Abstract
The demand for petroleum-based plastics is a major threat and has had several negative impacts on the environment. Biodegradable plastics are becoming increasingly attractive due to their cost-effectiveness and eco-friendly properties. This study aimed to develop and evaluate the properties of biodegradable plastic films produced using a low-cost starch source (potato) with a blend of glycerol and sorbitol as the plasticizers. A total of 10 bioplastic films were prepared by varying the type and concentration of plasticizers at a ratio of 0%, 10%, 20%, 30%, and the glycerol–sorbitol blend mixture at a ratio of 2:1, 1:2, and 1:1 (wt%) using the solution casting technique. Several characterization techniques were used to determine the morphological, structural, and chemical properties of the as-synthesized bioplastic films. Fourier transform infrared spectroscopy and morphology measurements verified the confirmation of potato starch bioplastics. Biofilms with glycerol: sorbitol blend plasticizer exhibited higher thermal stability and maximum tensile stress than films plasticized with glycerol. The results also showed that the films with a blend plasticizer exhibited better compatible morphology than those plasticized with glycerol. The results indicate that the synthesized bioplastic samples have great potential for various applications, especially as packaging materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, single-use plastics are used for a plethora of purposes. To meet the ever-increasing demand, plastic production increased from 1.5 million tons in 1950 to 335 million tons in 2016 (Plastics Europe 2018). Subsequently, global carbon dioxide emissions have increased due to the production of synthetic and semi-synthetic plastic polymers (Statista 2020). Moreover, synthetic plastics have introduced potentially hazardous pollutants into the environment, and they enter the food chain in the form of microplastics (MPs) (Sarker et al. 2020). Approximately 20–42% of total global plastics are currently deposited on the land (Zhang et al. 2019). Another 10% flow into aquatic environments, resulting in some ocean currents being termed ‘marine garbage belts” (Thompson 2006). These environmental concerns have led many researchers to develop biodegradable plastics. Agricultural waste has very often been indicated as a low-cost and renewable raw material alternative (Jain and Tiwari, 2015).
Starch is a semi-crystalline polymer consisting of two polysaccharides, amylopectin, and amylose. (Dufresne 2012). Amylopectin is a large branched polymer composed of α-1,4 and α-1,6 bonds located in the main polymer and side polymer chains. Amylose, on the other hand, is a linear or slightly branched polymer composed of glucose molecules interconnected mainly by α-1,4 glycosidic bonds. (Eliasson 2004; Perez et al. 2009). Starch is a natural, renewable, and sustainable polymer isolated from many potential botanic resources such as wheat, corn (maize), rice, potato, and tapioca (Perez et al. 2009). During photosynthesis, starch is produced in plants as the reserve food supply (Chen et al. 2013). Therefore, the benefit of starch-based biodegradable plastic is its abundance of raw materials and easy manufacturing process with low cost (Avella et al. 2009). Starch also has favorable thermoplastic properties and is biodegradable (Imre and Pukánszky 2015). Furthermore, starch-based bioplastic films showed tremendous optical (transparent, colorless), organoleptic (flavorless, odorless), and barrier properties (O2 and CO2 permeability) (Thakur et al. 2019). On the other hand, the strong intermolecular hydrogen bonding between amylopectin and amylose macromolecular network chains in native starches makes them stable and brittle (Ma and Yu 2004). Therefore, starch-based bioplastics could be a promising alternative to hazardous petroleum-based plastics (Shafqat et al. 2020).
However, the efficiency of starch as a raw material for bioplastics depends on its specific structure and composition (Kaseem et al. 2012). Therefore, it is crucial to optimize the starch-based bioplastic films so that they could satisfy the requirements of industrial applications. The addition of plasticizers increases the versatility of the starch-based bioplastics and, therefore, can be combined with several polymeric substances that ensure plentiful utility (Amin et al. 2019). However, the nature and constituents of plasticizers are precarious determinants that significantly impact biofilm properties (Vieira et al. 2011; Jost et al. 2014). These plasticizers are usually small molecules such as polyols (sorbitol and glycerol) that scatter and intercalate among and between polymer chains (Khoramnejadian et al. 2013), disrupting hydrogen bonding and spreading the chains apart, which escalate the biofilm flexibility, as well as intensifying water vapor and gas permeability (García et al. 2000). Therefore, this study was conducted with two main objectives—(a) the preparation and characterization of bioplastics using potato starch and plasticizers (glycerol and sorbitol) and (b) to examine the influence of plasticizers (glycerol and sorbitol) concentrations and/or combinations on the biofilm’s functional biofilm properties.
Materials and methods
Materials
Fresh potatoes were obtained from a local market in Patan, Gujarat, India. Glycerol (G5516) and sorbitol (240,850) were purchased from Sigma Aldrich. Sodium hydroxide (MB095), chloroform (AS040), diethyl ether (AS126), methanol (AS059), and ethanol were purchased from HiMedia.
Extraction of starch from potato
Due to its higher suitability for alkali medium (Xu et al. 2018), water was chosen as the medium for potato starch extraction. Briefly, 1 kg of fresh potato was thoroughly washed with tap water, peeled, grated, and then ground to a paste using a mortar pestle. The potato paste was volumed up to 1 L using distilled water and filtered twice using a cheesecloth. Then, this film-forming solution was allowed to settle at room temperature for 2 h. After discarding the excess water, the residue was collected and dried in an oven at 35 °C for 5 h. Subsequently, the dried residue was powdered with a commercial blender, sieved, and stored at room temperature.
Preparation of bioplastic films
Potato starch-based bioplastic films were prepared by following the protocols described by Farahnaky et al. (2013) with slight modifications. Briefly, 10 g of potato starch powder was mixed with 100 ml of distilled water and stirred mechanically for 15 min at room temperature (25 °C). Then, after adding plasticizer (glycerol or sorbitol) at different concentrations (0–30% w/w starch basis, these solutions were heated on a hot plate at 90 ± 2 °C with constant stirring (500 rpm) for 20 min to ensure complete gelatinization. Again, the glycerol–sorbitol blend was added at the ratios of 2:1, 1:2, and 1:1 (wt%), and these mixers were heated following the similar procedures described above. Later, the mixture was evenly spread on a Petri plate and allowed to dry in a hot air oven at 50 °C. The dried bioplastic films were peeled off from the plate and stored in desiccators until further study. The list of synthesized bioplastic samples is shown in Table 1.
Characterization
Fourier transform infrared (FTIR) spectra of prepared films were recorded by a Perkin Elmer spectrophotometer at wavenumbers ranging from 4000 to 500 cm−1. There were peaks at different positions on the graph, which confirmed the presence of different functional groups in the synthesized bioplastic samples. The surface of the biofilms was studied using a scanning electron microscope (SEM) at an accelerated voltage of 15 kV and up to 2000X magnification. For the SEM analysis, the biofilm samples were cut into 1 × 1 cm and dried in a hot air oven at 50 °C for 5 h. Thermogravimetric analysis (TGA) was executed using the Autograph AG100KNG universal testing machine. The analysis was carried out in a temperature range of 25–800 °C at a 10 °C/min rate and a flow rate of 20 ml/min.
The tensile test was performed using the Autograph AG100KNG universal test machine (Shimadzu, Kyoto, Japan) according to the standard test method for the tensile properties of plastic sheets (ASTM D882-02). The protocol described by (Krishnamurthy and Amritkumar 2019) was followed to adjust machine parameters, specimen settings, and conditioning. The biofilm samples were cut into 100 mm × 25 mm in dumbbell shape with a gauge length of 50 mm. The cross-head speed was modified and set at 3 mm/min. Three specimens were tested for each sample.
Evaluation of bioplastic properties
The thickness of the bioplastic films was determined according to Oluwasina et al. (2017) with slight modifications. Briefly, the biofilms were cut into small pieces (2 cm × 2 cm), and then, 15 measurements were taken at different points in the bioplastic films using a digital Vernier caliper. The thickness of the biofilms was calculated using the following equation.
The mass (M), area (A), and thickness (d) of the bioplastic films were determined. Then, the density of the biofilms was calculated using the equation below (Salgado et al. 2010).
The moisture content was calculated according to the procedures described by Oluwasina et al. (2017). The initial weight (W1) of the bioplastic films (2 cm × 2 cm) was measured, these films were oven-dried at 105 °C for 3 h, and then, the final weight (W2) was determined. The moisture content (MC) was determined using the following equation.
To perform water absorption tests on synthesized bioplastic films, a protocol previously described by Oluwasina et al. (2015) was followed by slight modifications. Briefly, the weight of the oven-dried (for 3 h at 105 °C) biofilm samples was measured, and then, the films were soaked in water for 24 h at room temperature (25 °C) with occasional agitation. Then, after the adhered water from the bioplastic films, the weight (Wt) of these films was measured again. The moisture absorption by the biofilms was calculated using the equation below.
Solubility and biodegradability tests
Bioplastic solubility was checked in water, 10% NaOH, chloroform, 10% H2SO4, diethyl ether, methanol, and ethanol solvents at room temperature and elevated temperature (60 °C) by following the protocol described by Pimpan et al. with slight modifications (Pimpan et al. 2001). Because all samples were soluble in water at both temperatures, the water solubility of the bioplastic samples was quantitatively measured according to the following procedures. Briefly, bioplastic films were cut into 2 cm × 2 cm, weighed, and immersed in 50 ml of the above-mentioned solutions for 24 h with constant shaking (120 rpm) at room temperature. Subsequently, the films were separated and kept in the oven for 24 h at 105 °C to dry. The final weight was measured, and the following equation was used to determine the percentage of weight loss.
The biodegradability of the bioplastic samples was determined using the soil burial degradation test. Films were cut into small pieces (2 cm × 2 cm) and weighed. The films were then buried in garden soil and weighed at regular intervals of 10, 15, 20, 25, and 30 days. Biodegradability was measured by comparing the final weight (Wf) with the initial weight (Wi) using the following equation.
where Wi is the initial weight of the sample and Wf is the final weight after the specific time.
Water vapor transmission rate (WVTR) test
The water vapor transmission test was conducted by utilizing the desiccant method according to the protocol described by Ng and colleagues (Ng et al. 2022). Briefy, desiccant silica was placed within an impermeable plastic cup, which was then tightly sealed with plastic film. A cup of distilled water and this dish were then placed inside a desiccator. Every 24 h, the sealed cups were weighed. The water vapor transmission rate (WVTR) was calculated using the following equation:
where A was the test area (m2) and G/t was the slope of the straight line (g/h) calculated using linear regression.
X-ray diffraction (XRD) test
A Shimadzu 7000 X-ray diffractometer was used for the XRD test, which was carried out using CuKα radiation (λ = 1.5405 Å), recorded between 0° and 60°, and operated at 30 kV and 10 mA.
Differential scanning calorimetry (DSC) test
The DSC test was carried out in accordance with the procedure given by (Yang et al. 2022), with minor adjustments. Dry samples (approximately 5–10 mg) were scanned by a DSC 1/500 (Mettler-Toledo (M) Sdn Bhd) after being held at 45 °C for 1 min and then heated from 45 to 220 °C at a heating rate of 10 °C/min in a sealed aluminum pan. A flow rate of 10 mL/min of high-quality nitrogen gas was employed. The DSC thermograms were used to calculate the glass transition temperature (Tg), melting temperature (Tm), and crystallization temperature (Tc).
Antimicrobial activity test
The disk diffusion technique (Kirby Baeur) was used to assess antimicrobial activity according to the methodology provided by Agustin and Padmawijaya (2017). In a brief, 1-cm-diameter bioplastics were placed on tryptone soya agar media [g/L—tryptone 17, soya peptone 3.0, sodium chloride 5.0, dextrose (Glucose) 2.5, dipotassium hydrogen phosphate 2.5, agar 15, final pH (at 25 °C) 7.3 ± 0.2]. The surface of this nutritional media was uniformly swabbed with the bacterial suspensions. Two gram-negative (Salmonella typhi, Escheritia coli) and three gram-positive (Bacillus subtilis, Bacillus megaterium, Bacillus haynesii) bacterial isolates (collected from the microbial culture stock of the Department of Life Sciences, Hemchandracharya North Gujarat University) were used in this test. The samples were then incubated for 24 h at 28 ± 2 °C. The inhibitory zone (clear zone) around the bioplastics samples was determined after incubation.
Results and discussion
Physicochemical properties of bioplastic films
To evaluate the effect of various combinations of plasticizers in the prepared biofilms, F-0 (containing 0% plasticizer), FG-10 (containing 10% glycerol), FS-10 (containing 10% sorbitol), and FG1-S1 (containing glycerol: sorbitol ratio = 1:1) samples were selected. FTIR analysis helps to determine any potential chemical alterations in the bioplastic samples. Also, additionally, it allows for quick, authentic, and efficient determination of functional groups (Pavia et al. 2001). In all samples, characteristic peaks (between 2900 and 3000 cm−1), which signify the (C-H) stretching due to the presence of starch, were observed (Figs. 1, 2, 3, 4 and Table 2). Previous studies have also reported similar peaks in starch-based bioplastic samples (Yin et al. 2005; Shafqat et al. 2021). In the case of the sample FG1-S1, peaks were observed that signify functional (O–H) groups at wavelength 1418 cm−1 (Fig. 4). This is because FG1-S1 was plasticized with both glycerol and sorbitol; both plasticizers are polyols (Ano et al. 2017) that contain a huge number of (O–H) groups (Chen et al. 2015a, b). The four bioplastic samples showed peaks (C = C) at the wavelength between 892 and 1664 cm−1 (Figs. 1, 2, 3, 4 and Table 2), similar to a previous report in which bioplastic films were generated using modified cassava starch (Harsojuwono et al. 2018). The 1,2,4-trisubstituted functional group was present only in the case of the samples plasticized with glycerol: sorbitol combinations (Table 2). The change in specific starch conformations induced by specific plasticizer combinations, which leads to polymer disorder, could be attributed to the altered intensity of specific peaks (Warren et al. 2016).
Thermogravimetric analysis (TGA) of potato starch-based bioplastic samples is shown in Fig. 5. The TGA diagram shows that the bioplastic samples have a continuous degradation pattern with a two-step process of decompositions. In the first step of decomposition, the light volatile compounds are lost and the evaporation of the water occurs at a temperature between 150 and 200 °C, which is similar to a previous report by Amin et al. (Amin et al. 2019). The decomposition graphs show that the T50% (the temperature at which 50% of weight loss occurred) for the film F-0 is 250–270 °C (Fig. 5a), for FG-10 it is 240–250 °C (Fig. 5b), and for FS-10 and FG1-S1 it is 300–320 °C (Fig. 5c and d). For the FS-10 and FG1-S1 films, a drastic weight loss was observed between temperatures 300 and 350 °C. In their study, Nurul et al. have also mentioned that for potato bioplastics, T50% are at 310 °C (Nurul et al. 2016). However, all bioplastic samples become fully decomposed at 450–500 °C. The overall results of the thermal analysis have shown that sorbitol-plasticized bioplastics have greater heat stability compared to those glycerol-plasticized bioplastics.
DSC was used to investigate the thermal characteristics of starch bioplastic and bioplastic composites such as melting temperature (Tm) as illustrated in Figs. 6, 7, 8, 9. The F-0 sample had the highest melting temperature (141.61 °C) (Fig. 6), followed by the FS-10 (138.46 °C, Fig. 8), FG1-S1 (135.11 °C, Fig. 9), and FG-10 (134.49 °C, Fig. 7). The crystallization temperature in these four bioplastic samples ranged from 147.69 °C to 156.66 °C (Figs. 6, 7, 8, 9), indicating that the starch-based bioplastics are semi-crystalline in nature (Amin et al. 2019). We were unable to identify any distinct Tg in the DSC thermograms for the tested bioplastic samples. Previous research had demonstrated that the Tg of starch-based bioplastics was seldom detected by DSC (Yokesahachart and Yoksan 2011). As a result, our findings are corroborated by previous reports.
Evaluation of the mechanical properties of bioplastics is crucial for assessing their utility (Azahari et al. 2011; Spiridon et al. 2013). Plasticizers significantly affected the tensile strength of the bioplastic samples (Fig. 10). The highest elongation (74.38%) was found in the FS-10 samples where sorbitol was used as a plasticizer (Fig. 10A). In FG1-S1 samples, the elongation was reduced to 13.56%, but the tensile strength was highest in this samples (4.12 MPa) (Fig. 10B). The results of the potato starch-based bioplastics tensile test have shown that the samples that have greater mechanical strength have less flexibility. Previous findings on plasticized biofilms of glycerol and sorbitol have shown similar trends for the tensile test (Ooi et al. 2012; Tapia-Blácido et al. 2013). The higher plasticization capacity of glycerol over sorbitol can be attributed to the fact that the molecular mass of glycerol is almost half that of sorbitol, therefore allowing the molecules of glycerol and starch to interact more efficiently (Shafqat et al. 2021). However, in the sample where a combination of the two plasticizers is added, both impact the bioplastic tensile simultaneously (Sanyang et al. 2016).
The XRD was employed to investigate the crystalline structures of the starch-based bioplastic samples. The results obtained from XRD analysis indicate the major pick at near 20° for all four bioplastic samples (Fig. 11A–D). Starch is a semi-crystalline material consisting of crystal units and amorphous units (Zhong and Xia 2008). There are two components in starch, namely amylose, which has a linear chain structure, and amylopectin, which has a branch chain structure (Zhaosheng et al. 2008). Therefore, our findings on the XRD analysis of the bioplastic samples are standard for starch-based materials (Jangong et al. 2019; Sangian et al. 2021).
Morphology
Figure 12A–D represents the digital images of the bioplastic films. The physical appearance of the bioplastics with different plasticizer concentrations is shown in Supplementary Figure S1. However, bioplastic should be tough enough to resist load-bearing pressure. The toughness could be assessed by the physico-tensile properties of bioplastics such as thickness, density, and tensile strength. As can be seen in the results from Fig. 12E, the bioplastic films formulated by the glycerol:sorbitol combination have the highest thickness followed by the sorbitol and glycerol-based samples. The lowest thickness was found in the samples without any plasticizer. The thicknesses obtained from the films in this work were greater than the 0.087–0.091 mm of potato starch nano-silica bioplastics reported by the Zhang et al. group Zhang et al. (2018). However, the differences in the thickness values recorded in this study may be related to the type and percentage concentration of added plasticizers (Oluwasina et al. 2021).
Furthermore, a directly proportional relationship between the thickness of the bioplastic film and the density was found (Fig. 12E). The high densities of bioplastic films may be related to the larger particle size of the bioplastic films (Reddy and Rhim 2014). Other findings have shown a correlation between increased density and the concentration of added plasticizers (Slavutsky et al. 2012; Abdullah et al. 2019).
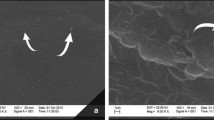
SEM micrographs are shown in Fig. 13A–H, which help to visually analyze the surface morphology and microstructure of the potato starch-based bioplastic samples. The study anticipated a comparison of bioplastics developed using different combinations of plasticizers. The results showed that the surface features consist of granules (the remaining part of the starch particle), which means that the starch was not fully gelatinized during the formation process (Fig. 13). These findings are similar to previous reports describing SEM images showing the presence of insoluble remains (i.e., ghosts) from the swelling starch granules (Hern'andez et al. 2017). The glycerol-based bioplastic sample (FG-10) contained some voids (Fig. 13D) that may have contributed to lower impact and tensile strength (Amin et al. 2019). However, the SEM images show that the potato starch and sorbitol plasticizers were relatively well homogenized, as the samples with sorbitol (FS-10 & FG1-S1) showed no phase separation, voids, or cracks (Fig. 13E, F). However, the incomplete gelatinization in bioplastic samples could be linked to the molecular rearrangements initiated by different starch: plasticizer ratios, which can induce amorphous, semi-crystalline, and crystalline arrangements of starch in the bioplastics (Capron et al. 2007).
Moisture content and water absorption study
The moisture content of the bioplastic samples increased when the plasticizers were added. From Fig. 14A, it can be found that the sample F-0 has a minimal value of moisture content. Bioplastic samples synthesized with glycerol as a plasticizer had the maximum values of moisture content, and bioplastics with sorbitol had the lowest value. However, in bioplastic samples where the glycerol–sorbitol combination was used, the moisture content had values lower than those of the glycerol samples but higher than those of the sorbitol-based samples. A previous study has also shown that the glycerol-based bioplastic formulation had a higher value than the sorbitol formulation, where two bioplastic samples were prepared from banana peel starch (BPP) and banana peel/corn starch/rice starch composite (COM) (Shafqat et al. 2021). This could be related to the fact that glycerol consists of a hydroxyl group that shows an affinity for water molecules that allows them to form hydrogen bonds and contain water in the structure (Cerqueira et al. 2012). However, sorbitol develops substantial hydrogen bonds with starch molecules, and therefore, the affinity to water molecules is reduced (Sanyang et al. 2016).
The water absorption values of the prepared bioplastic films are presented in Fig. 14B. As observed, films with no plasticizer (F-0) had the highest values (~ 80%) of water absorption. Among the plasticized samples, glycerol-based plasticization showed the highest water absorption, followed by a glycerol–sorbitol combination and then sorbitol. These findings are consistent with previous reports (Shafqat et al. 2021). Previous findings have also shown that glycerol has a relatively higher affinity for water compared to sorbitol (Cerqueira et al. 2012; Sanyang et al. 2016). This is because the hydroxyl group in starch has a strong affinity for water molecules and gelatinization also breaks up starch granules that allow water to diffuse (Azahari et al. 2011). Several reports have described a directly proportional relationship between the starch content and water uptake capacity of bioplastic films (Azahari et al. 2011; Aranda Garcia et al. 2015; Sujuthi and Liew 2016). Therefore, the addition of plasticizers reduces the absorption of water.
In the current work, the water vapor permeability of potato-based biodegradable plastic films made with various kinds and concentrations of plasticizers was tested using the WVTR method. Figure 15 displays the outcomes of the WVTR test. Due to their hydrophilic characteristics, biodegradable polymers derived from potatoes often have poor water vapor barriers (Othman et al. 2017). However, we discovered that the WVTR value is proportional to the concentration of plasticizers; when plasticizer concentration increased, WVTR increased as well, regardless of the type of plasticizer (Fig. 15). WVTR increased for sorbitol-based bioplastics from 76 to 104%, whereas it increased from 76 to 115% for glycerol-based bioplastics (Fig. 15). High plasticizer concentration increased the flexibility and mobility of starch polymer chains, resulting in a looser network through the structural change of starch–starch molecular interactions, could be one of the most plausible explanations for our findings (Sanyang et al. 2016; Ng et al. 2022).
Solubility and biodegradation investigation
Figure 16A and Table 3 show the solubility test results on bioplastic samples. Water solubility increased upon adding plasticizer. The reason why F-0 samples show the lowest value is that hydrogen bonds of the starch molecules’ crystalline structure of starch molecules significantly reduce their water solubility (Sarker et al. 2013). Similar to the moisture content, the water solubility of starch-based bioplastics was higher in samples with glycerol as a plasticizer, followed by the combination of glycerol–sorbitol and lowest in samples with sorbitol (Fig. 16A). Compared to sorbitol, glycerol has a higher affinity for water, and its molecular weight is also lighter, which allows water molecules to easily penetrate into polymer chains (Ghasemlou et al. 2011). These results are consistent with previous reports showing that it affects the solubility of bioplastic in water (Chiumarelli and Hubinger 2014; Shafqat et al. 2021).
From Table 3, it can be seen that all samples were soluble in water at both room temperature and elevated temperature (60 °C). At room temperature, bioplastic samples with sorbitol and glycerol–sorbitol combinations were insoluble in 10% NaOH and 10% H2SO4. However, with an increase in temperature, some samples became soluble in these solvents, and some showed partial swelling. All of these samples were insoluble in both methanol and ethanol, while some of them showed partial solubility and/or swelling in diethyl ether at elevated temperature. The current findings are also supported by previous reports by Pimpan et al. (2001).
The biodegradation ability of bioplastics is attributed to their physicochemical properties such as molecular weight, chemical structure, affinity to water, surface area, etc. (Tokiwa et al. 2009). The biodegradation ability of the bioplastic samples was evaluated in garden soil for up to 30 days. After every 5 days, the samples were observed, weighted, and the percent weight loss was calculated. From the time series data presented in Fig. 16A, the percentage of weight loss of all samples was observed to increase. Films with glycerol showed the highest percentage of biodegradation, after which samples with sorbitol and glycerol–sorbitol combination act as a plasticizer, respectively. The lowest weight loss was observed in the F-0 samples (Fig. 16B). However, all bioplastic samples degraded within 30 days and weight loss reached 100%. The addition of plasticizers that increases the biodegradation of samples can be attributed to the higher affinity of both the plasticizers (glycerol and sorbitol) toward water molecules (Shafqat et al. 2021). In this study, we excluded any fillers in the formulation of the bioplastic samples. A recent study has shown that the addition of filler to corn starch bioplastics reduces biodegradation (Mohan et al. 2016). However, the presence of filler provides a huge surface contact area through which degrading agents can enter the polymer (Rutkowska et al. 2002). The application of natural fillers has been reported to accelerate the biodegradation of bioplastics (Egute et al. 2009; Kumar and Sekaran 2014). Fillers demonstrate hydrophilic properties, and microorganisms can easily absorb them, resulting in enhanced adhesion of microorganisms to the surface of the polymer and its resultant biodegradation (Shah et al. 2008). Fillers that contain protein or easily soluble and hydrolyzed components improve biodegradation ability (Mastalygina et al. 2016). Therefore, as a future strategy, it is recommended to consider different kinds of waste materials to determine their efficiency as reinforcing filler in bioplastics.
The results of the antimicrobial test are shown in Table 4 and Fig. 17. The inhibitory response was calculated using the clear zone surrounding the bioplastic film disc. Measurement of clear zone diameter was started from the outside diameter of film bioplastics.
The absence of the clear zone indicates no inhibitory zone, and the diameter was valued as zero. The bioplastics have shown fair antimicrobial activity against the both gram-positive and gram-negative bacteria except B. haynesii where no clear zone was found. Also, FG1-S1 samples did not show any inhibitory activity against the S. typhii. A similar trend in data was also reported by Agustin and Padmawijaya (2017).
Conclusion
This study shows that potato starch, with or without the addition of different plasticizers, can be used to produce bioplastics with different physical and chemical properties. Differences in these properties will enhance the suitability of these bioplastics for various applications. The thickness values of the bioplastic films obtained in this study suggest that the bioplastic films have the stress-bearing strength needed for good packaging material. These potato starch-based bioplastics were biodegradable and environmentally friendly, making them a good substitute for petroleum-based plastics and an effective way to alleviate the problem of plastic pollution. However, further studies are needed to identify suitable raw materials for the synthesis of biodegradable biopolymers. It would be interesting to include different fillers obtained from a variety of waste materials and determine their efficiency as reinforcing fillers in bioplastics. The use of cheap raw materials for the production of bioplastics also increases their economic feasibility. Moreover, further research is needed to find agents that can impart hydrophobic properties to bioplastic films and enhance their moisture resistance.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdullah AH, Pudjiraharti MS, Karina M, Putri OD, Fauziyyah RH (2019) Fabrication and characterization of sweet potato starchbased bioplastics plasticized with glycerol. J Bio Sci 19:57–64. https://doi.org/10.3923/jbs.2019.57.64
Agustin YE, Padmawijaya KS (2017) Effect of glycerol and zinc oxide addition on antibacterial activity of biodegradable bioplastics from chitosan-kepok banana peel starch. IOP Conf Ser Mater Sci Eng 223:012046. https://doi.org/10.1088/1757-899X/223/1/01204
Amin MR, Chowdhury MA, Kowser MA (2019) Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 5(8):e02009. https://doi.org/10.1016/j.heliyon.2019.e02009
Ano Y, Hours RA, Akakabe Y, Kataoka N, Yakushi T, Matsushita K, Adachi O (2017) Membrane-bound glycerol dehydrogenase catalyzes oxidation of Dpentonates to 4-keto-D-pentonates, D-fructose to 5-keto-D-fructose, and Dpsicose to 5-keto-D-psicose. Biosci Biotechnol Biochem 81(2):411–418. https://doi.org/10.1080/09168451.2016.1254535
Aranda Garcia FJ, Gonzalez Nunez R, Jasso Gastinel CF, Mendizabal E (2015) Water absorption and thermomechanical characterization of extruded starch/poly (lactic acid)/agave bagasse fiber bioplastic composites. Int J Polym Sci 2015:343294. https://doi.org/10.1155/2015/343294
Avella M, Buzarovska A, Errico ME (2009) Gennaro gentile and grozdanovaeco challenges of bio-based polymer composites. Materials 2:911–925
Azahari NA, Othman N, Ismail H (2011) Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J Phys Sci 22(2):15–31
Capron I, Robert P, Colonna P, Brogly M, Planchot V (2007) Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydr Polym 68:249–259. https://doi.org/10.1016/j.carbpol.2006.12.015
Cerqueira MA, Souza BWS, Teixeira JA, Vicente AA (2012) Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocolloids 27(1):175–184. https://doi.org/10.1016/j.foodhyd.2011.07.007
Chen C, Zhao X, Yen H, Ho S, Cheng C, Lee D et al (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10. https://doi.org/10.1016/j.bej.2013.03.006
Chen R, Zhang C, Kessler MR (2015a) Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying OH numbers. J Appl Polym Sci 132(1):41213. https://doi.org/10.1002/app.41213
Chen X, Chen J, You T, Wang K, Xu F (2015b) Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr Polym 125:85–91. https://doi.org/10.1016/j.carbpol.2015.02.054
Chiumarelli M, Hubinger MD (2014) Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll 38:20–27. https://doi.org/10.1016/j.foodhyd.2013.11.013
Dufresne A (2012) Nanocellulose from nature to high performance tailored materials, 1st edn. Walter de Gruyter GmbH, Berlin, Boston, pp 413–414
Egute NS, Forster PL, Parra DF, Fermino DM, Santana S, Lugao AB (2009) Mechanical and thermal properties of polypropylene composites with curaua fibre irradiated with gamma radiation. Proc Int Nucl Atlantic Conf 41:35
Eliasson A (2004) Starch in food Structure function and applications. Woodhead Publishing Limited, Cambridge, pp 73–74
Farahnaky A, Saberi B, Majzoobi M (2013) Effect of glycerol on physical and mechanical properties of wheat starch edible films. J Texture Stud 44(3):176–186. https://doi.org/10.1111/jtxs.12007
García MA, Martino MN, Zaritzky N (2000) Microstructural characterization of plasticized starch-based films. Starch-Starke 52(4):118–124. https://doi.org/10.1002/1521-379X(200006)52:4%3c118::AID-STAR118%3e3.0.CO;2-0
Ghasemlou M, Khodaiyan F, Oromiehie A (2011) Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr Polym 84(1):477–483. https://doi.org/10.1016/j.carbpol.2010.12.010
Harsojuwono BA, Arnata IW, Mulyani S (2018) Bio-plastic characteristics from cassava starch modified in variations the temperature and pH of gelatinization. RJPBCS 9(2):290–296
Hern’andez CJ, Meraz M, Lara VH (2017) Acid hydrolysis of composites based on corn starch and trimethylene glycol as plasticizer. Revista Mexicana De Ingeniería Química 16(1):169–178. https://doi.org/10.24275/rmiq/Alim764
Imre B, Pukánszky B (2015) Compatibilization in bio-based and biodegradable polymer blends. Eur Polym J 49(6):1215–1233
Jain R, Tiwari A (2015) Biosynthesis of planet friendly bioplastics using renewable carbon source. J Environ Health Sci Eng 13(1):1–5
Jangong OS, Gareso PL, Mutmainna I, Tahir D (2019) Fabrication and characterization starch/chitosan reinforced polypropylene as biodegradable. J Phys: Conf Ser 1341:082022. https://doi.org/10.1088/1742-6596/1341/8/082022
Jost V, Kobsik K, Schmid M, Noller K (2014) Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr Polym 110:309–319. https://doi.org/10.1016/j.carbpol.2014.03.096
Kaseem M, Hamad K, Deri F (2012) Thermoplastic starch blends: a review of recent works. Polymer Sci Ser a 54:165–176
Khoramnejadian S, Zavareh JJ, Khoramnejadian S (2013) Effect of potato starch on thermal and mechanical properties on low-density polyethylene. Curr World Environ 8(2):215–220
Krishnamurthy A, Amritkumar P (2019) Synthesis and characterization of eco-friendly bioplastic from low-cost plant resources. SN Appl Sci. https://doi.org/10.1007/s42452-019-1460-x
Kumar KP, Sekaran ASJ (2014) Some natural fibers used in polymer composites and their extraction processes: a review. J Reinf Plast Compos 33(20):1879–1892. https://doi.org/10.1177/0731684414548612
Ma X, Yu J (2004) The plastcizers containing amide groups for thermoplastic starch. Carbohydr Polym 57(2):197–203. https://doi.org/10.1016/j.carbpol.2004.04.012
Mastalygina EE, Varyan IA, Kolesnikova NN, Monakhova TV, Karpova SG, Popov AA (2016) Effect of chemical composition and dimensional parameters of natural filler on structure formation and physical-chemical properties of polyethylene composites. AIP Conf Proc 1783(1):020150. https://doi.org/10.1063/1.4966443
Mohan TP, Devchand K, Kanny K (2016) Barrier and biodegradable properties of corn starch-derived biopolymer film filled with nanoclay fillers. J Plast Film Sheet 33(3):309–336. https://doi.org/10.1177/8756087916682553
Ng JS, Kiew PL, Lam MK et al (2022) Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics. Int J Environ Sci Technol 19:1545–1554. https://doi.org/10.1007/s13762-021-03213-5
Nurul AI, Tahir SM, Yahya N (2016) Synthesis and characterization of biodegradable starch-based bioplastics. Mater Sci Forum 846:673–678
Oluwasina OO, Lajide L, Owolabi BJ (2015) Performance of bonded boards using lignin-based resins. Wood Mater Sci Eng 10:168–177. https://doi.org/10.1080/17480272.2014.923044
Oluwasina OO, Falola T, Wahab JO, Idahagbon BN (2017) Enhancement of physical and mechanical properties of dioscorea dumetorum starch films with dialdehyde starch solution. Starch-Stärke 70(1–7):1700148. https://doi.org/10.1002/star.201700148
Oluwasina OO, Akinyele BP, Olusegun SJ, Oluwasina OO, Nelcy DSM (2021) Evaluation of the effects of additives on the properties of starch-based bioplastic film. SN Appl Sci 3:421. https://doi.org/10.1007/s42452-021-04433-7
Ooi ZX, Ismail H, Bakar AA, Aziz NAA (2012) The comparison effect of sorbitol and glycerol as plasticizing agents on the properties of biodegradable polyvinyl alcohol/rambutan skin waste flour blends. Polym-Plast Technol Eng 51(4):432–437. https://doi.org/10.1080/03602559.2011.639827
Othman SH, Edwalm SAM, Risyon NP, Basha RK, Talib RA (2017) Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci Technol 37(1):63–70. https://doi.org/10.1590/1678-457x.30216
Pavia DL, Lampman GM, Kriz GS (2001) Introduction to spectroscopy. Thomson Brooks/Cole, Philadelphia, USA, p 23
Perez S, Baldwin PM, Gallant DJ (2009) Starch Chemistry and Technology. Elsevier, Armsterdam, pp 149–192
Pimpan V, Ratanarat K, Pongchawanakul M (2001) Preliminary study on preparation of biodegradable plastic from modified cassava starch. J Sci Res Chula Univ 26(2):117–126
PlasticsEurope (2018) Plastics—the facts 2018: an analysis of European plastics production. Demand and waste data. https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf, Accessed date: 21 February, 2022
Reddy JP, Rhimm JW (2014) Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr Polym 110:480–488. https://doi.org/10.1016/j.carbpol.2014.04.056
Rutkowska M, Heimowska A, Krasowska K, Janik H (2002) Biodegradability of polyethylene starch blends in sea water. Polish J Environ Stud 11(3):267–272
Salgado PR, Ortiz SE, Petruccelli S, Mauri AN (2010) Biodegradable sunflower protein films naturally activated with antioxidant compounds. Food Hydrocoll 24:525–533. https://doi.org/10.1016/j.foodhyd.2009.12.002
Sangian HF, Maneking E, Tongkukut SHJ et al (2021) Study of SEM, XRD, TGA, and DSC of cassava bioplastics catalyzed by ethanol. IOP Conf Series Mater Sci Eng 1115:012052. https://doi.org/10.1088/1757-899X/1115/1/012052
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J Food Sci Technol 53(1):326–336. https://doi.org/10.1007/s13197-015-2009-7
Sarker MZI, Elgadir MA, Ferdosh S, Akanda MJH, Aditiawati P, Noda T (2013) Rheological behavior of starch-based biopolymer mixtures in selected processed foods. Starch-Stärke 65(1–2):73–81. https://doi.org/10.1002/star.201200072
Sarker A, Deepo DM, Nandi R, Rana J, Islam S, Rahman S, Hossain MN, Islam MS, Baroi A, Kim JE (2020) A review of microplastics pollution in the soil and terrestrial ecosystems: a global and Bangladesh perspective. Sci Total Environ 733:139296. https://doi.org/10.1016/j.scitotenv.2020.139296
Shafqat A, Tahir A, Mahmood A, Pugazhendhi A (2020) A review on environmental significance carbon foot prints of starch based bio-plastic: a substitute of conventional plastics. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2020.101540
Shafqat A, Al-Zaqri N, Tahir A, Alsalme A (2021) Synthesis and characterization of starch based bioplastics using varying plant-based ingredients, plasticizers and natural fillers. Saudi J Biol Sci 28(3):1739–1749. https://doi.org/10.1016/j.sjbs.2020.12.015
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Slavutsky AM, Bertuzzi MA, Armada M (2012) Water barrier properties of starch-clay nanocomposite films. Braz J Food Technol 15:208–218. https://doi.org/10.1590/S1981-67232012005000014
Spiridon I, Teacă CA, Bodîrlău R, Bercea M (2013) Behavior of cellulose reinforced cross linked starch composite films made with tartaric acid modified starch microparticles. J Polym Environ 21(2):431–440. https://doi.org/10.1007/s10924-012-0498-2
Statista (2020) https://www.statista.com/statistics/282732/global-production-ofplasticssince- 1950, Accessed date: 21 February, 2022
Sujuthi RAF, Liew KC (2016) Properties of bioplastic sheets made from different types of starch incorporated with recycled newspaper pulp. Trans Sci Technol 3(1–2):257–264
Tapia-Blácido DR, Sobral PJDA, Menegalli FC (2013) Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT - Food Sci Technol 50:392–400. https://doi.org/10.1016/j.lwt.2012.09.008
Thakur R, Pristijono P, Scarlett CJ, Bowyer M, Singh S, Vuong QV (2019) Starch-based films: major factors affecting their properties. Int J Biol Macromol 132:1079–1089. https://doi.org/10.1016/j.ijbiomac.2019.03.190
Thompson RC (2006) Plastic debris in the marine environment: consequences and solutions. Marine Nat Conserv Europe 193:107–115
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10(9):3722–3742. https://doi.org/10.3390/ijms10093722
Vieira MGA, da Silva MA, dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: a review. Eur Polym J 47(3):254–263. https://doi.org/10.1016/j.eurpolymj.2010.12.011
Warren F, Gidley M, Flanagan B (2016) Infrared spectroscopy as a tool to characterise starch ordered structure—a joint FTIR–ATR, NMR XRD and DSC Study. Carbohyd Polym 139:35–42. https://doi.org/10.1016/j.carbpol.2015.11.066
Xu A, Guo Ke, Liu T, Xiaofeng B, Zhang L, Wei C (2018) Effects of different isolation media on structural and functional properties of starches from root tubers of purple, yellow and white sweet potatoes. Molec (Basel, Switzerland). https://doi.org/10.3390/molecules23092135
Yang J, Ching YC, Julai JS, Chuah CH, Nguyenm DH, Linm P-C (2022) Comparative study on the properties of starch-based bioplastics incorporated with palm oil and epoxidized palm oil. Polym Polym Compos. https://doi.org/10.1177/09673911221087595
Yin Y, Li J, Liu Y, Li Z (2005) Starch crosslinked with poly(Vinyl Alcohol) by boric acid. J Appl Polym Sci 96:1394–1397. https://doi.org/10.1002/app.21569
Yokesahachart C, Yoksan R (2011) Effect of amphiphilic molecules on characteristics and tensile properties of thermoplastic starch and its blends with poly(lactic acid). Carbohydr Polym 83(1):22–31. https://doi.org/10.1016/j.carbpol.2010.07.020
Zhang Y, Rempel C, Liu Q (2018) Thermoplastic starch processing and characteristics-a review. Crit Rev Food Sci Nutr 54(10):1353–1370. https://doi.org/10.1080/104
Zhang SL, Wang JQ, Liu X, Qu FJ, Wang XS, Wang XR, Li Y, Sun YK (2019) Microplastics in the environment: a review of analytical methods, distribution, and biological effects. Trend Anal Chem 111:62–72. https://doi.org/10.1016/j.trac.2018.12.002
Zhaosheng Y, Xiaoqian M, Ao L (2008) Kinetic studies on catalytic combustion of rice and wheat straw under air-and oxygen-enriched atmospheres, by using thermogravimetric analysis. Biomass Bioenerg 32(11):1046–1055
Zhong QP, Xia WS (2008) Physicochemical properties of edible and preservative films from chitosan/cassava starch/gelatin blend plasticized with glycerol. Food Technol Biotechnol 46(3):262–269
Acknowledgements
The support of the Department of Biotechnology and the Department of Life Sciences of Hemchandracharya North Gujarat University, Patan, Gujarat, India, is greatly appreciated. This research was supported by a grant (Award No. RC2- 2018-009) from the Center for Membranes and Advanced Water Technology (CMAT) funded by Khalifa University of Science and Technology in Abu Dhabi (UAE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflcit of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: T.Karak.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, M., Islam, S., Kallem, P. et al. Potato starch-based bioplastics synthesized using glycerol–sorbitol blend as a plasticizer: characterization and performance analysis. Int. J. Environ. Sci. Technol. 20, 7843–7860 (2023). https://doi.org/10.1007/s13762-022-04492-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04492-2