (Table 1; Fig. 3a–c).
Stem Anatomy
The obtained results showed that stem epidermis and cortex thickness of S. marina was stimulated beginning from moderate to low salinity levels (Table 2). Stem anatomical parameters as thickness of phloem and xylem increased with the rise in salinity levels (Table 2). Pith width increased significantly ranging from 205.56 to 450.92 µm with the increase in salt concentration (Table 2; Fig. 4a–c). There was an increase in pith length with increasing salt levels (Table 2). However, the sclerenchyma thickness was not much affected by increasing salt levels but the high salt concentration caused a significant increase in this character (Table 2; Fig. 4a–c). A significant increase in the aeriferous cavities of the cortex parenchyma of the stem was observed with increasing salt levels (Table 2; Fig. 4a–c). Calcium oxalate crystals in the cortical parenchyma of the stem increased with an increase in salt levels (Table 2).
Leaf Anatomy
Leaf cuticle thickness increased with increase in salt intensity (Table 3; Fig. 5a–c). However, leaf epidermis thickness was not much affected by increasing salt level. Salt stress led to a significant increase in the thickness of the parenchyma in the leaves of S. marina. Their highest values were determined under highest salinity levels (18.4–26.2 dS/m) (Table 3; Fig. 6a–c). In addition, calcium oxalate crystals in the mesophyll of the leaf increased with the increase in salinity (Fig. 6).
Leaf cross-sections showing changes in S. marina plants grown at low salinity (a), moderate salinity (b) and high salinity (c), respectively. Bs bundle sheath, Mv main vein, Pr parenchyma. High salinity led to a significant increase in the thickness of parenchyma (c). Leaf bundle sheath was correlated with increasing salt levels (a–c)
Stomatal density on both adaxial and abaxial leaf surfaces showed almost a similar response to increasing salt levels (Fig. 7a–c). Stomata index both on the lower and upper surfaces was decreased with the increase in salinity (Table 4). Similarly, stomata length and width generally decreased with rise in salt levels (Table 3).
The length and width of the leaf main vein was unaffected by low and moderate salinity. However, this parameter significantly increased at high salinity (Table 3). Leaf sheath thickness increased gradually with increasing salinity (Table 3). On the other hand, salt stress imposed a distinct increase in sclerenchyma thickness (Fig. 8a–c).
Leaf main vein and bundle sheath showing changes in S. marina plants grown at low salinity (a), moderate salinity (b) and high salinity (c), respectively. Bs bundle sheath, Mv main vein. The length and width of the leaf main vein was correlated with increasing salt levels (a–c). Increased thickness at the leaf sheath is seen in c
Discussion
As observed in the present study different salt levels affected the anatomical structure of the investigated species. These results showed that the root cortex area of S. marina was decreased with increasing salt level (Table 1). The results of the study of Hajibagheri et al. [27] confirms the present results. It shows that the cortical thickness and root stele diameter of Suaeda maritima was greater in the presence of 340 mM NaCl as compared to those grown in the absence of NaCl. The halophyte, Kandelia candel showed greater root growth in plants grown in low and moderate salinities. It might indicate an advantageous effect of NaCl on the growth [28]. In addition, Boughalleb et al. [17] reported that the cortical thickness of Atriplex halimus and Nitraria retusa decreased significantly at salinity exceeding 200 mM NaCl. A higher number of cortical layers accompanied by small vacuolated parenchyma cells have been reported in N. retusa and A. halimus roots grown in moderate salinity. These results are in agreement with the results of Shannon et al. [6], reporting that in numerous halophytes salinity induces vacuolisation. The aeriferous cavities in vegetative organs are common features for more halophyte species such as Spergularia [29]. It is known that the presence of aerenchyma in Spergularia root may be correlated with poor ventilation of saline soils [30]. Such root aerenchyma structures have also been reported in Imperata cylindrica [31]. In the present study a remarkable increase in aerenchyma formation in the root of S. marina with increased salinity level has been noticed (Table 1; Fig. 2a–c). This structural formation can lead to an increase in storage tissue area with increased vacuolar volume storing toxic ions. This is an important strategy in coping with high salinities [32]. In the present study, the xylem vessel diameter for S. marina root was reduced significantly with increasing salt levels (Table 1; Fig. 3a–c). In wild barley [2] and in cotton and tomato plants [33] also the similar diminution of the diameter of xylem vessel was observed under saline conditions [2, 33]. As compared to non-saline conditions the number of xylem vessels are more and they are narrower than those found under saline conditions [34]. The selection for narrow vessels leads to improved water use capacity of leaves. The risk of xylem embolism will be reduced in saline habitats [17].
The obtained results in the present study revealed that with low to moderate salinity the stem cortex area of the species S. marina was increased, but declined severely at higher salinity levels (Table 2). In addition, increased sclerenchyma thickness was observed with increasing salt levels (Table 2; Fig. 4). It is known that the sclerenchymatic and lignified ring of Spergularia media stem can be related to an excessive salinity in soil [30, 35]. It was also suggested that the lignin may be a cellular resistance element against the high osmotic pressure inside the plant body [36]. Hameed et al. [37] described that at high salt level the sclerenchyma thickness of Cynodon dactylon was greater confirming the present results. This characteristic may offer some resistance to water loss through the stem and may play a crucial role in adaptation to unfavourable conditions [30, 37, 38]. The thickness of xylem and phloem increased clearly under high salinity conditions. (Table 2). It was suggested that increased xylem and phloem area plays an important role in the conduction of water and photosynthates, particularly under adverse saline conditions [37]. This has been supported by previous reports in different plant species such as rice [39], Kandelia candel [28], Ziziphus cultivars [40] and Arabidopsis thaliana [41].
Kozlowski [34] determined that the production of calcium oxalate increased under salinity. According to Grigore and Toma [30], calcium also plays an important role in maintaining the integrity of plant cell membrane. It is a physiological barrier to free diffusion of potentially toxic ions prevalent in a saline environment. In agreement with this idea, it was seen that calcium oxalate crystals in the cortical parenchyma of the stem of S. marina were significantly increased at higher salinity.
Salinity leads to significant changes in the leaf anatomy of S. marina. The thickness of leaf mesophyll parenchyma increases with high salinity and reduces the dimensions of leaf stomata. The greater leaf mesophyll parenchyma thickness was measured in S. marina at higher salinity (Table 3). Further published data that salinity increases succulence and leaf thickness in plants like Cakile maritima [42], Nitraria retusa and Atriplex halimus [17] are similar to the present results. Halophytes utilize different mechanism to deal with high internal ion concentrations. Debez et al. [42] showed that succulence is one of the adaptations to increased salinity. These results are consistent with the investigations on other halophytes as Atriplex patula [43] and Suaeda maritima [27] and glycophytes as Gossypium hirsutum [43] and Hordeum vulgare [19].
In the present study, the stomatal density and dimensions decreased particularly in the upper than in the lower surface under high salinity (Table 3). This could be explained with the changes in the leaf area under salt stress. This was further supported by Curtis and Lauchli [44] who reported a negative relationship between stomata density and leaf size under stress conditions. On the other hand, it has been known for a long time that high salinity has a decreasing effect on stomata number [45], stomata index [46] and this complies with the results of the present study. Robinson et al. [47] reported that the stomata get closed as a response to salt stress due to the increase in Na+ and Cl− ions and the decrease in K+ amount in the leaves of plants and so they can survive since transpiration and water loss decreases. The present data agrees with previous data which reports that the salt stress stimulates reduced stomatal density [16, 28].
Salt tolerant species generally possess thick epidermis and cuticle. This serves as an effective mechanism against water loss during limited moisture availability [48, 49]. In the present study, thickness of cuticle increases with the increasing salt level (Table 3).
Another remarkable leaf anatomical feature observed in S. marina under salt stress was a significant increase in the number of calcium oxalate crystals (Fig. 6). Therefore, it can be assumed that calcium ions are involved in increasing the salt tolerance in different ways. It ensures the preventing of water loss through the leaf. The same findings were obtained by Hajibagheri et al. [50, 51] in Suaeda maritima and by Bray and Reid [46] in Phaseolus vulgaris.
Leaves of S. marina showed modifications in the increase in the thickness of the midrib. (Table 3; Fig. 8a–c). Hameed et al. [38] stated that this anatomical feature may be helpful in the storage of ions inside the plant body. Examples of leaf succulence are common in Kandelia candel, a dicot species [28], grassland legumes [52] and halophytes [53].
Increased leaf bundle sheath thickness under the high salinity level may be of importance as it would provide rigidity to the leaf. These results show that the leaf bundle sheath thickness of S. marina increased with high salinity (Table 3; Fig. 8a–c). Similar results have been shown in Nitraria retusa, Atriplex halimus and Medicago arborea [17].
In the present study the changes in the root, stem and leaf anatomy of S. marina under increasing salinity levels were investigated. It can be stated that the root and stem anatomical mechanisms in the toleration of salinity are increasing aerenchyma, thickness of periderm, vascular tissues and sclerenchyma. This can be described as an adaptive strategy in the facilitation of water transport. In the studied species leaf anatomical parameters showed significant changes with high salt concentrations. The results obtained in the present study indicate that salt stress resulted in an increase of cuticle and parenchyma thickness (succulence) of the leaf. Furthermore, thickness of midribs and vascular bundle sheath increased under high salinity. The anatomical strategy like reducing the stomatal dimensions and density seems to be an efficient strategy regarding their tolerance against salinity and reducing water loss. It can be assumed that a significant increase in the number of calcium oxalate crystals in S. marina leaf under salt stress may be to ensure prevention of water loss through the leaf. From the present results, it can be concluded that the anatomical mechanisms used by S. marina to cope up with salinity might play a crucial role in adaptation to unfavourable conditions of plants.
References
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stres responses in plants: present and future. In: Ahmad P, Prasad MNV (eds) Abiotic stres responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 1–19
Huang J, Redmann RE (1995) Response of growth, morphology and anatomy to salinity and calcium supply in cultivated and wild barley. Can J Bot 73:1859–1866
Shannon MC (1997) Adaptation of plants to salinity. Adv Agron 60:76–119
Isla R, Agragues R, Royo A (1998) Validity of various physiological traits as screening criteria for salt tolerance in barley. Field Crops Res 58:97–107
Muscolo A, Panuccio MR, Sidari M (2003) Effects of salinity on growth, carbohydrate metabolism and nutritive properties of kikuyu grass (Pennisetum clandestinum Hochst). Plant Sci 164:1103–1110
Shannon MC, Grieve CM., Francois LE (1994) Plant environment interaction. In: Wilkinson RE, Dekker M (ed) Whole plant response to salinity. New York, pp 199–24
Popp M (1995) Salt resistance in herbaceous halophytes and mangroves. In: Behnke HD, Luttge U, Esser K, Kadereit JW, Runge M (eds) Progress in botany. Springer, Berlin, pp 416–429
Poljakoff-Mayber A (1988) Ecological-physiological studies on the responses of higher plants to salinity and drought. Arid Zone Res 6:163–183
Akram M, Akhtar S, Javed IH, Wahid A, Rasul E (2002) Anatomical attributes of different wheat (Triticum aestivum) accessions/varieties to NaCl salinity. Int J Agric Biol 4:166–168
Wahid A (2003) Physiological significance of morpho-anatomical features of halophytes with particular reference to Cholistan flora. Int J Agri Bio 5:207–212
Choat B, Ball MC, Luly JG, Holtum JAM (2005) Hydraulic architecture of deciduous and evergreen dry forest tree species from north-eastern Australia. Trees (Berl) 19:305–311
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Polic D, Lukovic J, Zorići L, Boza P, Merkulov L, Knezević A (2009) Morpho-anatomical differentiation of Suaeda maritima (L.) Dumort. (Chenopodiaceae) populations from inland and maritime saline area. Cent Eur J Biol 4(1):117–129
Hameed M, Batool S, Naz N, Nawaz T, Ashraf M (2012) Leaf structural modifications for drought tolerance in some differentially adapted ecotypes of blue panic (Paniculum antidotale Retz.). Acta Physiol Plant 34:1479–1491
Çavuşoğlu K, Kılıç S, Kabar K (2007) Some morphological and anatomical observations during alleviation of salinity (NaCl) stress on seed germination and seedling growth of barley by polyamines. Acta Physiol Plant 29:551–557
Çavuşoğlu K, Kılıç S, Kabar K (2008) Effects of some plant growth regulators on leaf anatomy of radish seedlings grown under saline conditions. J Appl Biol Sci 2:47–50
Boughalleb F, Denden M, Ben Tiba B (2009) Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol Plant 31:947–960
Koyro HW (2006) Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ Exp Bot 56:136–146
Kılıç S, Çavuşoğlu K, Kabar K (2007) Effects of 24-epibrassinolide on salinity stress induced inhibition of seed germination, seedling growth and leaf anatomy of barley. Suleyman Demirel Univ Fac Arts Sci J Sci 2:41–52
Vijayan K, Chakraborti SP, Ercisli S, Ghosh PD (2008) NaCl induced morpho-biochemical and anatomical changes in mulberry (Morus spp.). Plant Growth Regul 56:61–69
Ungar IA (1967) Influence of salinity and temperature on seed germination. Ohio J Sci 67:120–123
Cheeseman JM, Bloebaum P, Enkoji C, Wickens LK (1985) Salinity tolerance in Spergularia marina. Can J Bot 63:1762–1768
Ghazanfar SA, Nasir YJ (1986) Caryophyllaceae. In: Nasir E, Ali SE (ed) Flora of Pakistan, Department of Botany, University of Karachi, No 175, pp 1–125
Khan MA, Gul B (2006) Halophyte seed germination. In: Khan MA, Weber DJ (eds) Eco-physiology of high salinity tolerant plants. Springer Publications, Netherlands, pp 11–30
Keiffer CH, Ungar IA (1997) The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am J Bot 84:104
Mesdner H, Mansfield TA (1968) Physiology of stomata. McGraw-Hill, London
Hajibagheri MA, Yeo AR, Flowers TJ (1985) Salt tolerance in Suaeda maritima (L.) Dum.: fine structure and ion concentrations in the apical region of roots. New Phytol 99:331–343
Hwang YH, Chen SC (1995) Anatomical responses in Kandelia candel (L.) druce seedlings growing in the presence of different concentrations of NaCI. Bot Bulletin Acad Sin 36:181–188
Anderson CE (1974) A review of structure in several north Narolina salt marsh plants. In: Reimold RJ, Queen WH (eds) Ecology of halophytes. Academic Press, New York, pp 307–344
Grigore MN, Toma C (2008) Ecological anatomy investigation related to some halophyte species from Moldovia. Plant Biol 53:23–30
Cheng KT, Chou CH (1997) Ecotypic variation of Imperata cylindrica populations in Taiwan: I. Morphological and molecular evidences. Bot Bull Acad Sinica (Taiwan) 38:215–223
Akhtar J, Gorham J, Qureshi RH, Aslam M (1998) Does tolerance of wheat to salinity and hypoxia correlate with root dehydrogenase activities or aerenchyma formation? Plant Soil 201:275–284
Strogonov BP (1962) Fisioloithcheskie osnovy soleustoitchivosti rastenii (Physiological bases of salt tolerance in plants). Akademia Nauk SSSR, Moskova
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree physiology monograph No. 1. Heron Publishing, Victoria, pp 1–29
Waisel Y (1972) Biology of halophytes. Academic Press, New York
Grigore MN, Toma C (2007) Histo-anatomical strategies of Chenopodiaceae halophytes: adaptive, ecological and evolutionary implications. WSEAS Trans Biol Biomed 4:204–218
Hameed M, Ashraf M, Naz N, Al-Qurainy F (2010) Anatomical adaptations of Cynodon dactylon (L.) Pers., from the salt range Pakistan, to salinity stress. I. Root and stem anatomy. Pak J Bot 42(1):279–289
Hameed M, Ashraf M, Naz N (2009) Anatomical adaptations to salinity in cogon grass [Imperata cylindrica (L.) Raeuschel] from the salt range. Pak Plant Soil 322:229–238
Datta SK, Som J (1973) Note on the effect of salinity on the structural changes in the stem of rice varieties. Ind J Agric Sci 43:614–617
Awasthi O, Pathak RK (1999) Effect of salinity levels on survival and anatomy of four scion cultivars budded on Indian jujuba. Hort J 12:53–59
Baloch AH, Gates PJ, Baloch V (1998) Anatomical changes brought about by salinity in stem, leaf and root of Arabidopsis thaliana (L.) Heynh (thale cress). Sarhad J Agric 14:131–142
Debez A, Saadaoui D, Ramanib B, Ouerghi Z, Koyro HW, Huchzermeyer B, Abdelly C (2006) Leaf H-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot 57:285–295
Longstreth DJ, Nobel PS (1979) Salinity effects on leaf anatomy. Plant Physiol 63:700–703
Curtis PS, Lauchli A (1987) The effect of moderate salt stress on leaf anatomy in Hibiscus cannabinus (Kenaf) and its relation to leaf area. Am J Bot 74:538–542
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Q Rev Biol 61:313–337
Bray S, Reid DM (2002) The effect of salinity and CO2 enrichment on the growth and anatomy of the second trifoliate leaf of Phaseolus vulgaris. Can J Bot 80:349–359
Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol 73:238–242
Yujing Z, Yong Z, Zizhi H, Shunguo Y (2000) Studies on microscopic structure of Puccinellia tenuiflora stem under salinity stress. Grassl China 5:6–9
Ristic Z, Jenks MA (2002) Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J Plant Physiol 159:645–651
Hajibagheri MA, Hall JL, Flowers TJ (1983) The structure of the cuticle in relation to cuticular transpiration in leaves of the halophyte Suaeda maritima (L.) Dum. New Phytol 94:125–131
Hajibagheri MA, Hall JL, Flowers TJ (1984) Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum. J Exp Bot 35:1547–1557
González LM, López RC, Fonseca I, Ramýrez R (2000) Growth, stomatal frequency, DM yield and accumulation of ions in nine species of grassland legumes grown under saline conditions. Pastos Forrajes 23:299–308
Flowers TJ, Colmer TDA (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akcin, T.A., Akcin, A. & Yalcin, E. Anatomical Adaptations to Salinity in Spergularia marina (Caryophyllaceae) from Turkey. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 85, 625–634 (2015). https://doi.org/10.1007/s40011-014-0386-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-014-0386-8