Abstract
A huge amount of plastic wastes caused disastrous effects to the environment. Pertaining to the adverse effects imposed by these wastes, a significant progress is observed in the development of biodegradable plastics using natural raw materials such as starch from potato or corn to produce biodegradable plastic materials with similar functionalities to that of petroleum-based polymers. The present study aimed to investigate the effects of glycerol and sorbitol as plasticizing agents on the properties and biodegradability of potato-based bioplastic. Bioplastic films were produced by varying the type and concentration of plasticizers at the ratio of 15, 30 and 45 (wt%) using solution casting technique. The characterization results revealed that plasticized films were less fragile and more flexible compared to those of 0% plasticizer. Plasticized film with higher concentration of plasticizers demonstrated lower tensile strength with increased elongation at break value. Higher water vapour transmission was also observed for plasticized films as the concentration of plasticizers was increased, indicated higher water permeability but lower water absorption ability of the films. The addition of plasticizers effectively reduced the swelling and water retaining capacity of potato-based biodegradable plastic films. From the Fourier Transform Infrared spectra, similar absorption bands were obtained for both the pure potato starch powder and plasticized films, indicating no significant chemical interaction between the starch and plasticizers which could alter their respective functional groups. Insignificant biodegradation was observed using soil burial test for one week, but slight mass reduction and plastic swelling were observed at low plasticizer concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum based plastics are artificially created by humans to produce packaging materials, bottles, and parts of vehicle such as side door due to their outstanding versatility, mechanical strength, and barrier properties. These carbon-based polymers are mainly produced from petroleum that is non-renewable such as polyethylene and polyolefins (Kuruppalil 2011). Apart from good tensile properties, petroleum based plastics are also high in chemical stability and demonstrate good resistance to microbial degradation in the atmosphere. In fact, Ooi et al. (2012) reported that approximately 4 centuries are required to fully decompose these plastics. Packaging wastes disposed by human are categorized as municipal solid wastes (MSW) that require proper treatment to prevent solid waste generation and accumulation in the environment. These packaging materials are mostly synthetic polymeric bags manufactured by petrochemical-based polymer which are virtually non-biodegradable (Song et al. 2009). As a result of increasing plastic waste production rate, the demand for landfill also increases in recent years.
Biodegradable plastic has been developed to overcome these issues particularly to reduce or replace the utilization of synthetic plastic. The comparison between synthetic and biodegradable plastic was reported by Sandhu and Shakya (2019), concluding that biodegradable plastic is more environmental friendly because it becomes a food source for other living organisms upon its decomposition. In contrast, synthetic plastic poses threat to living organisms such as marine birds (Franeker and Law 2015), sea turtles (Nelms et al. 2016) and filter feeders (Bonello et al. 2018) as they are made of toxic compounds such as hydrocarbon. Apart from the risk of become entangled in plastic debris, Azzarello and van Vleet (1987) reported that ingestion of these debris causes gastrointestinal blockage and affects the level of gastric enzymes and steroid hormones in the organisms, especially marine birds. Nevertheless, the commercial deployment of biodegradable plastic at industrial scale to replace the utilization of conventional plastic remains as a challenge. Based on the recent report by Sandhu and Shakya (2019), about 200 million tons of synthetic plastic are produced every year with only 0.7 million tons of biodegradable plastic are synthesized. Pertaining to this issue, poor physical performance, variability of feedstock associated with geographical location, time of harvest for feedstock, and complexity of production procedures are the main factors that contributed to the low production rate of biodegradable plastics (Mekonnen et al. 2013).
It is worth to highlight that biodegradable plastic has surpassed synthetic plastic in terms of some major aspects or properties such as higher rate of biodegradability, non-toxic to the environment, and serves as organic food source for living organisms (Song et al. 2009). However, there are problems associated with biodegradable plastics that include its properties and high manufacturing cost. In terms of properties, biodegradable plastics are often correlated to weaker mechanical properties as compared to synthetic plastics such as tensile strength, elongation at break and flexibility. Moreover, according to Song et al. (2009), the cost of bioplastic polymers production from various renewable sources are relatively higher in comparison with the conventional plastic as it requires complex processes to produce a stable biodegradable film.
Various researches are conducted on starch based biodegradable plastic which is one of the alternatives for petroleum based plastic. Starch is considered as one of the most promising natural raw materials to produce biodegradable plastic due to its abundant availability and high biodegradability characteristic. Starch can be extracted from corn, cereal grain, rice, potato and etc. In recent years, there has been an increasing trend in exploring the physicochemical characteristics of biodegradable film synthesized using the starch of banana (Zamudio-Flores et al. 2006); cassava (Souza et al. 2012; Maran et al. 2013), corn (Monero et al. 2014; Dai et al. 2015), potato (Talja et al. 2007; Hu et al. 2009; Fonseca et al. 2015) and sago (Abdorreza et al. 2011).
Starch biodegradable plastics are widely used in packaging application due to good barrier properties towards oxygen and carbon dioxide (Ballesteros-Mártinez et al. 2020). Nevertheless, native starches are stable and brittle due to the strong intermolecular hydrogen bonding between amylopectin and amylose macromolecular network chains which is not process-able (Ma and Yu 2004). Therefore, addition of plasticizers is needed to improve the properties and characteristics of starch based biodegradable plastic. Numerous plasticizers are available such as glycerol, sorbitol, ethylene, urea, and formamide for this purpose. Though the role of plasticizers to overcome the issue of brittleness with starch based biodegradable plastic is widely reported, direct comparison of the effects of different plasticizers as well as its compositions in the starch blend towards the tensile, thermal and barrier properties of the plastic is still scarce in the literature.
In the present work, glycerol and sorbitol are used as the plasticizing agent to enhance the plasticity of the bioplastic produced. It is expected that with the synthesis of bioplastic, the conventional petrochemical-based polymers can be replaced which can contribute to a more sustainable environment and offers a new direction for the plastic industry to venture into. By comparing the properties and characteristics of the bioplastic synthesized using different plasticizers of varying concentrations, different formulations of the bioplastic blends can be further explored to make them a fit for different applications such as packaging, food services, agriculture and horticulture etc. The experiments of this work were conducted at the Chemical Engineering Laboratory of UCSI University, Kuala Lumpur, Malaysia from January to March 2020. The tensile tests were performed at Universiti Tunku Abdul Rahman, Perak, Malaysia.
Materials and methods
Bioplastics with glycerol as plasticizer
300 g of pure starch powder was mixed with distilled water at the ratio of 8% (w/w). The mixture was then heated at 95 ± 2 °C for 15 mins under constant stirring. This was to provide homogenous dispersion by disintegrating the starch granules. After that, glycerol plasticizers were added into the dispersion at different concentrations, namely 0%, 15%, 30% and 45% (w/w) by using the starch amount as the basis. The heating process was then resumed for another 15 mins at 95 ± 2 °C. Subsequently, the heated mixture was evenly spread onto a casting plate. All bioplastic films were dried at 45 °C until constant weight was obtained. Lastly, the dried films were peeled off from the casting plate and stored in desiccators prior to the characterization studies.
Bioplastics with sorbitol as plasticizer
The procedures involved in the synthesis of biodegradable film in this section were similar to the one of glycerol as plasticizer, with the exception that sorbitol was added into the dispersion at different concentrations ranging from 15%, 30% to 45% (w/w) by using starch amount as the basis.
Characterization of bioplastics
Fourier transform infrared (FTIR) spectroscopy
The FTIR analysis was performed to identify the functional groups present in the bioplastics produced. The spectra were obtained using a Nicolet iS 5 FTIR Spectrometer (Thermo Fisher Scientific) in the range 4000 cm−1 to 400 cm−1.
Tensile test
The tensile test was carried out using a Universal Instron Testing Machine. The parameters of the tensile machine, specimen, and conditioning were done according to ASTM D882. The crosshead speed was set at 200 mm/min. The average value of three tests were taken for each bioplastic composition. Some of the important parameters taken from the tensile tests were the tensile strength, which was the maximum tensile stress sustained, percent elongation at break, which was the elongation of the sample at the point of rupture, and Young’s Modulus, which was the stress to strain ratio below the elastic limit.
Water vapour transmission test
The water vapour transmission test was conducted according to ASTM E96 using the desiccant method. An impermeable plastic cup was filled with desiccant silica and firmly sealed with the plastic film. This dish was then being placed in a controlled environment (desiccator) along with a cup of distilled water. The sealed cups were weighed every 24 h. The water vapour transmission rate (WVTR) was calculated using Eq. 1.
where A was the test area (m2) and G/t was the slope of the straight line (g/h) calculated using linear regression.
Water absorption test
The water absorption of the bioplastic films was determined using the procedures as reported by Azahari et al. (2011) with some modifications. Generally, all dried films were cut into 2 cm × 2 cm and the initial weight was recorded. They were then immersed in distilled water at room temperature. The final weight measurement was performed after 24 h. In order to calculate the amount of water absorbed, the moisture on the surface of the film was removed and then weighed. The moisture absorbed by each sample was calculated using Eq. 2.
Biodegradability study—soil burial test
All bioplastic films were cut into 2 cm × 2 cm and then buried in a pot filled with garden soil at a depth of 10 cm. The pot was left inside the laboratory and the soil was wet with water at regular intervals to maintain its humidity. The film was removed from the pot after 1 week and the final weight was measured and recorded.
Results and discussion
General appearance potato starch plasticized films
Figure 1 shows the image of potato-based biodegradable plastics synthesized using different types and concentrations of plasticizing agent. It was found that bioplastic film without the addition of plasticizer was brittle and fragile, coupled with hard and cracked surface. As explained by Sanyang et al. (2016), this observation could be correlated to the strong hydrogen bonds within the inter- and intra- molecular structure of potato starch which resulted in less mobility of the macromolecular chains. On the contrary, bioplastic films with the addition of plasticizers (both glycerol and sorbitol) were found to be less fragile with smoother surface. The descriptions of the general appearance of all bioplastics synthesized in this study are summarized in Table 1.
Based on the observations recorded in Table 1, bioplastic films with 45% plasticizing agent regardless of the type, demonstrated higher flexibility compared to those of 30% and 15%. This was due to the low molecular size of plasticizers which were able to penetrate into the intermolecular spaces of polymer chains (George 2012). As a result, the molecular mobility was increased by weakening the hydrogen bonding of potato-based biodegradable plastics. To compare the effect of different plasticizers used, it was observed that glycerol plasticized films were more flexible and sticky, yet less transparent compared to those of sorbitol plasticized films, at the same concentration (Fig. 1).
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of potato-based biodegradable plastics with different concentrations of plasticizing agents were compared. Figure 2 shows the FTIR spectra obtained from potato starch powder and 0% plasticized film. The peaks of both samples were similar to each other with the exception that the 0% plasticized film showed higher band intensities compared to the potato starch powder. In other word, there is no change in terms of functional groups before and after the potato starch powder was used to synthesize the bioplastics.
The FTIR spectra of potato-based bioplastics with glycerol and sorbitol as plasticizing agent at different concentrations are presented in Fig. 3a and b, respectively. It was observed that the broad absorption bands of all spectra in Figs. 2 and 3 were similar. This indicated that there was no significant chemical interaction between the starch and plasticizers which could alter their respective functional groups, regardless of the type and concentration of plasticizer used. According to Sultan and Johari (2017), the peaks within the range of 3262–3289 cm−1 were attributed by the presence of O–H group involved in the hydrogen bond formation at the end of the polymer chains of starch and plasticizing agents. From Fig. 3a and b, it was observed that the plasticized bioplastics demonstrated higher band intensity in this region compared to the sample of 0% plasticizing agent. As reported by Ooi et al. (2012), this could be due to the addition of polyol plasticizing agents such as glycerol and sorbitol that increased the concentration of O–H functional group in the bioplastics.
The peaks observed at the range of 2919–2929 cm−1 in Figs. 2 and 3 were classified as C-H aliphatic absorption peaks which indicated the presence of C–H bond stretching of CH2 groups in the starch structure (Sanyang et al. 2016). In addition, the peaks within the region 1639–1645 cm−1 were referred to as the result of water molecules adsorbed in the amorphous region of starch (Sultan and Johari 2017). The most intense peak was consistently observed within the range of 990–1000 cm−1 for all samples (Figs. 2 and 3). It was correlated by Sanyang et al. (2016) and Sultan and Johari (2017) to the presence of C–O bond stretching of C–O–C groups in the anhydro-glucose rings of starch.
In short, the results of FTIR analysis revealed that all bioplastic films showed the absorption peaks at similar regions irrespective of the concentration and type of plasticizing agent used. The main characteristic of these bands remained similar except for the band intensities. Therefore, it can be deduced that the functional groups did not change before and after the addition of plasticizers during synthesis of the plastic films.
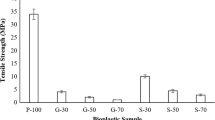
Tensile strength
The effects of different types and concentrations of plasticizing agent on the tensile strength of potato-based biodegradable plastics films are presented in Fig. 4. It was found that the samples with lower concentration of plasticizer (15% glycerol and 15% sorbitol) demonstrated the highest tensile strength compared to other concentrations of plasticizers added. This was probably due to the domination of strong hydrogen bonding within starch-starch intermolecular interaction without much disruption by the starch-plasticizer attraction at lower concentrations of plasticizer (Zahiruddin et al. 2019). However, a significant decrease in the tensile strength was observed when both plasticizers were added at increasing concentration from 15 to 45%. The results were consistent with the work of Salit et al. (2016) whereby the authors reported that the increase of plasticizer concentration resulted in a decrease of tensile strength of cassava‐starch‐based films. Based on Fig. 4, the tensile strength of sorbitol plasticized films reduced from 25 to 6 MPa whereby that of glycerol plasticized film decreased from 11 to 5 MPa as the concentration was increased from 15 to 45%. According to Ballesteros-Mártinez et al. (2020), this phenomenon occurred mainly due to the effect of plasticizing agents which weakened the strong intra-molecular attraction within the starch polymer chain. Moreover, plasticizers promoted the formation of hydrogen bonds between starch and plasticizer molecules. As a result, the tensile strength of potato-based biodegradable plastics films in this study decreased due to the disruption and weakening of hydrogen bonds between the starch polymer chains (Muscat et al. 2012).
In comparing the effect of different plasticizers, it was observed that at the same concentration (with the most significant difference at 15%), the glycerol plasticized film showed lower tensile strength as compared to the sorbitol counterpart. These results could be explained by the difference in molecular weight of glycerol (92 g/mol) and sorbitol (182 g/mol) in which glycerol molecules could facilitate better interaction between the starch-glycerol molecular chains with its smaller molecular size. As a result, glycerol demonstrated higher efficiency in plasticizing the potato-based biodegradable plastic films as compared to sorbitol. The tensile strength profiles presented in Fig. 4 were consistent with the findings of Chéret et al. (2005) whereby the authors reported that sorbitol plasticized films were highly resistant to breakage compared to glycerol plasticized films at the same plasticizer concentration. Moreover, the presence of sorbitol at 15% resulted in higher tensile strength than the 0% plasticized film. This observation was in agreement with the report by Aguirre et al. (2013) which stated that sorbitol enhanced the properties of plastic film by increasing the resistance toward breakage.
On the other hand, elongation at break is defined as the extendibility of plastic film starting from initial length to the maximum breaking point (Djafari 2016). This parameter indicates the stretchability and flexibility of plastic films which is important for packaging industry depending on their intended application. The results of elongation at break for all types of bioplastic film in this study were of opposite trend to the tensile strength. As presented in Fig. 5, the elongation at break of bioplastic films increased with the increasing plasticizer concentration for both glycerol and sorbitol. Ballesteros-Mártinez et al. (2020) explained that this was mainly due to the reduction of intermolecular bonds between amylose and amylopectin of the starch matrix when plasticizers were added. Thus, there was formation of hydrogen bonds between the plasticizer and starch molecules. As a result, reconstruction of starch molecular chains reduced the crystallinity and thus enhanced the flexibility of plastic films.
Figure 6 presents the results of Young’s modulus which is an important parameter implying the flexibility and brittleness of plastic films. In this study, the results revealed that as the concentration of plasticizers increased, the Young’s modulus value reduced consistently for both glycerol and sorbitol. In fact, the highest Young’s modulus (990 MPa) was recorded for the bioplastic films without plasticizing agent, implying its high brittleness. In comparing the effects of glycerol and sorbitol, both plasticized films resulted in the highest Young’s modulus value at the concentration of 15%. As the concentration of plasticizers was further increased up to 45%, similar trend was observed for both glycerol and sorbitol whereby the Young’s modulus values dropped. The results presented in Fig. 6 proved that the addition of plasticizers enhanced the flexibility and durability of the bioplastic films which resulted in slower rate towards the fracture point. This was in accordance to the findings by Sanyang et al. (2015) who reported that the presence of plasticizers modified the starch’s fracture mechanism from swift brittle fracture at low strains to elastoplastic fracture at higher strain.
Water vapour transmission
Generally, potato-based biodegradable plastics exhibit weak water vapour barrier due to their hydrophilic properties (Othman et al. 2017). In the present study, water vapour transmission rate (WVTR) test was carried out to investigate the water vapour permeability of potato-based biodegradable plastic films produced from different types and concentrations of plasticizer. The results of the WVTR are presented in Fig. 7.
Based on Fig. 7, when the concentration of plasticizer was increased from 0 to 45%, the WVTR increased from 62 to 160 g day−1 m−2 for glycerol plasticized films and 62 to 131 g day−1 m−2 for the sorbitol plasticized films. Positive correlation between the concentration of plasticizer with the WVTR of bioplastic films regardless of the type of plasticizing agent was clearly observed where similar findings had also been reported by Sanyang et al. (2015). One of the most plausible explanations for the observation was that high plasticizer concentration enhanced the flexibility and mobility of starch polymer chains (as proven in tensile strength measurement) resulted in looser network through the structural modification of starch-starch molecular interactions. In contrast, bioplastic films without addition of plasticizing agent (0% plasticizer) showed the lowest WVTR which could be due to more compact and denser starch network in the structure. Also, 15% plasticized films demonstrated relatively lower WVTR compared to the one with 30% and 45% plasticizers due to weaker disruption of starch-starch molecule interaction by low concentration of plasticizer molecules.
In comparing the effect of glycerol and sorbitol as the plasticizing agent, it can be seen that sorbitol plasticized films consistently resulted in lower WVTR compared to glycerol plasticized films. This could be attributed by the hydrophilic nature of glycerol which induced better absorption of water compared to sorbitol (Pagliaro and Rossi 2010). Moreover, the high molecular structure of sorbitol which was similar to the glucose units resulting into stronger intermolecular interactions between the sorbitol and starch polymer chains (Zahiruddin et al. 2019). Consequently, the sorbitol plasticized films demonstrated lower interaction with water molecules. The WVTR measures the transmission of water vapour through the bioplastic. With higher WVTR value, it indicates possible higher loss of water vapour from the content of the plastic. As explained by Basha et al. (2011), packaging material with high water vapour permeability has a high potential for use in packaging of fresh produce (leafy vegetables and strawberries) whereby the shelf life of these products can be extended. The growth of mold or fungus can be reduced as well. Based on the WVTR results in the present study, glycerol is the better plasticizer option for the synthesis of potato-based bioplastic designed for the application of packaging of fresh produce and food waste compared to sorbitol.
Water absorption test
One of the major concerns of potato-based biodegradable plastic films is the resistance and diffusivity when in contact with water molecules, particularly for packaging application. In this study, the water absorption test was performed for 7 days at room temperature and the mass of all the synthesized bioplastic films was recorded every day. This testing is important to investigate the stability of plastic film under humid and moisture conditions. Generally, all bioplastic films were immersed in distilled water in order to investigate the effects of the presence of plasticizing agents with varied concentrations on the hydrophilic nature of starch plastic films. When the bioplastic films were immersed into the distilled water, water molecules diffused into the network chains of the plastic. As a result, the bioplastic films absorbed water and started to swell. At the initial stage of the absorption process, the mass of the films increased gradually owing to high availability of vacant sites of the active hydroxyl groups of the bioplastic films. As time goes by, the water absorption eventually reached an equilibrium state whereby the bioplastic films could not absorb any more water molecules due to saturation of active sites (Mali et al. 2005).
Table 2 tabulates the result of water absorption and mass gained by all the bioplastic films synthesized using different types and concentrations of plasticizer. The results showed that the ability to absorb water reduced accordingly when the concentration of plasticizers was increased, with the 0% plasticized film demonstrated high water absorption rate due to the hydrophilic properties of starch-based films. This proved that the addition of plasticizer reduced the hydrophilic nature of potato-based biodegradable plastic films hence resulted in reduced water absorption ability of the films.
When glycerol was used as the plasticizer, at the concentration of 15%, it was found that the water absorption characteristic was not significantly different from the un-plasticized film. However, as the concentration was further increased to 30% and 45%, significant reduction in the water absorption percentage was recorded. Similar trend was observed in sorbitol plasticized films in which the water absorption ability gradually decreased due to the increasing sorbitol concentration. Based on the results obtained, it was also obvious that glycerol plasticized films demonstrated higher water absorption and mass gained compared to sorbitol plasticized films at all concentrations investigated in this study. This was similar to the work of Sanyang et al. (2015) and Müller et al. (2008) who reported that sorbitol plasticized films were more resistant to water absorption due to their stronger hydrogen bonding within the starch intermolecular structure. In other word, sorbitol reduced the interactions between intermolecular hydrophilic functional groups of starch and water molecules more effectively compared to glycerol. The addition of both plasticizers, however, was proven to effectively reduce the swelling and water retaining capacity of potato-based biodegradable plastic films in this study.
Soil burial test
All synthesized bioplastic films were subjected to soil burial test in order to investigate their respective biodegradability. The testing was performed for one week, with moisture content of the soil was maintained throughout the period. Table 3 tabulates the weight loss of all bioplastic samples investigated after one week. It was observed that 0% plasticized film showed the highest weight reduction followed by the sample with 15% glycerol and sorbitol plasticized films, respectively. The result indicated the possibility of enzymatic biodegradation within the plastic films which consisted of higher concentration of starch but lower concentration of plasticizers. Naturally, starch consists of highly complex polymer chains. The polymer chains may experience cleavage enzymatically and disintegrate in the soil. This led to the formation of monomers (short chains) that could penetrate into the membranes of microorganism and subsequently served as carbon source for the microorganism. At higher concentrations of plasticizers, the biodegradability of the bioplastics was hampered due to lower concentration of starch carbon sources. Though the soil burial test was conducted for only one week in this study, the effect of the presence of plasticizers onto the biodegradability of the synthesized bioplastics was significant.
Conclusion
In this work, potato-based biodegradable plastic films were successfully produced from pure potato starch powder. The FTIR spectra showed similar functional groups obtained from the pure starch powder and plasticized films regardless of the type of plasticizing agent added. Also, at lower plasticizer concentrations, the synthesized bioplastics consistently demonstrated higher tensile strength, lower elongation at breaks and higher Young’s modulus value. The role of glycerol and sorbitol as the plasticizer in modifying the brittle characteristic of starch film into ductile was evidenced. It was found that glycerol plasticized films showed higher WVTR compared to sorbitol plasticized film due to the difference in hydrophilicity which affected the starch intermolecular network of the films. In water absorption test, as the concentration of plasticizers increased, the water adsorption ability of all bioplastic films reduced, with glycerol plasticized films demonstrated better water absorption ability compared to sorbitol plasticized films. Lastly, the biodegradability study via soil burial test revealed that the bioplastic films did not biodegrade significantly within the first 7 days of the experiment. However, the effect of the presence of plasticizers onto the biodegradability of the synthesized bioplastics was noticeable with the highest weight loss recorded for un-plasticized film, followed by the lowest concentration (15%) of both glycerol and sorbitol plasticized films. The preliminary results obtained in the present study provide insights into the future work to focus specifically on either glycerol or sorbitol as the suitable plasticizer to produce bioplastics that demonstrate the required characteristics for a specific application (packaging, food services, agriculture and horticulture).
Availability of data and material (data transparency)
All data and materials provided in the manuscript originated from the experimental work performed by the authors.
References
Abdorreza MN, Cheng LH, Karim AA (2011) Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll 25(1):56–60. https://doi.org/10.1016/j.foodhyd.2010.05.005
Aguirre A, Borneo R, León AE (2013) Properties of triticale protein films and their relation to plasticizing–antiplasticizing effects of glycerol and sorbitol. Ind Crops Prod 50:297–303. https://doi.org/10.1016/j.indcrop.2013.07.043
Azahari NA, Othman N, Ismail H (2011) Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J Phys Sci 22(2):15–31
Azzarello M, van Vleet ES (1987) Marine birds and plastic pollution. Mar Ecol Prog Ser 37:295–303
Ballesteros-Mártinez L, Pérez-Cervera C, Andrade-Pizarro R (2020) Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J 20:1–9. https://doi.org/10.1016/j.nfs.2020.06.002
Basha RK, Konno K, Kani H, Kimura T (2011) Water vapor transmission rate of biomass based film materials. Eng Agr Environ Food 4(2):37–42. https://doi.org/10.1016/S1881-8366(11)80018-2
Bonello G, Varrella P, Pane L (2018) First evaluation of microplastic content in benthic filter-feeders of the Gulf of La Spezia (Ligurian Sea). J Aquat Food Prod Technol 27(3):284–291. https://doi.org/10.1080/10498850.2018.1427820
Chéret R, Chapleau N, Delbarre-Ladrat C, Verrez-Bagnis V, Lamballerie M (2005) Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. J Food Sci 70(8):E477–E483. https://doi.org/10.1111/j.1365-2621.2005.tb11518.x
Dai L, Qiu C, Xiong L, Sun Q (2015) Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem 174:82–88. https://doi.org/10.1016/j.foodchem.2014.11.005
Djafari SR (2016) Physical and mechnical properties of natural fibers. In: Fan M, Fu F (eds) Advanced high strength fibre composites in construction. Elsevier, Amsterdam
Fonseca LM, Gonçalves JR, El Halal SLM, Pinto VZ, Dias ARG, Jacques AC, Zavareze ER (2015) Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT Food Sci Technol 60(2):714–720. https://doi.org/10.1016/j.lwt.2014.10.052
Franeker JA, Law KL (2015) Seabirds, gyres and global trends in plastic pollution. Environ Pollut 203:89–96. https://doi.org/10.1016/j.envpol.2015.02.034
George W (2012) Effect of plasticizers on properties of plasticized materials. In: George E (ed) Handbook of plasticizers, 2nd edn. ChemTec Publishing, Toronto, pp 209–306
Hu G, Chen J, Gao J (2009) Preparation and characteristics of oxidized potato starch films. Carbohydr Polym 76(2):291–298. https://doi.org/10.1016/j.carbpol.2008.10.032
Kuruppalil Z (2011) Green plastics: an emerging alternative for petroleum based plastics?. In: Proceedings of the 2011 IAJC-ASEE International Conference Paper 036: ENT202. https://ijme.us/cd_11/PDF/Paper%2036%20ENT%20202.pdf
Ma X, Yu J (2004) The plastcizers containing amide groups for thermoplastic starch. Carbohydr Polym 57(2):197–203. https://doi.org/10.1016/j.carbpol.2004.04.012
Mali S, Sakanaka LS, Yamashita F, Grossmann MVE (2005) Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr Polym 60(3):283–289. https://doi.org/10.1016/j.carbpol.2005.01.003
Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K (2013) Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym 92(2):1335–1347. https://doi.org/10.1016/j.carbpol.2012.09.069
Mekonnen T, Mussone P, Khalil H, Bressler D (2013) Progress in bio-based plastics and plasticizing modifications. J Mater Chem A 1:13379–13398. https://doi.org/10.1039/C3TA12555F
Moreno O, Pastor C, Muller J, Atarés L, González C, Chiralt A (2014) Physical and bioactiveproperties of corn starch—Buttermilk edible films. J Food Eng 141:27–36. https://doi.org/10.1016/j.jfoodeng.2014.05.015
Müller CMO, Yamashita F, Laurindo JB (2008) Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr Polym 72(1):82–87. https://doi.org/10.1016/j.carbpol.2007.07.026
Muscat D, Adhikari B, Adhikari R, Chaudhary DS (2012) Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J Food Eng 109(2):189–201. https://doi.org/10.1016/j.jfoodeng.2011.10.019
Nelms SE, Duncan EM, Broderick AC, Galloway TS, Godfrey MH, Hamann M, Lindeque PK, Godley BJ (2016) Plastic and marine turtles: a review and call for research. ICES J Mar Sci 73(2):165–181. https://doi.org/10.1093/icesjms/fsv165
Ooi ZX, Ismail H, Bakar AA, Aziz NAA (2012) The comparison effect of sorbitol and glycerol as plasticizing agents on the properties of biodegradable polyvinyl alcohol/rambutan skin waste flour blends. Polym Plast Technol Eng 51(4):432–437. https://doi.org/10.1080/03602559.2011.639827
Othman SH, Edwal SAM, Risyon NP, Basha RK, Talib RA (2017) Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci Technol 37(1):63–70. https://doi.org/10.1590/1678-457x.30216
Pagliaro M, Rossi M (2010) The future of glycerol, 2nd edn. Royal Society of Chemistry, Cambridge
Salit AE, Sapuan M, Jawaid M, Zahari NI (2016) Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch 68:1–11. https://doi.org/10.1002/star.201500366
Sandhu RS, Shakya M (2019) Comparative study of synthetic plastics and biodegradable plastics. Global J Bio-Sci Biotechnol 8(1):107–112
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2015) Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 7(1):1106–1124. https://doi.org/10.3390/polym70x000x
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J Food Sci Technol 53(1):326–536. https://doi.org/10.1007/s13197-015-2009-7
Song JH, Murphy RJ, Narayan R, Davies GBH (2009) Biodegradable and compostable alternatives to conventional plastics. Philos Trans R Soc Lond B Biol Sci 364:2127–2139. https://doi.org/10.1098/rstb.2008.0289
Souza AC, Benze R, Ferrão ES, Ditch C, Coelho ACV, Tadini CC (2012) Cassava starch biodegradable films: Influence of glycerol and clay nanoparticles content on tensile and barrierproperties and glass transition temperature. LWT Food Sci Technol 46(1):110–117. https://doi.org/10.1016/j.lwt.2011.10.018
Sultan NFK, Johari WLW (2017) The development of banana peel/corn starch bioplastic film: a preliminary study. Bioremediat Sci Technol Res 5(2):12–17
Talja RA, Helén H, Roos YH, Jouppila K (2007) Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr Polym 67(3):288–295. https://doi.org/10.1016/j.carbpol.2006.05.019
Zahiruddin SMM, Othman SH, Tawakkal ISMA, Talib RA (2019) Mechanical and thermal properties of tapioca starch films plasticized with glycerol and sorbitol. Food Res 3(2):157–163
Zamudio-Flores PB, Vargas-Torres A, Pérez-González J, Bosquez-Molina E, Bello-Pérez LA (2006) Films prepared with oxidized banana starch: Mechanical and barrier properties. Starch 58(6):274–282. https://doi.org/10.1002/star.200500474
Acknowledgement
This work is financially supported by the UCSI University Research Excellence & Innovation Grant (REIG) with the grant no. REIG-FETBE-2020/019.
Funding
This work is financially supported by the UCSI University Research Excellence & Innovation Grant (REIG) with the grant no. REIG-FETBE-2020/019.
Author information
Authors and Affiliations
Contributions
All authors contributed to the experimental design and results analysis. Material preparation, data collection and analysis were performed by Ng Jun Siang, Kiew Peck Loo, Lam Man Kee, Yeoh Wei Ming and Ho Mui Yen. The first draft of the manuscript was written by Ng Jun Siang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
This paper has not been published and is not being considered for publication elsewhere. All authors have been agreed to submit this paper to International Journal of Environmental Science and Technology.
Consent for publication (include appropriate statements)
This paper has not been published and is not being considered for publication elsewhere. All authors have been agreed to submit this paper to International Journal of Environmental Science and Technology.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Ng, J.S., Kiew, P.L., Lam, M.K. et al. Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics. Int. J. Environ. Sci. Technol. 19, 1545–1554 (2022). https://doi.org/10.1007/s13762-021-03213-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03213-5